Abstract
Probiotics such as bifidobacteria have been given to low-birth-weight neonates (LBWNs) at risk for a disrupted gut microbiota leading to the development of serious diseases such necrotizing enterocolitis. Recently prebiotics such as lactulose are used together with bifidobacteria as synbiotics. However, faster and more powerful bifidobacteria growth is desired for better LBWN outcomes. The prebiotic 1-kestose has a higher selective growth-promoting effect on bifidobacteria and lactic acid bacteria in vitro among several oligosaccharides. Twenty-six premature neonates (less than 2,000 g) admitted to a neonatal intensive care unit (NICU) were randomly assigned to receive Bifidobacterium breve M16-V with either 1-kestose or lactulose once a day for four weeks from birth. A 16S rRNA gene analysis revealed similar increases in alpha-diversity from 7 to 28 days in both groups. The most dominant genus on both days was Bifidobacterium in both groups, with no significant difference between the two groups. Quantitative PCR analysis revealed that the number of Staphylococcus aureus tended to be lower in the 1-kestose group than in the lactulose group at 28 days. The number of Escherichia coli was higher in the 1-kestose group at 7 days. The copy number of total bacteria in the 1-kestose group was significantly higher than that in the lactulose group at 3 time points, 7, 14, and 28 days. No severe adverse events occurred in either group during the study period. l-Ketose may offer an alternative option to lactulose as a prebiotic to promote the development of gut microbiota in LBWNs.
Keywords: 1-kestose, lactulose, low birth weight neonates, bifidobacteria, Staphylococcus aureus, gut microbiota
INTRODUCTION
The intestinal microbiota in early life is important for reducing neonatal morbidity and mortality [1], and it also has impacts on later health [2]. Low-birth-weight neonates (LBWNs) are at risk for disrupted normal microbiome development related to prematurity, and this disruption, called dysbiosis, can result in necrotizing enterocolitis (NEC) and sepsis [3, 4]. In recent years, LBWNs admitted to neonatal intensive care units (NICUs) have been given probiotics from an early stage [5]. Several reports have suggested that probiotics promote normal flora and may contribute to the prevention of NEC and other neonatal infections [6,7,8]. Furthermore, prebiotics have recently been administered to promote the growth of bifidobacteria [9, 10]. The combination of Bifidobacterium breve and lactulose is routinely given to LBWNs currently in Japan [11]. However, faster and more powerful bifidobacteria growth are still desired.
1-Kestose is a fructo-oligosaccharide that is a prebiotic with a trisaccharide structure. It has a higher selective growth-promoting effect on bifidobacteria and lactic acid bacteria than other prebiotics in vitro and in vivo in animal models [12, 13]. In clinical studies, 1-kestose has been found to have some beneficial effects in infants with atopic dermatitis [14, 15] and in adults with obesity [16]. However, there have been no studies examining the effects of 1-kestose on bifidobacteria growth and gut microbiota in neonates, especially in LBWNs. Therefore, we conducted a randomized control study to investigate the efficacy with respect to the development of gut microbiota and the safety of 1-kestose in LBWNs in comparison with lactulose.
PARTICIPANTS AND METHODS
Patients and study design
The eligible neonates included in this study were LBWNs who weighed less than 2,000 g and were born and admitted to the NICU of Kimitsu-Chuo Hospital in Chiba Prefecture, Japan, from May 2019 to March 2020. Neonates who were not expected to be given enteral nutrition or internal medication soon at birth due to surgical disorders, such as esophageal obstruction or diaphragmatic hernia, were excluded in this study. Written informed consent was obtained from the guardians of all subjects after an explanation of the study. The study neonates were randomly assigned into two groups using a computer before the first enteral feeding, and both groups received 5.0 × 108B. breve M16-V as a probiotic. As a prebiotic, 0.3 g of 1-kestose (Kestose 95, B Food Science Co., Ltd., Aichi, Japan) dissolved in water or 0.2 g of lactulose (Lactulose Syrup 65%, TAKATA Pharmaceutical Co., Ltd., Saitama, Japan) was given by oral or tubal nutrition once a day from day 1 to day 28. Fecal samples were collected at 0, 7, 14, and 28 days of age, immediately stored at −30°C for about 1 month, and kept at −80°C until used for analysis. This study was designed according to the Declaration of Helsinki for experiments with human beings, approved by the ethical committee of the Graduate School of Medicine of Chiba University (No. 3133), and registered in UMIN-CTR (UMIN000034023).
DNA extraction
Bacterial DNA was extracted from feces as described by Takahashi et al. [17]. Briefly, frozen fecal samples (0.1 g) were added to tubes that contained 4 M guanidium thiocyanate, 100 mM Tris-HCl (pH 9.0), and 40 mM EDTA 800 µL, and the samples were then homogenized with zirconia beads using a Precellys Evolution instrument (Bertin Instruments, Paris, France). DNA was extracted from the bead-treated suspensions using a Magtration System 12GC and GC series MagDEA DNA 200 (Precision System Science Co., Ltd., Chiba, Japan). DNA was purified using PI-480 and NR-201 (Kurabo Industries, Ltd., Osaka, Japan), and DNA concentrations were estimated by spectrophotometry using a NanoDrop ND-8000 instrument (Thermo Fisher Scientific, Waltham, MA, USA).
16S rRNA gene sequence analysis using next generation sequencing
Fecal bacterial 16S rRNA genes (16S rDNA) were analyzed by next generation sequencing (NGS) using the MiSeq system (Illumina, San Diego, CA, USA) as previously described [17]. The V3–V4 hypervariable regions of 16S rDNA were amplified from microbial genomic DNA by polymerase chain reaction (PCR) using universal primers for bacteria (341f and R806, Supplementary Table 1) and the dual-index method [18]. Barcoded amplicons were sequenced using the paired-end method and were modified to 2 × 284-bp cycle runs on the MiSeq system using MiSeq Reagent Kit version 3 (600 cycle; Illumina). After alignment, the overlapping regions within the paired-end reads were merged, and primer regions were omitted, which resulted in a 430 bp sequence. Only reads with ≥99% of their sequence with quality value scores of ≥20 were extracted for further analysis. The chimeric sequence detected by Usearch6.1.544_i86 was excluded [18]. Based on the sequences, the taxonomic positions of the sequences were identified at 97% similarity using the Metagenome@KIN analysis software (World Fusion, Tokyo, Japan) and TechnoSuruga Lab Microbial Identification database DB-BA 13.0 (TechnoSuruga Laboratory, Shizuoka, Japan) [19, 20]. The sequences were deposited in a public database (DRA Submission ID: bfs_rdc-0001).
Quantitative real-time PCR analysis of intestinal microorganisms
Using the extracted DNA samples used in NGS analysis, quantitative analysis of the following intestinal organisms was performed by real-time PCR: Bifidobacterium spp., Staphylococcus spp., Bifidobacterium longum, B. breve, Staphylococcus aureus, Escherichia coli, and Klebsiella pneumoniae. The primers are listed in Supplementary Table 1. A standard curve for each bacterium was prepared using the PCR product of a known concentration as a template. The quantitative real-time PCR (qPCR) analysis was performed using the extracted DNA as a template and TB Green fast qPCR Mix (Takara bio). The obtained Ct value was applied to a standard curve to quantify the copy number of the target bacteria contained in the extracted DNA.
Safety monitoring
For the safety of dosing, we checked for adverse events during the study period. The number of daily stools was counted each week to evaluate adverse gastrointestinal symptoms, and weight trends also were checked to evaluate weight gain failure.
Statistical analysis
Statistical analyses were performed using the IBM SPSS Statistics 26 software package (IBM Corp., Armonk, NY, USA). The normality of data was examined by the Shapiro–Wilk test. Paired two-group comparisons were made using the Wilcoxon signed-rank test. The Mann–Whitney U test was performed for comparisons between two groups without correspondence. Pearson’s correlation coefficient was calculated to evaluate data. Differences were considered to be significant at p<0.05 and to be a tendency at p<0.1.
RESULTS
Characteristics of the study subjects
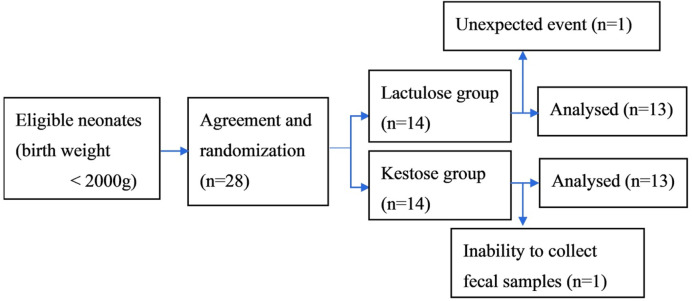
Out of 28 eligible neonates, two were unable to complete the study due to unexpected events or inability to collect fecal samples. Therefore, 26 children were analyzed as subjects in this study: 13 neonates in the 1-kestose group and 13 in the lactulose group (Fig. 1). Table 1 and Supplementary Table 2 shows the baseline characteristics of the subjects who completed the study. The mean birth weights of the 1-kestose group and lactulose group were 1,544 g (range 650–1,772 g) and 1,470 g (range 847–1,998 g), respectively. The mean gestational ages at birth of the 1-kestose group and lactulose group were 31.0 weeks (range 24–33 weeks) and 31.0 weeks (range 24–34 weeks), respectively. At baseline, no differences between the 2 groups were identified for sex, gestational age, birth weight, mode of delivery, enteral feeding, or antibiotic use.
Fig. 1.
Flow diagram.
Table 1. Patient characteristics.
| 1-kestose | Lactulose | p-value | |
|---|---|---|---|
| N=13, N (%) | N=13, N (%) | ||
| Male sex, n (%) | 9 (69.2) | 8 (61.5) | 0.680 |
| Gestational age (weeks), median [range] | 31.0 [24–33] | 31.0 [24–34] | 0.429 |
| <32 weeks of gestation age, n (%) | 9 (69.2) | 8 (61.5) | |
| Birth weight (g), median [range] | 1,544 [650–1,772] | 1,470 [847–1,998] | 0.798 |
| <1,500 g of birth weight, n (%) | 6 (46.2) | 7 (53.8) | |
| Caesarean section, n (%) | 11 (84.6) | 11 (84.6) | 1.000 |
| Own mother’s milk only, n (%) | 2 (15.4) | 3 (23.1) | 0.618 |
| Cow milk-based formula only, n (%) | 0 (0.0) | 2 (15.4) | 0.086 |
| Mixed (formula and any breast milk), n (%) | 11 (84.6) | 8 (61.5) | 0.179 |
| Antibiotic use, n (%) | 3 (23.1) | 3 (23.1) | 1.000 |
16S rRNA gene analysis of intestinal microbiota
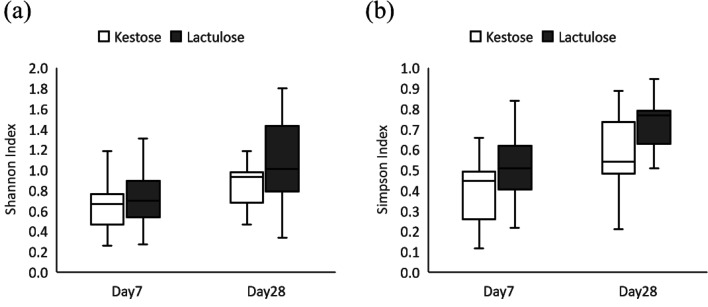
Many of the day 0 samples were not subjected to 16S rRNA analysis and real-time PCR analysis, because sufficient DNA concentrations were not obtained. The total numbers of reads analyzed for each sample at the different time points (days 7 and 28) were 18,277 ± 6,122 and 36,167 ± 10,559 (mean ± SD), respectively. The results for gut microbial diversity (alpha-diversity) calculated from the number of reads for each genus are shown in Fig. 2. Alpha-diversity indices slightly increased from 7 to 28 days, and no significant difference was identified between the groups at the same time points.
Fig. 2.
Alpha-diversity index of gut microbiota in each group. (a) Shannon alpha-diversity index, (b) Simpson alpha-diversity index.
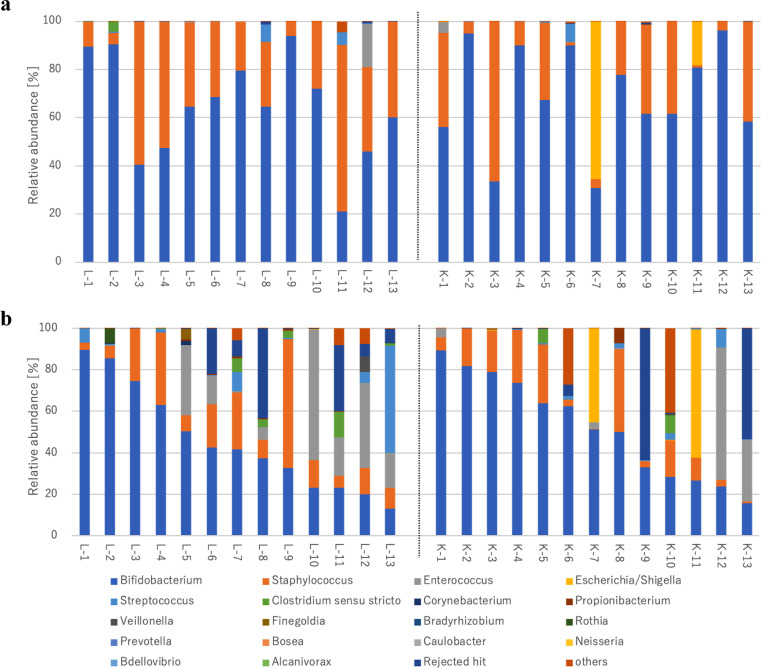
The relative abundance ratios of intestinal microbiota at 7 and 28 days, calculated from the number of reads, are shown in Table 2. The most dominant genus in both groups and on both days was Bifidobacterium followed by Staphylococcus. The ratios of both Bifidobacterium spp. and Staphylococcus spp. decreased from at 7 days to at 28 days. A comparison between 2 groups at the same age showed no significant difference in any of the relative abundance ratios. The relative abundance ratios for each neonate at 7 days and at 28 days are shown in Fig. 3a, 3b.
Table 2. Relative abundance ratios of gut microbiota at the genus level.
| Relative abundance median (25%–75%) (% detection ratio) | ||||
|---|---|---|---|---|
| 1-kestose | Lactulose | |||
| Day 7 | Day 28 | Day 7 | Day 28 | |
| Bifidobacterium | 67.3 (58.4–89.9) | 51.1 (28.5–73.6) | 64.5 (47.4–79.6) | 41.6 (23.3–62.9) |
| Staphylococcus | 22.0 (3.9–38.5) | 10.9 (3–20.1) | 31.2 (20.4–39.8) | 12.8 (7.8–25.5) |
| Enterococcus | 0.0 (0.0–0.0) | 0.1 (0.0–3.4) | 0.0 (0.0–0.0) | 6.0 (0.1–18.6) |
| Streptococcus | 0.1 (0.0–0.2) | 0.5 (0.1–1.6) | 0.1 (0–0.4) | 0.4 (0.1–5.3) |
| Clostridium sensu stricto | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.1 (0.0–3.6) |
The results are shown for five bacteria in which one of the median values at the same time point in the same group was 0.1% or higher.
Fig. 3.
Relative abundance ratios of gut microbiota at the genus level for each neonate at 7 days (3a), and at 28 days (3b). The relative abundance ratios of each neonates in the lactulose group (L-) were shown in the left side of this figure and those in the 1-kestose group (K-) were shown in the right side of this figure.
Quantitation of the number of intestinal bacteria by real-time PCR
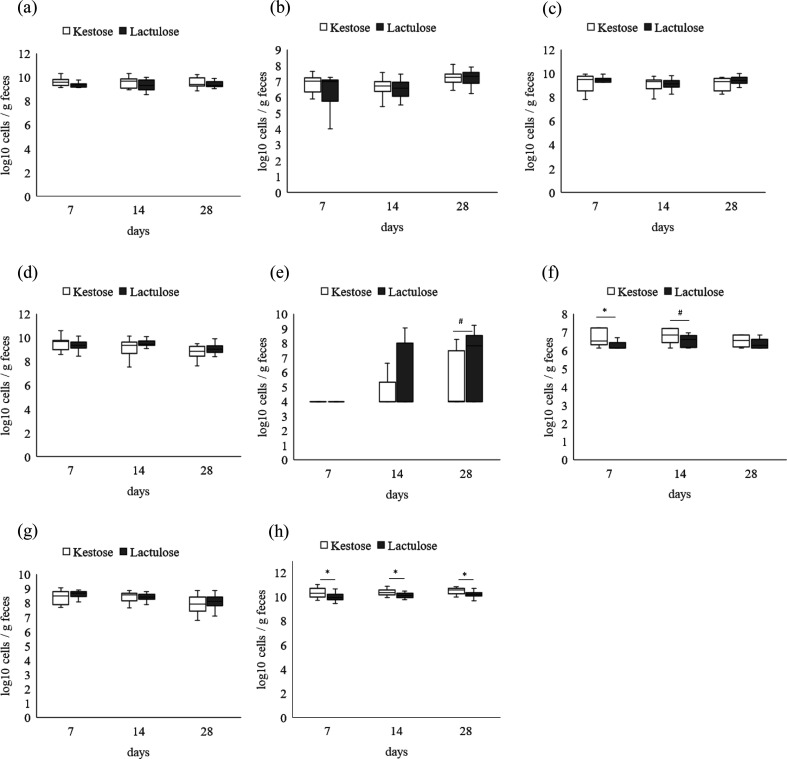
Quantitative results for each bacterium are shown in Fig. 3. No significant difference was observed between the two groups for Bifidobacterium, B. longum, B. breve, Staphylococcus, and K. pneumoniae at 3 time points (Fig. 4a–4d, 4g). The number of S. aureus tended to be lower in the 1-kestose group than in the lactulose group at 28 days (Fig. 4e). E. coli was significantly higher in the 1-kestose group at 7 days and tended to be higher at 14 days (Fig. 4f). The copy number of total bacteria in the 1-kestose group was significantly higher than that in lactulose groups at 3 time points, 7, 14, and 28 days (Fig. 4h).
Fig. 4.
Bacterial counts of representative species at 7 and 28 days detected by RT-PCR of 16S rDNA from genomic DNA extracted from fecal samples. (a) Bifidobacterium spp., (b) Bifidobacterium longum, (c) Bifidobacterium breve, (d) Staphylococcus spp., (e) Staphylococcus aureus, (f) Escherichia coli, (g) Klebsiella pneumoniae. (h) All Bacteria. Median ± interquartile range and complete range, *p<0.05, ♯p<0.1.
Adverse events of 1-kestose
During the study period, there were no severe adverse events, such as NEC, sepsis, or death, in either group. The mean numbers of stools at day 28 (mean ± SD) in the 1-kestose and lactulose groups were 3.6 ± 1.7 and 3.6 ± 1.5, respectively. Weight gain was almost normal in both groups: the mean gain rates at 28 days (g/day, mean ± SD) in the 1-kestose and lactulose groups were 10.9 ± 3.6 and 11.7 ± 4.6, and the number of days to regain to birth weight (days, mean ± SD) were 14.1 ± 3.9 and 15.5 ± 3.9, respectively. There were no significant differences in these parameters between the two groups during the study period.
DISCUSSION
In this study, we compared the effect of 1-kestose, which has a higher ability to grow bifidobacteria and other useful bacteria among existing prebiotics [21], on intestinal microbiota in LBWNs with that of lactulose, which is routinely used as a prebiotic for infants under care in NICUs. There was no difference in bacterial diversity between the two groups. 1-Kestose was similar to lactulose in terms of bifidobacterial growth at 3 time points between 1 to 4 weeks of age. S. aureus is one of the most critical pathogenic bacteria that can cause NEC in low-birth-weight neonates [22, 23]. There was a trend toward suppression of S. aureus colonization in the 1-kestose group at 4 weeks of age, but it was not statistically significant compared with the lactulose group. This may be due to the higher total number of bacteria in the 1-kestose group. Although bacterial count and copy number are not synonymous, qPCR is currently the most commonly used method of quantification [24]. Several papers reported quantification of total bacterial count [25, 26]. Probiotics have been reported to increase total bacterial counts at 2 and 3 weeks of age compared with controls [27], suggesting the high potential of 1-kestose as a prebiotic to promote the effects of the probiotic B. breve M16-V.
Regarding the higher number of E. coli at 7 and 14 days in the 1-kestose group, this may be attributed to two neonates in the 1-kestose group having extremely high abundances of E. coli in their floras, which seem to have been in the process of sequential transition from Enterobacteriaceae- to Bifidobacteriaceae-dominated microbiota [28]. However, because of the limited number of subjects in this pilot study, it is not possible to conclude whether these results are due to individual differences or intervention.
Safety as well as efficacy of prebiotics is important in clinical practice. Many previous studies have shown that prebiotics are safe [29]; however, studies on the safety of 1-kestose are limited. Previous clinical studies on 1-kestose have not described severe adverse events for infants over six months of age or in animal experiments [14,15,16]. Although small doses of 1-kestose (0.3 g/day) administered to infants did not cause severe diarrhea, weight loss, or other adverse events, further studies with larger numbers of subjects are needed to determine the appropriate dosage, efficacy, and safety as a prebiotic in LBWNs.
A major limitation of this study is the small sample size. Due to the exploratory nature of this study, an acceptable sample size could not be calculated. Therefore, this clinical trial was a pilot study with a small sample size. The study was conducted in a single-blinded fashion. Although gut microbiota analysis was performed in a blinded fashion after processing the information anonymously, double blinding would be preferable for future larger studies.
In conclusion, 1-kestose may offer an alternative option to lactulose as a prebiotic to promote the development of gut microbiota in LBWNs. Larger studies are needed to elucidate the potential of 1-kestose as a prebiotic in LBWNs.
FUNDING
This work was supported by B Food Science-Chiba University joint research expenses (J20KK00061).
CONFLICT OF INTEREST
M. Takahashi, Y. Kadota, and T. Tochio are employed by B Food Science Co., Ltd., which produces the 1-kestose used in the present study. This study was funded by B Food Science Co., Ltd.
Supplementary Material
Acknowledgments
We thank all the babies and families involved in this study, as well as all the staff in the NICU who cared for the patients and collected study samples.
REFERENCES
- 1.Morgan RL, Preidis GA, Kashyap PC, Weizman AV, Sadeghirad B, McMaster Probiotic, Prebiotic, and Synbiotic Work Group 2020. Probiotics reduce mortality and morbidity in preterm, low-birth-weight infants: a systematic review and network meta-analysis of randomized trials. Gastroenterology 159: 467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derrien M, Alvarez AS, de Vos WM. 2019. The gut microbiota in the first decade of life. Trends Microbiol 27: 997–1010. [DOI] [PubMed] [Google Scholar]
- 3.Stewart CJ, Marrs ECL, Magorrian S, Nelson A, Lanyon C, Perry JD, Embleton ND, Cummings SP, Berrington JE. 2012. The preterm gut microbiota: changes associated with necrotizing enterocolitis and infection. Acta Paediatr 101: 1121–1127. [DOI] [PubMed] [Google Scholar]
- 4.Mai V, Torrazza RM, Ukhanova M, Wang X, Sun Y, Li N, Shuster J, Sharma R, Hudak ML, Neu J. 2013. Distortions in development of intestinal microbiota associated with late onset sepsis in preterm infants. PLoS One 8: e52876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horigome A, Hisata K, Odamaki T, Iwabuchi N, Xiao JZ, Shimizu T. 2021. Colonization of supplemented Bifidobacterium breve M-16V in low birth weight infants and its effects on their gut microbiota weeks post-administration. Front Microbiol 12: 610080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aceti A, Maggio L, Beghetti I, Gori D, Barone G, Callegari ML, Fantini MP, Indrio F, Meneghin F, Morelli L, et al. Italian Society of Neonatology. 2017. Probiotics prevent late-onset sepsis in human milk-fed, very low birth weight preterm infants: systematic review and meta-analysis. Nutrients 9: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gómez-Rodríguez G, Amador-Licona N, Daza-Benítez L, Barbosa-Sabanero G, Carballo-Magdaleno D, Aguilar-Padilla R, González-Ramirez E. 2019. Single strain versus multispecies probiotic on necrotizing enterocolitis and faecal IgA levels in very low birth weight preterm neonates: a randomized clinical trial. Pediatr Neonatol 60: 564–569. [DOI] [PubMed] [Google Scholar]
- 8.Thomas JP, Raine T, Reddy S, Belteki G. 2017. Probiotics for the prevention of necrotising enterocolitis in very low-birth-weight infants: a meta-analysis and systematic review. Acta Paediatr 106: 1729–1741. [DOI] [PubMed] [Google Scholar]
- 9.Rada V, Nevoral J, Trojanová I, Tománková E, Smehilová M, Killer J. 2008. Growth of infant faecal bifidobacteria and clostridia on prebiotic oligosaccharides in in vitro conditions. Anaerobe 14: 205–208. [DOI] [PubMed] [Google Scholar]
- 10.Karakan T, Tuohy KM, Janssen-van Solingen G. 2021. Low-dose lactulose as a prebiotic for improved gut health and enhanced mineral absorption. Front Nutr 8: 672925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riskin A, Hochwald O, Bader D, Srugo I, Naftali G, Kugelman A, Cohen E, Mor F, Kaufman B, Shaoul R. 2010. The effects of lactulose supplementation to enteral feedings in premature infants: a pilot study. J Pediatr 156: 209–214. [DOI] [PubMed] [Google Scholar]
- 12.Patterson JA, Orban JI, Sutton AL, Richards GN. 1997. Selective enrichment of bifidobacteria in the intestinal tract of broilers by thermally produced kestoses and effect on broiler performance. Poult Sci 76: 497–500. [DOI] [PubMed] [Google Scholar]
- 13.Endo A, Hirano K, Ose R, Maeno S, Tochio T. 2020. Impact of kestose supplementation on the healthy adult microbiota in in vitro fecal batch cultures. Anaerobe 61: 102076. [DOI] [PubMed] [Google Scholar]
- 14.Shibata R, Kimura M, Takahashi H, Mikami K, Aiba Y, Takeda H, Koga Y. 2009. Clinical effects of kestose, a prebiotic oligosaccharide, on the treatment of atopic dermatitis in infants. Clin Exp Allergy 39: 1397–1403. [DOI] [PubMed] [Google Scholar]
- 15.Koga Y, Tokunaga S, Nagano J, Sato F, Konishi K, Tochio T, Murakami Y, Masumoto N, Tezuka JI, Sudo N, et al. 2016. Age-associated effect of kestose on Faecalibacterium prausnitzii and symptoms in the atopic dermatitis infants. Pediatr Res 80: 844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe A, Tochio T, Kadota Y, Takahashi M, Kitaura Y, Ishikawa H, Yasutake T, Nakano M, Shinohara H, Kudo T, et al. 2021. Supplementation of 1-kestose modulates the gut microbiota composition to ameliorate glucose metabolism in obesity-prone hosts. Nutrients 13: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi S, Tomita J, Nishioka K, Hisada T, Nishijima M. 2014. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS One 9: e105592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27: 2194–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hisada T, Endoh K, Kuriki K. 2015. Inter- and intra-individual variations in seasonal and daily stabilities of the human gut microbiota in Japanese. Arch Microbiol 197: 919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasai C, Sugimoto K, Moritani I, Tanaka J, Oya Y, Inoue H, Tameda M, Shiraki K, Ito M, Takei Y, et al. 2015. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol 15: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ose R, Hirano K, Maeno S, Nakagawa J, Salminen S, Tochio T, Endo A. 2018. The ability of human intestinal anaerobes to metabolize different oligosaccharides: novel means for microbiota modulation? Anaerobe 51: 110–119. [DOI] [PubMed] [Google Scholar]
- 22.Rozé JC, Ancel PY, Lepage P, Martin-Marchand L, Al Nabhani Z, Delannoy J, Picaud JC, Lapillonne A, Aires J, Durox M, et al. Nutrition EPIPAGE 2 study group EPIFLORE Study Group 2017. Nutritional strategies and gut microbiota composition as risk factors for necrotizing enterocolitis in very-preterm infants. Am J Clin Nutr 106: 821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brehin C, Dubois D, Dicky O, Breinig S, Oswald E, Serino M. 2020. Evolution of gut microbiome and metabolome in suspected necrotizing enterocolitis: a case-control study. J Clin Med 9: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fogel GB, Collins CR, Li J, Brunk CF. 1999. Prokaryotic genome size and SSU rDNA copy number: estimation of microbial relative abundance from a mixed population. Microb Ecol 38: 93–113. [DOI] [PubMed] [Google Scholar]
- 25.Funahara M, Yamaguchi R, Honda H, Matsuo M, Fujii W, Nakamichi A. 2023. Factors affecting the number of bacteria in saliva and oral care methods for the recovery of bacteria in contaminated saliva after brushing: a randomized controlled trial. BMC Oral Health 23: 917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koirala R, Gargari G, Arioli S, Taverniti V, Fiore W, Grossi E, Anelli GM, Cetin I, Guglielmetti S. 2020. Effect of oral consumption of capsules containing Lactobacillus paracasei LPC-S01 on the vaginal microbiota of healthy adult women: a randomized, placebo-controlled, double-blind crossover study. FEMS Microbiol Ecol 96: fiaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurath-Koller S, Neumann C, Moissl-Eichinger C, Kraschl R, Kanduth C, Hopfer B, Pausan MR, Urlesberger B, Resch B. 2020. Hospital regimens including probiotics guide the individual development of the gut microbiome of very low birth weight infants in the first two weeks of life. Nutrients 12: 1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuki T, Yahagi K, Mori H, Matsumoto H, Hara T, Tajima S, Ogawa E, Kodama H, Yamamoto K, Yamada T, et al. 2016. A key genetic factor for fucosyllactose utilization affects infant gut microbiota development. Nat Commun 7: 11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srinivasjois R, Rao S, Patole S. 2009. Prebiotic supplementation of formula in preterm neonates: a systematic review and meta-analysis of randomised controlled trials. Clin Nutr 28: 237–242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.