Abstract
Objective:
Experiencing potentially traumatic events across one’s lifecourse increases risk for poor physical health outcomes. Existing models emphasize the effects of any lifetime trauma exposure, risk accumulation (multiple traumas over time), and sensitive periods of exposure (specific exposure timepoints leading to lasting consequences). We examined how different indices of trauma exposure across the lifecourse were associated with later life arthritis, a common and debilitating health condition.
Methods:
Data include 5,717 Health and Retirement Study participants (age mean=65.3, SD=12.9) who reported on lifetime adversity and trauma in 2006–2008. Lifetime trauma exposure was modeled as any trauma, accumulation of traumas, and lifecourse profiles (no exposure, childhood only, adulthood only, childhood and adulthood exposure). Outcomes included prevalent arthritis at baseline and incident arthritis across 12 years of follow-up. Covariate-adjusted generalized linear models for prevalence ratios (PR) and Cox proportional hazards models for hazard ratios (HR) were conducted.
Results:
Any lifetime trauma was associated with both prevalent arthritis at baseline (PR=1.13, 95%CI 1.05–1.22) and incident arthritis over 12 years (HR=1.25, 95%CI 1.17–1.47). Greater trauma accumulation was significantly associated with both prevalent and incident arthritis. Childhood exposure was particularly strongly associated with prevalent and incident cases, with adulthood exposure being unassociated with incident arthritis. Across models, trauma exposure was associated with prevalent cases of both immune-related and osteoarthritis types.
Conclusions:
Higher lifetime trauma burden, especially during childhood, may predispose individuals to arthritis later in life. Early intervention or prevention efforts should identify trauma as an important risk factor for musculoskeletal health across the lifecourse.
Keywords: trauma, accumulation of risk, sensitive periods, arthritis
Introduction
Exposure to potentially traumatic events (hereafter referred to as trauma),1 where individuals experience actual or threatened death or serious injury or violence, is a major risk factor for negative psychological and physical outcomes.2,3 One chronic condition potentially linked to experiences of trauma is arthritis, a category of musculoskeletal disorders resulting from inflammatory or degenerative processes affecting musculoskeletal tissue. Lifetime trauma exposure may influence risk for arthritis via multiple pathways, including physiological factors like stress response system dysregulation leading to increased allostatic load; psychological responses involving posttraumatic stress, depressive, or anxiety symptoms; and behavioral reactions such as engagement in risky health or adverse coping behaviors.4–6 Examining whether and how traumatic experiences across the lifecourse impact arthritis risk may highlight opportunities for early intervention and prevention.
Arthritis is common, impacting an estimated 24% of US adults and projected to increase in prevalence in coming years with an aging population.7 Indeed, about 50% of adults over age 65 report experiencing arthritis, and arthritis is socially patterned with women and those with lower socio-economic status more likely to be affected.7 The burden of arthritis on individuals and the population is substantial, leading to pain, social and work-related impairment, and lower physical activity and well-being, representing the most frequently reported main cause of disability in US adults.7–9 Common risk factors for arthritis generally include biological (e.g., body mass index, genetic predisposition, hormones), behavioral (e.g., smoking, diet quality), and psychosocial (e.g., stress) factors.10–12 However, there are distinct etiologies for different forms of arthritis. Osteoarthritis is most common, defined by joint symptoms including tissue damage, loss of cartilage, and inflammation,11 with risk factors including genetics, obesity, hormones, bone mineral density, diet, and acute injury.9,11 In contrast, immune-related arthritis, like rheumatoid arthritis (RA), represents systemic autoimmune conditions characterized by loss of self-tolerance and joint inflammation.10,13 Although the exact etiology of RA is unknown, genetic, behavioral, environmental, and psychosocial risk factors have been identified.8,10,14 Psychological trauma may predispose individuals to osteoarthritis or immune-related arthritis to a different extent and through distinct mechanistic pathways. While some studies link trauma with arthritis outcomes,15–19 little work has examined how trauma may impact different forms of arthritis.
Three key models have been proposed in the epidemiological literature to explain how experiences across the lifecourse impact health.20 First, in any lifetime trauma exposure models, any exposure at any time is expected to lead to shifts in psychological and biological setpoints that confer increased lifetime risk of poor health outcomes.5 Foundational work on trauma exposure and physical health indicates that experience of trauma (not accounting for timing, chronicity, type, etc.) can impact health, through psychological (e.g., distress symptoms), biological (e.g., dysregulated stress-response systems), and behavioral (e.g., health risk behaviors) mechanisms.21 Exposure is operationalized as examining any lifetime trauma with risk outcomes. Second, in accumulation of risk models, stressors give rise to additional stressors across time, with different types of experiences accumulating with age across the lifecourse and increasing risk of poor health generally linearly.22 Cumulative experience of different traumatic events may be due to particularly adverse environments, and/or to a chain of risk whereby trauma tends to beget more trauma.23 The higher burden of exposures may thus lead to increased behavioral, psychological, or physiological effects and fewer resources to cope.23 Accumulation of risk is often operationalized as count of total adversities or trauma types experienced.1 Third, in sensitive period models, experience during certain periods leads to chronic alterations that result in negative health outcomes.24 Experiences in specific developmental periods compared to others may differentially impact health risk, with childhood and adolescence broadly reflecting a time of heightened risk due to developing neurobiological systems and psychosocial needs.25,26 For example, assaults on one’s safety or integrity during childhood may occur during important brain developmental stages, resulting in psychological and physiological changes (e.g., epigenetic modifications) that could influence health outcomes even decades later.27,28 Generally, sensitive periods are operationalized by classifying exposures to traumatic events during key developmental periods versus not to create lifecourse profiles of exposure. Much of the evidence examining lifecourse trauma has focused on psychiatric or psychological health outcomes (e.g., depression, posttraumatic stress, mental health impairment),23,29 with less identifying the physical health impacts of such exposure and no empirical studies to our knowledge focusing on arthritis outcomes in particular.
Existing evidence suggests that trauma at various points in ones’ life may increase the risk for arthritis in adulthood. Early life traumatic experiences, including childhood adversity (e.g., household dysfunction) and maltreatment (e.g., physical, sexual, and emotional abuse), are associated with increased risk for arthritis in adulthood.17–19 One prior analysis of the Health and Retirement Study (HRS) found various negative childhood experiences (including socioeconomic disadvantage, risky behaviors, chronic disease, substance use, and psychological distress) were associated with osteoarthritis and RA in adulthood.30 These findings in conjunction with other work demonstrating specific and lasting impacts of childhood experiences on adult health,3 suggest the importance of early life trauma for later life physical health. Despite the potential importance of considering lifecourse timing, fewer studies have examined adult trauma, or both childhood and adult trauma concurrently, with risk for arthritis.15,16 A separate body of evidence indicates that individuals perceive that arthritis onset or flares, particularly in RA, may have been triggered by acute stressors or adverse experiences in adulthood31,32; however little work has directly examined this empirically. Both childhood experiences, adult trauma, and more proximal stressful events may impact one’s risk for arthritis, supporting the need to take a lifecourse approach to understand risk more comprehensively. There are often high levels of co-occurrence of childhood adversities and adult traumas,22,33 further suggesting that both early life and more recent or adulthood experiences should be taken into account. Additionally, though lifecourse models focus on how timing and accumulation of experiences impact health, the type of trauma (e.g., interpersonal versus not) also may differentially impact health.34
In the current study, we used data from older adults in the HRS cohort to test three different lifecourse models of trauma and arthritis. Specifically, we estimated associations between any lifetime trauma, accumulation of trauma, and lifecourse profiles of sensitive periods of trauma and diagnoses of arthritis. We hypothesized that any exposure, greater accumulation of lifetime trauma, and childhood exposure, compared to no exposure, lower, or only adult exposure, would be associated with greater risk for arthritis. Lifetime trauma experiences were retrospectively reported by HRS participants in 2006–2008, a relatively arbitrary timepoint in one’s lifecourse with respect to both trauma and arthritis development. Therefore, we chose to perform both prevalent and incident analyses. First, we examined cross-sectional associations between lifetime trauma variables and prevalent arthritis as of 2008, recognizing the potential lack of temporal precedence (e.g., arthritis may have onset prior to reported trauma). Next, we examined prospective associations between lifetime trauma variables and incident arthritis, restricting to individuals without any prior reported arthritis as of our study baseline. In all models, we first examined any arthritis diagnoses, and then estimated associations with immune-related and osteoarthritis types separately. Without prior evidence and given certain shared risk factors (e.g., stress and psychosocial risk, smoking) known to be linked to trauma, we did not have directional hypotheses regarding the differences in associations between trauma and osteoarthritis versus immune-related arthritis. As epidemiological data suggests arthritis is more prevalent in women,35,36 and there is equivocal evidence of gender differences in associations between childhood adversity or trauma and arthritis,17,37 we performed exploratory follow-up analyses testing for gender interactions. Similarly, race and ethnicity in the US are fundamental social constructs that shape access to resources and opportunities, which, in turn, result in significant differences in rates of both trauma and arthritis across groups.38,39 Therefore, we also tested exploratory racial and ethnic interactions.
Methods
Sample Population
Data came from HRS, a population-based longitudinal study of US adults over aged 50 and their spouses. HRS began in 1992 with the inclusion of US adults born in 1931–1941, and has been refreshed with new birth cohorts to retain a steady-state design. HRS is sponsored by the National Institute on Aging (U01AG009740) and is conducted by the University of Michigan and made publicly available (https://hrsdata.isr.umich.edu/data-products/public-survey-data). Ethical approval for the HRS was granted from the University of Michigan Institutional Review Board and participants provided informed consent. Study procedures include phone-based structured interviews assessing factors related to health, labor force participation, and aging every two years. Beginning in 2006, psychosocial factors were assessed in supplemental leave-behind questionnaires administered to half of the HRS sample at alternating years (e.g., half the sample completed the questionnaire in 2006, while the other half completed the questionnaire in 2008). Psychosocial questionnaires included assessment of adversity and trauma, thus 2008 was considered the current analysis baseline. Follow-up assessments every two years were included to determine arthritis outcomes over time through 2020. The analytic sample was restricted to individuals who participated in 2006/2008 and completed adversity and trauma assessments, which included 5,702 individuals. For prospective analyses of incident arthritis, we excluded anyone who reported arthritis diagnoses as of 2008, resulting in 2,265 individuals.
Measures
Lifetime Trauma Exposure
Lifetime trauma exposure was assessed in 2006 or 2008 using ten items from the questionnaire developed by Krause et al.40 Participants were asked whether they had ever experienced any of three events before age 18, reflecting childhood events (i.e., trouble with police, family problems due to parental alcohol or drug use, physical abuse by a parent) or any of seven events ever in their lives (i.e., death of a child; experienced a major natural disaster; combat exposure; spouse, partner, or child addicted to drugs or alcohol; experienced serious physical attack; experienced life threatening illness or accident; spouse or child experiencing a life threatening illness or accident).41 All items were binary variables (yes/no) for exposure versus no exposure. For events that could have occurred at any time, a follow-up item assessed the year one experienced the event. We considered all reported events as potentially traumatic given evidence of associations with poor sequelae42,43 and thus use the term “lifetime trauma”, but acknowledge that severity and impact of different events will vary by experience and context. In secondary analyses, we examined trauma exposure types: interpersonal violence (i.e., childhood physical abuse, physical attack); other traumatic event to self (i.e., trouble with police in childhood, combat exposure, major natural disaster, life threatening illness or accident); and traumatic event occurring to others (i.e., parental alcohol or drug use; death of a child; spouse, partner, or child addicted to drugs or alcohol; and spouse or child experiencing a life-threatening illness or accident).34
To evaluate different lifecourse models of trauma, we defined three trauma exposures: 1) any lifetime trauma, 2) accumulation of trauma; and 3) lifecourse profile of trauma. Any lifetime trauma was defined as endorsing experience of any event versus none. Accumulation of trauma was defined by the count of total types of events experienced (potential range 0–11). Lifecourse profile of trauma was defined as a four-level categorical variable of no exposure at any time point, childhood only exposure (before age 18), adult only exposure (after age 18), and both childhood and adult exposure. For lifecourse profiles analyses, there was missingness of year of exposure among individuals who endorsed exposure (23.3% for prevalent and 22.5% for incident). Thus, we restricted to 4,386 individuals in prevalent and 1,758 individuals in incident analyses for complete case analyses (e.g., rather than imputing and thus adding artificial, predicted timing of exposures).
Arthritis
Self-reported arthritis diagnosis was assessed at each year of data collection, “have you ever had, or has a doctor ever told you that you have arthritis or rheumatism?”, yes or no. Starting in 2004, a follow-up question assessed the type of arthritis, with the following categories presented: 1) rheumatoid arthritis (autoimmune arthritis), 2) osteoarthritis (degenerative or “wear and tear” arthritis), and 3) arthritis due to injury (arthritis related to a previous injury). Given the potential for misclassification of specific disease diagnoses in self-report data, we grouped conditions by those primarily driven by immune-related mechanisms (i.e., autoimmune conditions including RA) or by mechanical mechanisms (i.e., osteoarthritis, arthritis of specific joints, injury). Our main outcome was any arthritis (any type of arthritis), with secondary outcomes including arthritis types immune-related arthritis (RA) and osteoarthritis (osteoarthritis or arthritis due to injury). There was some missingness for type of arthritis (13.0% missing prevalent type, 38.0% missing incident type), thus prevalence of “any arthritis” was greater than those represented in individual categories. Outcomes were defined as of baseline (e.g., any diagnosis of arthritis by 2008 versus no diagnoses by 2008) for prevalent analyses and at each follow-up assessment (i.e., any diagnosis at each assessment versus no diagnoses by that time) for incident analyses.
Covariates
Covariates included sociodemographic factors at our analysis baseline that were conceptualized as possible confounders of our primary relationship. Confounders included age (included continuously, categorized by quartiles for descriptive purposes), gender (self-reported binary gender: woman, man), race (Black or African American, White, other [American Indian or Alaskan Native, Asian or Pacific Islander, or other race]), and ethnicity (Hispanic, not Hispanic). As our lifetime trauma variables incorporate experiences as early as early childhood, we focused on factors that are expected to precede exposures or are relatively stable across time.
Additional covariates were included as potential pathway variables reported as of analysis baseline. These included adult social factors (i.e., educational attainment, marital status), biobehavioral factors (i.e., body mass index [BMI; kg/m2], alcohol use, smoking, physical activity), and depressive symptoms (i.e., 8-item sum score from the Center for Epidemiological Studies-Depression scale).44 There was no missingness among confounders, and less than 3% missingness among the additional covariates (i.e., educational attainment n=1, BMI n=14, physical activity n=126, alcohol use n=120, smoking n=125, depressive symptoms n=77), thus mean imputation was used.
Analyses
We conducted descriptive statistics of baseline covariates, lifetime trauma exposure, and prevalent and incident arthritis cases. For prevalent analyses, we used Poisson regression with robust standard errors to derive prevalence ratios (PR) of binary arthritis outcomes cross-sectionally as of 2008. Predictors included any lifetime trauma, accumulation of trauma (i.e., continuous trauma count), and lifetime trauma profiles, examined in separate models. We modelled each outcome separately, starting from any arthritis, followed by immune-related arthritis and osteoarthritis. For incident analyses, we used Cox proportional hazards models to derive hazard ratios (HR) for associations of predictors with time to arthritis diagnosis among the incident analytic sample. Study participants contributed person-time (in years) from baseline in 2008 to the year of follow-up when the outcome occurred (i.e., reporting incident arthritis) or they were censored (i.e., lost to follow-up due to reasons other than death, lost to follow-up due to death, or end of follow-up in 2020, whichever occurred first). Like prevalent analyses, each trauma exposure and arthritis outcome were evaluated in separate models adjusting for covariates. We tested and found no evidence of violation of the proportional hazards assumption using Global Schoenfeld Tests (all p>.05) for Cox models. To nominally compare models on the amount of variance explained, we calculated pseudo-R2 values (R2 Nagelkerke) which are estimates of coefficients of determination for generalized regression models.45 Models were conducted increasingly adjusted for covariates: crude (unadjusted), confounders (age, gender, race, ethnicity; considered the primary models), adult social factors (confounders and educational attainment and marital status), biobehavioral factors (confounders, adult social factors, and BMI, alcohol use, smoking, and physical activity), and depressive symptoms (confounders, adult social factors, biobehavioral factors, and depressive symptoms).
We calculated inverse probability weights (IPW) to account for selection and attrition bias, given that socio-demographic characteristics differed between both the prevalent and incident samples versus those excluded, and between the incident sample versus those lost to follow-up. Similar to prior work,46 we modeled the odds of inclusion in the sample (for prevalent and incident analytic samples); and not being lost to follow-up because of death and not being lost to follow-up due to reasons other than death (for the incident sample) predicted by baseline study variables. The inverse probability of inclusion was derived and extracted as a weight for each participant in the prevalent sample for selection bias. For the incident sample, the inverse probabilities of not being lost to follow up (i.e., for death or reasons other than death) were also derived. All three probabilities were multiplied to create overall weights for each participant in the incident sample for selection bias and loss to follow-up. All analytic models included IPW which weight the effect estimates to more closely reflect the initial HRS population prior to selection or lost to follow-up.
We performed a series of exploratory analyses. The primary models were rerun separately using trauma type specific exposure variables (e.g., any interpersonal violence exposure, accumulation of interpersonal violence, lifecourse profiles of interpersonal violence), to identify whether particular categories of traumatic experience were more strongly associated with arthritis outcomes. Some experiences of trauma could directly influence arthritis risk through injury sustained during the traumatic event. Thus, we reran primary models with alternative trauma variables that excluded traumatic events feasibly associated with physical injury, including physical abuse by a parent, combat exposure, serious physical attack, and life-threatening illness or accident. We tested gender-trauma variable interactions and then performed gender-stratified primary prevalent and incident models. We additionally tested race- and ethnicity-trauma interactions and performed race and ethnicity stratified models. All hypothesis testing was two-sided with a priori determined significance threshold of p<.05. All analyses were conducted in R, version 4.2.3.
Results
The baseline analytic sample was 65.4 years old on average (SD=12.9) and included mostly women (60.2%) (see Table 1). The sample was mostly White (84.1%), with fewer Black or African American (10.8%) or other race (5.1%) individuals, and 8.2% identified as Hispanic. A majority of the sample experienced at least one lifetime traumatic event (71.6%). Any lifetime trauma exposure was less likely among younger (age ≤55) adults, more common among men versus women, but was similarly distributed across race and ethnicity. The average count of lifetime trauma types was 1.5 (SD=1.4), with 28.4% experiencing no trauma, 30.3% experiencing one trauma type, 19.8% experiencing two, 12.4% experiencing three, and 9.1% experiencing four or more. Among those with information on timing of exposures, 25.3% experienced exposures during childhood only, 25.5% in adulthood only, and 12.2% in both childhood and adulthood.
Table 1.
Distribution of covariates among the analytic sample and by trauma exposure in the Health and Retirement Study (n=5,717)
| Full Sample | No Lifetime Trauma, n=1,623 (28.4%) |
Any Lifetime Trauma, n=4,094 (71.6%) |
|
|---|---|---|---|
| Covariate | N (%) | N (%) | N (%) |
| Age, m (SD) | 65.4 (12.9) | 64.9 (13.2) | 65.5 (12.8) |
| Age Category | |||
| ≤55 years old | 1,545 (27.0) | 509 (31.4) | 1,036 (25.3)* |
| 56–60 years old | 1,380 (24.1) | 359 (22.1) | 1,021 (24.9) |
| 60–78 years old | 1,396 (24.4) | 369 (22.7) | 1,027 (25.1) |
| >78 years old | 1,396 (24.4) | 386 (23.8) | 1,010 (24.7) |
| Gender | |||
| Women | 3,443 (60.2) | 1,041 (64.1) | 2,402 (58.7)* |
| Men | 2,274 (39.8) | 582 (35.9) | 1,692 (41.3) |
| Race | |||
| White | 4,807 (84.1) | 1,349 (83.1) | 3,458 (84.5) |
| Black or African American | 617 (10.8) | 174 (10.7) | 443 (10.8) |
| Other | 293 (5.1) | 100 (6.2) | 193 (4.7) |
| Ethnicity | |||
| Hispanic | 467 (8.2) | 148 (9.1) | 319 (7.8) |
| Not Hispanic | 5,250 (91.8) | 1,475 (90.9) | 3,775 (92.2) |
| Educational Attainment | |||
| Less than High School | 880 (15.4) | 258 (15.9) | 622 (15.2)* |
| High School Graduate | 1,701 (29.8) | 515 (31.7) | 1,186 (29.0) |
| GED | 224 (3.9) | 53 (3.3) | 171 (4.2) |
| Some College | 1,432 (25.0) | 357 (22.0) | 1,075 (26.3) |
| 4-year College or More | 1,480 (25.9) | 440 (27.1) | 1,040 (25.4) |
| Marital Status | |||
| Married or partnered | 3,817 (66.8) | 1,137 (70.1) | 2,680 (65.5)* |
| Never married | 174 (3.0) | 61 (3.8) | 113 (2.8) |
| Separated or divorced | 644 (11.3) | 147 (9.1) | 497 (12.1) |
| Widowed | 1,082 (18.9) | 278 (17.1) | 804 (19.6) |
| BMI, m (SD) | 28.0 (5.9) | 27.7 (5.8) | 28.1 (5.9)* |
| Physical Activity | |||
| Never | 3,262 (57.1) | 916 (56.4) | 2,346 (57.3) |
| <1 times per month | 426 (7.5) | 121 (7.5) | 305 (7.4) |
| 1–3 times per month | 504 (8.8) | 165 (10.2) | 339 (8.3) |
| 1–2 times per week | 1,222 (21.4) | 346 (21.3) | 876 (21.4) |
| ≥3 times per week | 177 (3.1) | 41 (2.5) | 136 (3.3) |
| Missing | 126 (2.2) | 34 (2.1) | 92 (2.2) |
| Alcohol Consumption | |||
| Never drinks | 2,536 (44.4) | 745 (45.9) | 1,791 (43.7) |
| Drinks alcohol | 3,061 (53.5) | 848 (52.2) | 2,213 (54.1) |
| Missing | 120 (2.1) | 30 (1.8) | 90 (2.2) |
| Smoking | |||
| Non-smoker | 4,855 (84.9) | 1,407 (86.7) | 3,448 (84.2) |
| Current smoker | 737 (12.9) | 183 (11.3) | 554 (13.5) |
| Missing | 125 (2.2) | 33 (2.0) | 92 (2.2) |
| Depressive Symptoms, m (SD) | 1.5 (2.0) | 1.2 (1.7) | 1.6 (2.1)* |
p<.05 for t-tests or Chi-square tests between covariates and lifetime trauma exposure
Lifecourse Trauma and Prevalent Arthritis Cross-sectionally at Baseline
Among the analytic sample for prevalent analyses, 3,448 (60.3%) individuals had a diagnosis of any arthritis as of our study baseline. With respect to arthritis type, 781 (13.7%) individuals reported immune-related arthritis and 2,692 (47.1%) reported osteoarthritis. Notably, 502 (8.8%) individuals reported both immune-related and osteoarthritis.
Any lifetime trauma was associated with 13% higher likelihood of having any prevalent arthritis (confounder-adjusted PR=1.13, 95%CI 1.05, 1.22; Table 2). Accumulation of trauma was also associated with higher likelihood of prevalent arthritis, with one additional traumatic event exposure associated with 6% higher likelihood of arthritis (confounder-adjusted PR=1.06, 95%CI 1.04, 1.09). Considering lifecourse trauma profiles, exposures during childhood, adulthood, or both were each associated with higher likelihood of arthritis relative to those unexposed to trauma. Estimates overall were relatively similar and had overlapping confidence intervals, though the magnitude of risk was highest among those who experienced trauma in both childhood and adulthood. Accounting for potential pathway variables (i.e., adult social factors, biobehavioral factors, depressive symptoms) only slightly attenuated the magnitude of associations. Associations were generally similar for immune-related arthritis and osteoarthritis, with higher magnitude associations for immune-related arthritis.
Table 2.
Cross-sectional associations between trauma exposure and prevalent arthritis as of baseline in the Health and Retirement Study (n=5,717)
| Any Arthritis | |||||
|---|---|---|---|---|---|
| Crude | Confounder | Adult Social Factors | Biobehavioral Factors | Depressive Symptoms | |
| Trauma Variables | PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) |
| Any Lifetime Trauma | |||||
| No Trauma | Ref | Ref | Ref | Ref | Ref |
| Any Lifetime Trauma | 1.13 (1.04, 1.22) | 1.13 (1.05, 1.22) | 1.13 (1.04, 1.22) | 1.12 (1.03, 1.21) | 1.10 (1.01, 1.19) |
| R2 Nagelkerke | 0.004 | 0.101 | 0.111 | 0.136 | 0.146 |
| Lifecourse Trauma Accumulation | |||||
| Count of Trauma, M (SD) | 1.06 (1.03, 1.08) | 1.06 (1.04, 1.09) | 1.06 (1.04, 1.09) | 1.06 (1.03, 1.08) | 1.05 (1.02, 1.07) |
| R2 Nagelkerke | 0.008 | 0.107 | 0.116 | 0.140 | 0.149 |
| Lifecourse Trauma Profiles a | |||||
| No Trauma | Ref | Ref | Ref | Ref | Ref |
| Childhood Only | 1.04 (0.94, 1.15) | 1.16 (1.05, 1.29) | 1.14 (1.03, 1.27) | 1.13 (1.02, 1.26) | 1.12 (1.01, 1.24) |
| Adult Only | 1.21 (1.09, 1.33) | 1.14 (1.03, 1.25) | 1.14 (1.03, 1.26) | 1.14 (1.03, 1.26) | 1.12 (1.01, 1.24) |
| Both Childhood and Adult | 1.18 (1.04, 1.33) | 1.24 (1.09, 1.41) | 1.23 (1.08, 1.39) | 1.21 (1.07, 1.37) | 1.17 (1.03, 1.33) |
| R2 Nagelkerke | 0.008 | 0.102 | 0.110 | 0.139 | 0.147 |
| Immune-Related Arthritis | |||||
| Crude | Confounder | Adult Social Factors | Biobehavioral Factors | Depressive Symptoms | |
| Trauma Variables | PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) |
| Any Lifetime Trauma | |||||
| No Trauma | Ref | Ref | Ref | Ref | Ref |
| Any Lifetime Trauma | 1.28 (1.08, 1.51) | 1.29 (1.10, 1.52) | 1.28 (1.08, 1.51) | 1.26 (1.07, 1.49) | 1.21 (1.03, 1.44) |
| R2 Nagelkerke | 0.004 | 0.026 | 0.055 | 0.074 | 0.086 |
| Lifecourse Trauma Accumulation | |||||
| Count of Trauma, M (SD) | 1.14 (1.09, 1.19) | 1.14 (1.09, 1.19) | 1.13 (1.08, 1.18) | 1.12 (1.06, 1.17) | 1.10 (1.05, 1.15) |
| R2 Nagelkerke | 0.012 | 0.034 | 0.061 | 0.079 | 0.089 |
| Lifecourse Trauma Profiles a | |||||
| No Trauma | Ref | Ref | Ref | Ref | Ref |
| Childhood Only | 1.37 (1.11, 1.69) | 1.52 (1.23, 1.87) | 1.44 (1.16, 1.78) | 1.42 (1.14, 1.75) | 1.37 (1.11, 1.70) |
| Adult Only | 1.30 (1.05, 1.60) | 1.24 (1.01, 1.54) | 1.24 (1.00, 1.54) | 1.25 (1.01, 1.55) | 1.20 (0.97, 1.48) |
| Both Childhood and Adult | 1.39 (1.07, 1.80) | 1.44 (1.10, 1.86) | 1.39 (1.07, 1.80) | 1.36 (1.04, 1.76) | 1.26 (0.96, 1.63) |
| R2 Nagelkerke | 0.007 | 0.035 | 0.064 | 0.085 | 0.096 |
| Osteoarthritis | |||||
| Crude | Confounder | Adult Social Factors | Biobehavioral Factors | Depressive Symptoms | |
| Trauma Variables | PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) |
| Any Lifetime Trauma | |||||
| No Trauma | Ref | Ref | Ref | Ref | Ref |
| Any Lifetime Trauma | 1.19 (1.09, 1.30) | 1.20 (1.10, 1.31) | 1.19 (1.09, 1.30) | 1.18 (1.08, 1.29) | 1.15 (1.05, 1.26) |
| R2 Nagelkerke | 0.005 | 0.073 | 0.078 | 0.104 | 0.114 |
| Lifecourse Trauma Accumulation | |||||
| Count of Trauma, M (SD) | 1.08 (1.06, 1.11) | 1.09 (1.07, 1.12) | 1.09 (1.06, 1.12) | 1.08 (1.06, 1.11) | 1.07 (1.04, 1.10) |
| R2 Nagelkerke | 0.012 | 0.082 | 0.086 | 0.110 | 0.119 |
| Lifecourse Trauma Profiles a | |||||
| No Trauma | Ref | Ref | Ref | Ref | Ref |
| Childhood Only | 1.12 (1.00, 1.26 | 1.24 (1.11, 1.40) | 1.23 (1.09, 1.38) | 1.22 (1.08, 1.37) | 1.20 (1.06, 1.35) |
| Adult Only | 1.26 (1.13, 1.41) | 1.20 (1.07, 1.35) | 1.20 (1.07, 1.34) | 1.20 (1.07, 1.34) | 1.18 (1.05, 1.32) |
| Both Childhood and Adult | 1.29 (1.12, 1.49) | 1.37 (1.19, 1.58) | 1.35 (1.17, 1.56) | 1.33 (1.15, 1.53) | 1.28 (1.11, 1.47) |
| R2 Nagelkerke | 0.010 | 0.080 | 0.083 | 0.111 | 0.119 |
n=4,386 due to missing timing of exposures
CI = confidence intervals; PR = prevalence ratio.
Model adjustment: Crude (no covariates), Confounder (age, gender, race, ethnicity), Adult Social Factors (confounders plus educational attainment and marital status), Biobehavioral Factors (confounders, adult social factors, and BMI, alcohol use, smoking, and physical activity), Depressive Symptoms (confounders, adult social factors, biobehavioral factors, and depressive symptoms). Models are adjusted for inverse probability weighting for selection bias. Bolded estimates are significant at p<.05. Immune-related arthritis includes rheumatoid arthritis, gout, and lupus; Osteoarthritis includes osteoarthritis and arthritis due to injury.
Lifecourse Trauma and Incident Arthritis in Prospective Data
Across follow-up, 810 (35.7%) individuals in the incident analytic sample developed any arthritis, with 85 (3.8%) cases were reported as immune-related arthritis and 443 (19.5%) as osteoarthritis.
Considering incident cases, any lifetime trauma was associated with 25% higher hazards of any arthritis (HR=1.25, 95%CI 1.07, 1.47; Table 3 and Figure 1) and each additional lifetime traumatic event was associated with 13% higher hazards (HR=1.13, 95%CI 1.07, 1.19) in confounder-adjusted models. Exposure to trauma particularly in childhood was associated with higher hazards of any incident arthritis, relative to no exposure. Similar to cross-sectional models, there was a minor attenuation of associations when additionally adjusting for potential pathway variables. Findings diverged by arthritis type, with no significant associations between trauma and immune-related arthritis (though directions of associations were similar, except for adult trauma which appeared protective). In contrast, there were significant associations for any lifetime trauma, trauma count, and childhood trauma exposure with increased hazards of incident osteoarthritis.
Table 3.
Prospective associations between trauma exposure and incident arthritis over 12 years of follow-up in the Health and Retirement Study (n=2,269)
| Any Arthritis | |||||
|---|---|---|---|---|---|
| Crude | Confounder | Adult Social Factors | Biobehavioral Factors | Depressive Symptoms | |
| Trauma Variables | HR (91%CI) | HR (95%CI) | HR (91%CI) | HR (91%CI) | HR (91%CI) |
| Any Lifetime Trauma | |||||
| No Trauma | Ref | Ref | Ref | Ref | Ref |
| Any Lifetime Trauma | 1.25 (1.07, 1.46) | 1.25 (1.07, 1.47) | 1.25 (1.06, 1.46) | 1.22 (1.04, 1.44) | 1.20 (1.02, 1.42) |
| R2 Nagelkerke | 0.004 | 0.011 | 0.015 | 0.032 | 0.040 |
| Lifecourse Trauma Accumulation | |||||
| Count of Trauma, M (SD) | 1.12 (1.07, 1.19) | 1.13 (1.07, 1.19) | 1.13 (1.07, 1.19) | 1.13 (1.07, 1.19) | 1.11 (1.05, 1.18) |
| R2 Nagelkerke | 0.009 | 0.016 | 0.021 | 0.038 | 0.045 |
| Lifecourse Trauma Profiles a | |||||
| No Trauma | Ref | Ref | Ref | Ref | Ref |
| Childhood Only | 1.41 (1.16, 1.71) | 1.45 (1.19, 1.76) | 1.44 (1.18, 1.75) | 1.41 (1.16, 1.73) | 1.41 (1.15, 1.72) |
| Adult Only | 1.21 (0.97, 1.51) | 1.19 (0.96, 1.48) | 1.20 (0.97, 1.50) | 1.19 (0.95, 1.48) | 1.17 (0.94, 1.46) |
| Both Childhood and Adult | 1.17 (0.88, 1.56) | 1.21 (0.90, 1.61) | 1.19 (0.89, 1.59) | 1.17 (0.87, 1.58) | 1.11 (0.82, 1.49) |
| R2 Nagelkerke | 0.007 | 0.017 | 0.021 | 0.032 | 0.041 |
| Immune-Related Arthritis | |||||
| Crude | Confounder | Adult Social Factors | Biobehavioral Factors | Depressive Symptoms | |
| Trauma Variables | HR (91%CI) | HR (95%CI) | HR (91%CI) | HR (91%CI) | HR (91%CI) |
| Any Lifetime Trauma | |||||
| No Trauma | Ref | Ref | Ref | Ref | Ref |
| Any Lifetime Trauma | 1.00 (0.71, 1.42) | 1.02 (0.72, 1.43) | 1.01 (0.72, 1.43) | 0.95 (0.66. 1.35) | 0.92 (0.65, 1.30) |
| R2 Nagelkerke | 0.000 | 0.017 | 0.019 | 0.026 | 0.032 |
| Lifecourse Trauma Accumulation | |||||
| Count of Trauma, M (SD) | 1.08 (0.95, 1.22) | 1.07 (0.95, 1.21) | 1.07 (0.94, 1.21) | 1.05 (0.92, 1.19) | 1.03 (0.90, 1.17) |
| R2 Nagelkerke | 0.001 | 0.017 | 0.019 | 0.026 | 0.032 |
| Lifecourse Trauma Profiles a | |||||
| No Trauma | Ref | Ref | Ref | Ref | Ref |
| Childhood Only | 1.24 (0.81, 1.90) | 1.29 (0.85, 1.96) | 1.23 (0.81, 1.89) | 1.16 (0.75, 1.80) | 1.16 (0.75, 1.80) |
| Adult Only | 0.71 (0.42, 1.24) | 0.73 (0.42, 1.25) | 0.75 (0.44, 1.30) | 0.71 (0.40, 1.25) | 0.71 (0.41, 1.24) |
| Both Childhood and Adult | 0.61 (0.30, 1.26) | 0.61 (0.30, 1.24) | 0.57 (0.28, 1.18) | 0.54 (0.25, 1.13) | 0.48 (0.23, 1.01) |
| R2 Nagelkerke | 0.004 | 0.026 | 0.031 | 0.035 | 0.042 |
| Osteoarthritis | |||||
| Crude | Confounder | Adult Social Factors | Biobehavioral Factors | Depressive Symptoms | |
| Trauma Variables | HR (91%CI) | HR (95%CI) | HR (91%CI) | HR (91%CI) | HR (91%CI) |
| Any Lifetime Trauma | |||||
| No Trauma | Ref | Ref | Ref | Ref | Ref |
| Any Lifetime Trauma | 1.22 (1.02, 1.48) | 1.23 (1.02, 1.49) | 1.24 (1.03, 1.50) | 1.20 (1.00, 1.45) | 1.18 (0.98, 1.43) |
| R2 Nagelkerke | 0.002 | 0.008 | 0.011 | 0.029 | 0.037 |
| Lifecourse Trauma Accumulation | |||||
| Count of Trauma, M (SD) | 1.13 (1.07, 1.21) | 1.14 (1.07, 1.22) | 1.14 (1.07, 1.22) | 1.14 (1.07, 1.21) | 1.12 (1.05, 1.20) |
| R2 Nagelkerke | 0.007 | 0.013 | 0.016 | 0.035 | 0.041 |
| Lifecourse Trauma Profiles a | |||||
| No Trauma | Ref | Ref | Ref | Ref | Ref |
| Childhood Only | 1.48 (1.18, 1.85) | 1.50 (1.20, 1.87) | 1.51 (1.20, 1.89) | 1.47 (1.17, 1.85) | 1.45 (1.16, 1.83) |
| Adult Only | 1.12 (0.86, 1.46) | 1.13 (0.87, 1.48) | 1.14 (0.87, 1.49) | 1.12 (0.85, 1.46) | 1.10 (0.84, 1.44) |
| Both Childhood and Adult | 1.25 (0.90, 1.74) | 1.30 (0.94, 1.80) | 1.28 (0.93, 1.78) | 1.24 (0.89, 1.72) | 1.17 (0.83, 1.63) |
| R2 Nagelkerke | 0.007 | 0.016 | 0.018 | 0.031 | 0.040 |
n=1,758 due to missing timing of exposures
CI = confidence intervals; PR = prevalence ratio.
Model adjustment: Crude (no covariates), Confounder (age, gender, race, ethnicity), Adult Social Factors (confounders plus educational attainment and marital status), Biobehavioral Factors (confounders, adult social factors, and BMI, alcohol use, smoking, and physical activity), Depressive Symptoms (confounders, adult social factors, biobehavioral factors, and depressive symptoms). Models are adjusted for inverse probability weighting for selection bias. Bolded estimates are significant at p<.05. Immune-related arthritis includes rheumatoid arthritis, gout, and lupus; Osteoarthritis includes osteoarthritis and arthritis due to injury.
Figure 1.
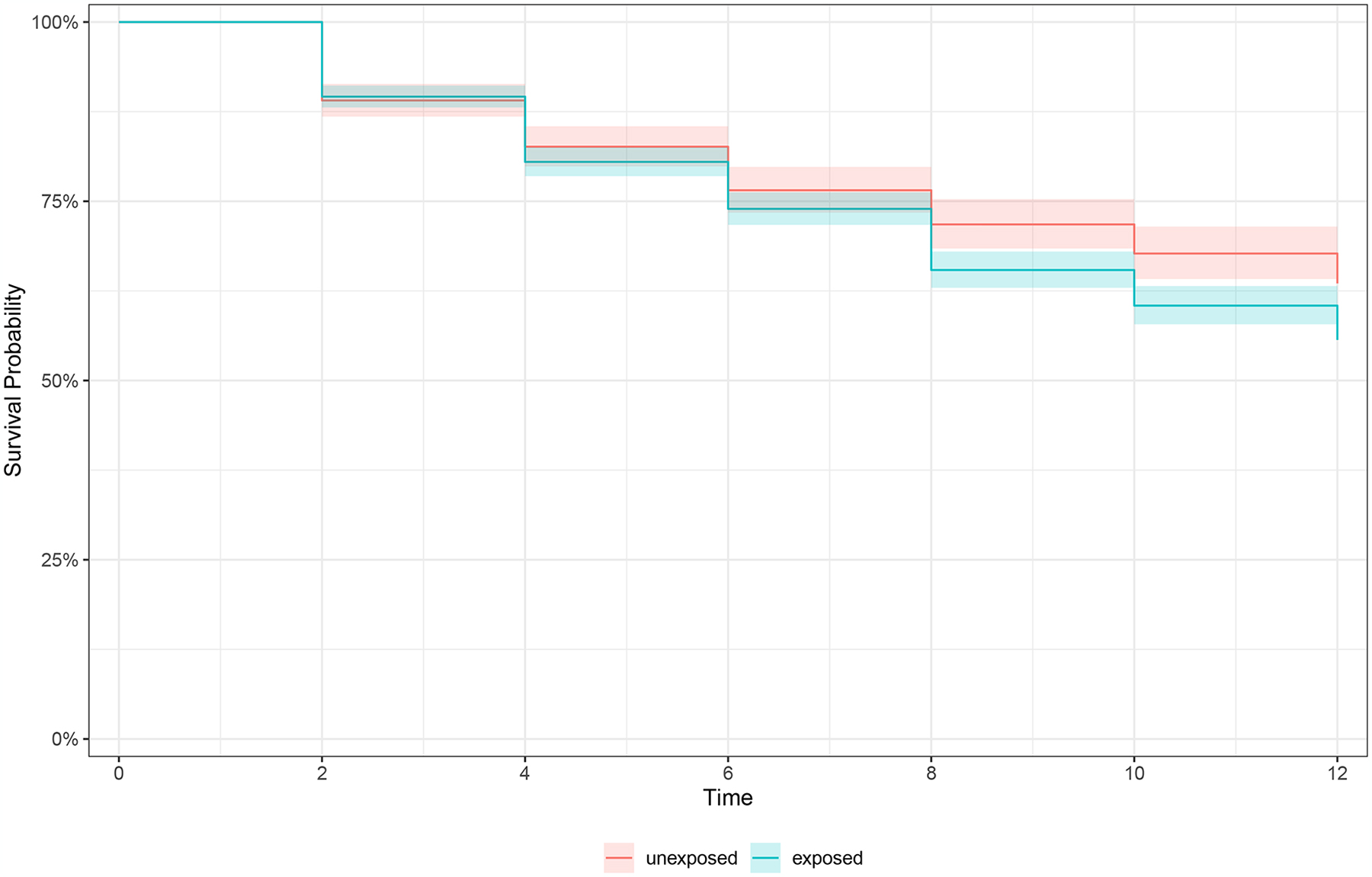
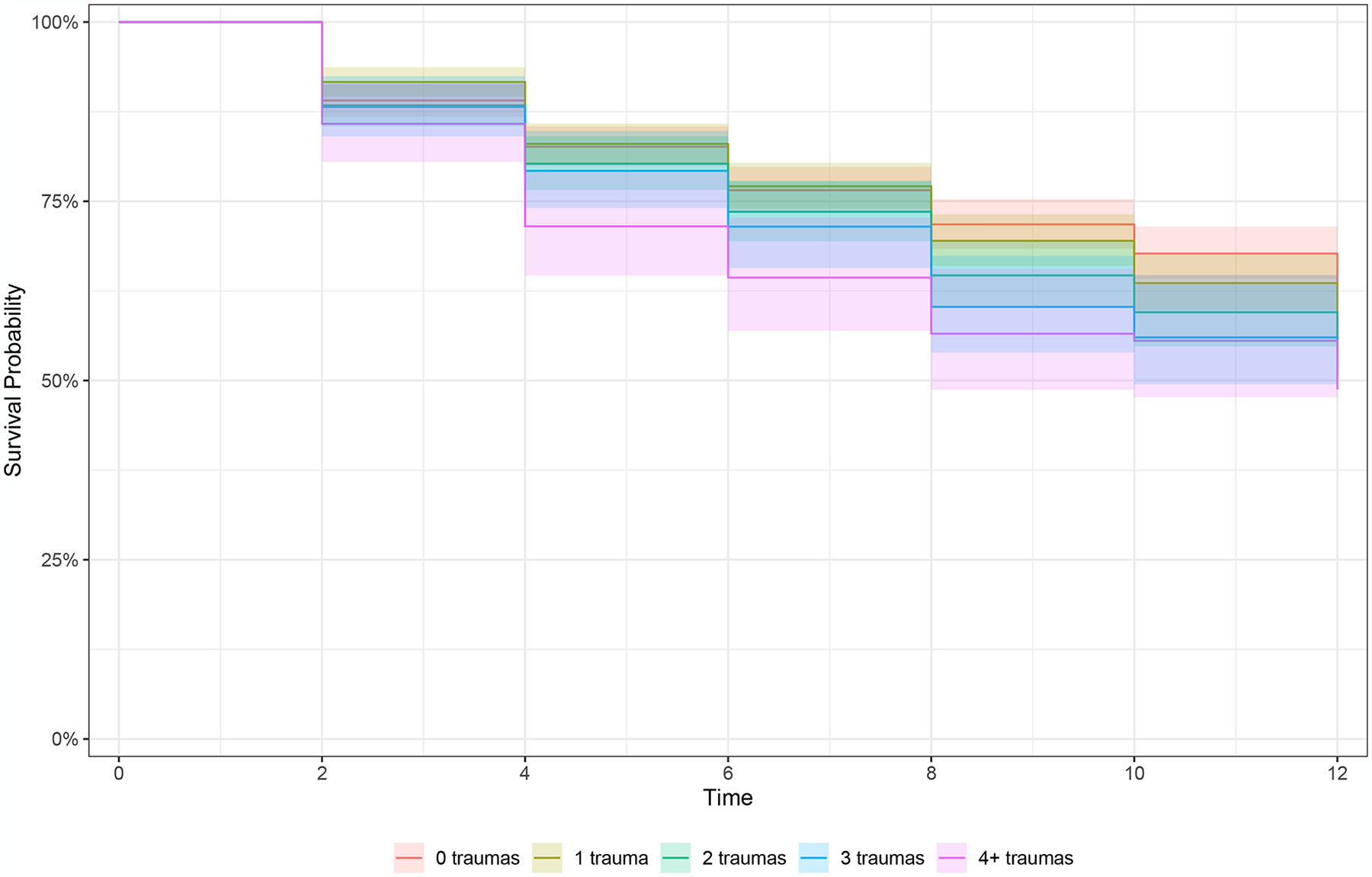
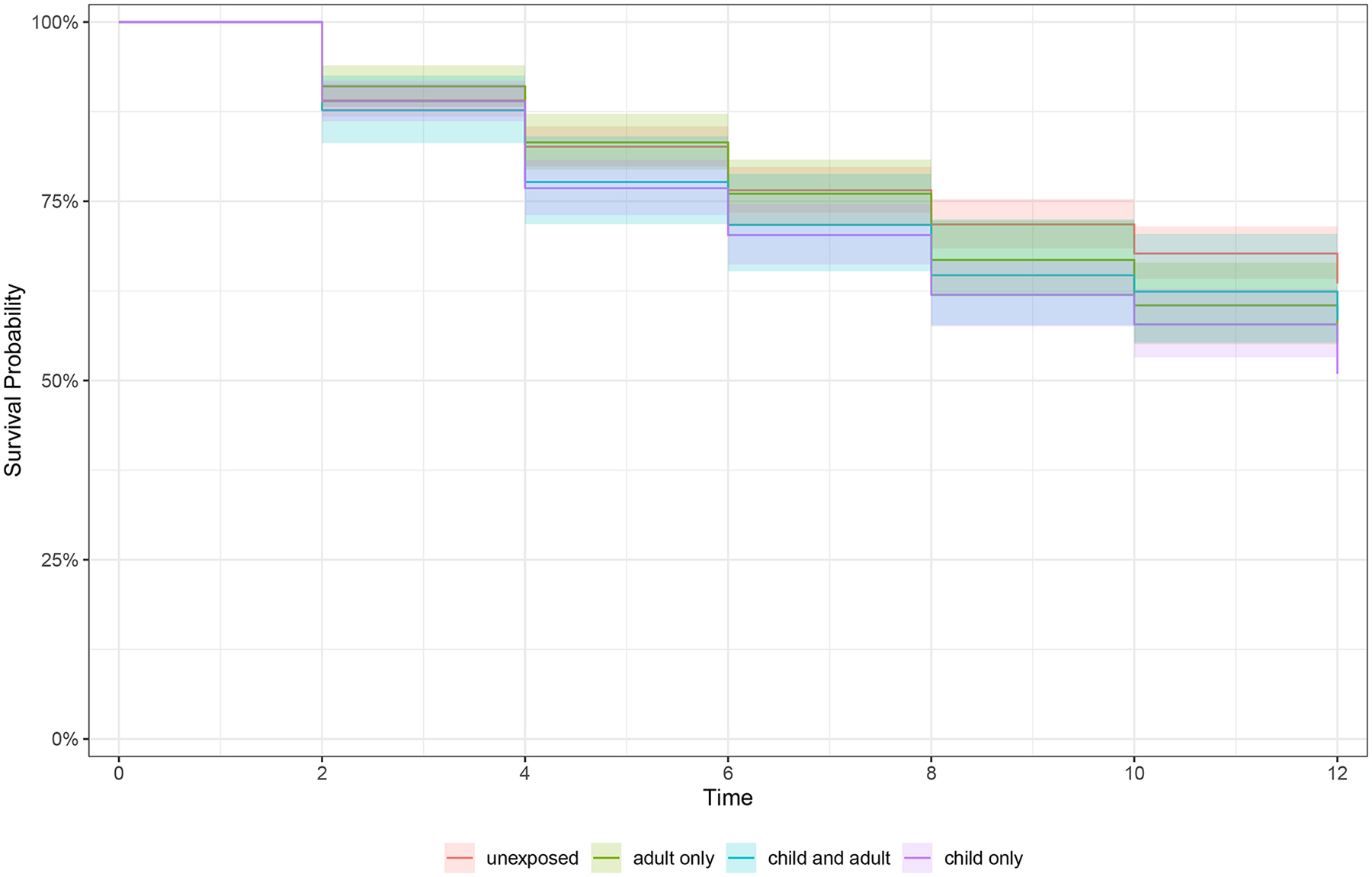
Kaplan Meier curves of survival rates for incidence of any arthritis in prospective data by a) any lifetime trauma exposure, b) trauma accumulation, and c) lifecourse trauma profiles
a) Survival to arthritis over follow-up stratified by lifetime trauma exposed versus unexposed, n=2,269
b) Survival to arthritis over follow-up stratified by trauma accumulation (count 0–4+), n=2,269
c) Survival to arthritis over follow-up stratified by lifecourse trauma profile (unexposed, child only, adult only, child and adult exposure), n=1,758
Color images are available online-only at the journal website, https://doi.org/10.1097/PSY.0000000000001331
Exploratory and Sensitivity Analyses
Examining different trauma types, any trauma exposure and higher trauma accumulation across trauma types were associated with prevalent arthritis cases (Supplemental Digital Content, Table S1). For lifecourse trauma profiles, there was some suggestion that childhood exposure to interpersonal trauma was more strongly associated with prevalent arthritis than either other trauma to self or trauma to others, however, patterns were generally similar. In prospective models, associations were similar across different trauma types, with some indication of stronger magnitude of effects for interpersonal violence (Table S2). When excluding injury-specific trauma variables, associations between trauma and prevalent arthritis outcomes were generally similar but slightly smaller magnitude compared to primary models (Table S3). Likewise, in incident models, estimates were generally comparable for any arthritis and attenuated for immune-related and osteoarthritis (Table S4). We largely did not identify significant trauma-gender interactions, suggesting that overall patterns of associations between trauma and arthritis outcomes were similar between men and women (Tables S5 and S6). There were few differences in the primary associations across different racial (Tables S7 and S8) and ethnic groups (Tables S9 and S10).
Discussion
In this large, community-based sample of older adults, we found that lifetime trauma exposure was associated with higher risk for prevalent and incident arthritis. Similar to other community-based estimates,47,48 a large majority of our sample experienced at least one lifetime trauma and exposures during childhood were common. Moreover, our large sample of older adults had high levels of prevalent arthritis (60.3% by baseline), which is slightly higher than US prevalence estimates of 50.4% of adults age 65 or older having doctor-diagnosed arthritis.49 We probed associations of different lifecourse models with arthritis in general and by arthritis type. Generally, experiencing any lifetime trauma versus none, higher accumulation of trauma, and trauma occurring in childhood or across multiple life periods (childhood and adulthood) were associated with higher likelihood of both having and developing arthritis in older adults, suggesting all three models were supported by the data.
Considering different lifecourse trauma models, we found evidence for any exposure, accumulation, and sensitive periods with arthritis. In prevalent models, any exposure as well as each additional accumulation of trauma was associated with higher likelihood of having arthritis. Exposures at each developmental period assessed were associated with having arthritis, relative to unexposed individuals. There was some indication that individuals exposed both in childhood and adulthood had highest likelihood of prevalent arthritis, suggesting accumulation of trauma across different developmental time periods may be a potent risk factor for having prevalent arthritis. These findings align with models of accumulation and sensitive periods of risk;20,23,25 it is possible that elevated risk was due to heightened trauma burden over time exceeding one’s coping resources and causing accumulation of biological or psychological consequences, or to disrupted biological, psychosocial, or behavioral development from exposure in childhood. While closer examination of mechanisms and better precision with respect to timing is necessary to disentangle these lifecourse models, the findings imply that prevention efforts should target trauma across the lifecourse, but limited resources may be best focused in childhood, particularly among children in potentially riskier environments who may face additional trauma. Prospective models similarly provided support for any exposure and accumulation of trauma and increased risk for incident osteoarthritis. Prospective analyses had relatively few individuals who experienced both childhood and adulthood trauma, therefore we may have been underpowered to identify these associations. Additionally, incident immune-related arthritis was uncommon and thus estimates for specific developmental periods were imprecise. Pseudo R2 values suggested that explanatory values across different lifecourse models were similar, and effect estimates within models were generally similar in magnitude (e.g., childhood only versus childhood and adulthood). Exploratory models examining categories of trauma types showed largely comparable associations, with some suggestion that interpersonal violence is particularly impactful. Future work should examine trauma history more comprehensively to assess both trauma type and lifecourse timing in relation to health outcomes. Therefore, while there is some suggestion that childhood exposures may be particularly detrimental, exposure to more trauma types at any time in one’s life appears detrimental to musculoskeletal and rheumatological health in later adulthood.
We identified similar patterns when examining separate arthritis types as endpoints. Trauma exposure variables tended to be associated with both arthritis types in the same directions and to similar magnitudes. The main exception was prospective models of immune-related arthritis, of which there were relatively few incident cases (n=85). Both arthritis types in the current study are characterized by damage to musculoskeletal tissue, but vary in pathophysiology with RA classified as a systemic autoimmune inflammatory condition involving loss of self-tolerance, and osteoarthritis a largely degenerative joint disease involving protein or tissue breakdown. There may be different mechanistic pathways linking exposure to trauma and development of immune-related versus osteoarthritis. For example, the pathogenesis of some but not all RA cases involves circulating autoantibodies in combination with genetic predisposition and environmental triggers, such as behaviors or stress.35,50 Other population-based studies tend to collapse “arthritis” as a general category, with many participants not reporting or unaware of arthritis type.51,52 However, despite the potential lack of precision in arthritis disease definitions, our findings suggest that experiences of trauma across the lifecourse may increase arthritis risk generally, highlighting the potential shared inflammatory, immune-related, and metabolic factors that may mechanistically link trauma exposure and arthritis.
Our models adjusted for a series of potential pathway variables, that could reflect mechanisms linking trauma exposures to arthritis including social (e.g., marital status), biobehavioral (e.g., BMI, smoking), and psychological (e.g., depressive symptoms) factors. Adjusting for these variables, in addition to our probable confounders, only modestly attenuated associations between trauma variables and both prevalent and incident arthritis cases. This suggests that these factors, as measured at baseline, did not fully explain the associations we identified and other potential mechanisms may be at work. Little difference was identified in associations between lifecourse trauma variables and arthritis endpoints across gender, race, and ethnic groups, indicating trauma may similarly impact arthritis risk broadly in different socio-demographic groups. Notably, these analyses were exploratory and some subgroup analyses were small and potentially underpowered (e.g., only 151 individuals of other race in prospective analyses), thus future work could directly address potential socio-demographic disparities.
An important consideration when examining the impacts of trauma on health outcomes, especially rheumatological or musculoskeletal conditions, is the potential for the traumatic experience to lead to injury directly causing physical damage to joints or muscles.53 While this relationship is indeed important (e.g., car accident injuries causing osteoarthritis), much of the research linking trauma and health outcomes is focused on psychological, behavioral, and physiological implications of the psychological experience of trauma. For example, experiencing traumatic stress and related chronic stress activation can influence dysregulated hypothalamic-pituitary-adrenal axis or sympathetic nervous system functioning, unhealthy behaviors, and maladaptive coping strategies, heightening allostatic load burden (e.g., cumulative physiological wear and tear from multiple systems) and ultimately impacting susceptibility to diseases like arthritis.6,37 Thus, even witnessing or learning about trauma can impact one’s later health through alternative pathways besides direct physical damage. Indeed, is possible that psychological consequences of trauma are key drivers of arthritis risk. For example, some evidence suggests that psychological sequelae of trauma, such as depression or posttraumatic stress disorder, is associated with increased risk for RA.54–56 In sensitivity analyses, we removed traumatic experiences that feasibly could lead to direct injury from the trauma definition. The associations were generally robust and similar to primary estimates, particularly associations between trauma and osteoarthritis, which could most commonly be a consequence of bodily injury. These analyses suggest that associations seen between trauma and arthritis are not solely due to injury from physical trauma, but may reflect additional pathophysiological mechanisms.
Incorporating both prevalent and incident models provided complementary evidence of the association of lifetime trauma and arthritis. Our prevalent analyses suggested that lifetime trauma was associated with higher likelihood of having any type of arthritis in older adulthood. However, the timing is imprecise; arthritis diagnosis may have occurred at any point before our study baseline and potentially before trauma exposures. Even for early life trauma, it is possible retrospective recall can be influenced by current mental or physical health status.57,58 Therefore, while childhood experiences should temporally precede adult arthritis, it is possible one’s current arthritis status could influence retrospective recall, potentially biasing associations. Our incident models found that lifetime trauma was associated with elevated risk for developing incident arthritis (particularly osteoarthritis) over 12 years of follow-up. However, lifetime trauma was reported in 2006–2008 (mean age 65), a relatively arbitrary time point to classify as baseline in relation to the development of arthritis. Over 60% of the analytic sample had already developed arthritis by 2008 and thus restricting to those without arthritis selected a healthy sample. For example, those with higher lifetime trauma burden may have already developed arthritis, thus the incident sample likely reflected healthier individuals who were generally at lower risk for arthritis. This is particularly important for RA which tends to onset between ages 30 and 50,59 as opposed to osteoarthritis which tends to increase in prevalence starting around age 50.60 This difference may partially explain the lack of significant associations between trauma and incident immune-related arthritis in prospective analyses. Future work with longer follow-up periods earlier in the lifecourse should test these associations. Despite inherent drawbacks for both strategies, comparing analyses provides converging evidence supporting an association between lifetime trauma exposure and diagnosis of arthritis.
Our study benefitted from a relatively large community-based sample of older adults, included a range of adversities and traumatic events, incorporated different lifecourse models, and examined different forms of arthritis. However, there are several limitations. Lifetime trauma was retrospectively reported in mid to late adulthood, which can result in recall bias and misclassification. The trauma measure was not comprehensive and important details (e.g., severity, chronicity) of experiences were not assessed, particularly in trauma type analyses (e.g., only two exposures were included in “interpersonal violence”). Missingness for timing of endorsed events could have caused selection bias especially if missingness was related to aspects of exposure (e.g., chronic exposures over years may be missing “year” of exposure). Modeling accumulation of risk as count of trauma types assumes a linear association between each additional trauma, which may not best represent associations if there are threshold effects. Arthritis outcomes were self-reported thus subject to reporting biases; missingness for specific arthritis types (especially incident cases) lowered our power and could have resulted in misclassification error. Our analyses were limited with respect to timing of trauma and arthritis measures, with lifetime trauma being reported once during adulthood. As described, application of complimentary methods allowed for comparison of associations given the limitations of both. Missing data and loss to follow-up could have induced selection bias in our analyses, which we attempted to statistically adjust for using IPW. Although HRS was designed to be representative of older US community-dwelling adults, given our focused research question, we did not apply sampling weights and thus our findings are not directly generalizable to the HRS target population.
Conclusions
Given the aging population and the high burden of arthritis in older adults, it is important to understand psychosocial risk factors and to highlight groups that might be at heightened risk for developing arthritis. Future studies should continue to examine the potential mechanisms linking lifetime trauma exposure with various arthritis-related outcomes, to best understand how to guide treatment or prevention efforts.
Supplementary Material
Conflicts of Interest and Sources of Funding:
There are no conflicts of interest. Dr. Nishimi is supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Medical Research Service of the SFVAHCS, the Department of Veterans Affairs Sierra-Pacific Mental Illness Research, Education, and Clinical Center (MIRECC), and the VA Office of Research and Development (IK2CX002627). Dr. O’Donovan is supported by the National Institutes of Mental Health (K01MH109871) and the Department of Defense (W81XWH19C0093). Dr. Chen is supported by the National Institute on Aging (K00 AG068431). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations
- BMI
Body mass index
- CI
Confidence interval
- HR
Hazard ratios
- HRS
Health and Retirement Study
- IPW
Inverse probability weights
- PR
Prevalence ratio
- RA
Rheumatoid arthritis
- SD
Standard deviation
References
- 1.Duchowny KA, Hicken MT, Cawthon PM, Glymour MM, Clarke P. Life course trauma and muscle weakness in older adults by gender and race/ethnicity: Results from the U.S. health and Retirement Study. SSM - Popul Health. 2020;11:100587. doi: 10.1016/j.ssmph.2020.100587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.López-Martínez AE, Serrano-Ibáñez ER, Ruiz-Párraga GT, Gómez-Pérez L, Ramírez-Maestre C, Esteve R. Physical Health Consequences of Interpersonal Trauma: A Systematic Review of the Role of Psychological Variables. Trauma Violence Abuse. 2018;19(3):305–322. doi: 10.1177/1524838016659488 [DOI] [PubMed] [Google Scholar]
- 3.Nelson CA, Bhutta ZA, Harris NB, Danese A, Samara M. Adversity in childhood is linked to mental and physical health throughout life. BMJ. 2020;371:m3048. doi: 10.1136/bmj.m3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suglia SF, Koenen KC, Boynton-Jarrett R, Chan PS, Clark CJ, Danese A, et al. Childhood and Adolescent Adversity and Cardiometabolic Outcomes: A Scientific Statement From the American Heart Association. Circulation. 2018;137(5):e15–e28. doi: 10.1161/CIR.0000000000000536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sowder KL, Knight LA, Fishalow J. Trauma Exposure and Health: A Review of Outcomes and Pathways. J Aggress Maltreatment Trauma. 2018;27(10):1041–1059. doi: 10.1080/10926771.2017.1422841 [DOI] [Google Scholar]
- 6.Sumner JA, Cleveland S, Chen T, Gradus JL. Psychological and biological mechanisms linking trauma with cardiovascular disease risk. Transl Psychiatry. 2023;13(1):1–16. doi: 10.1038/s41398-023-02330-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theis KA, Murphy LB, Guglielmo D, Boring MA, Okoro CA, Duca LM, et al. Prevalence of Arthritis and Arthritis-Attributable Activity Limitation — United States, 2016–2018. Morb Mortal Wkly Rep. 2021;70(40):1401–1407. doi: 10.15585/mmwr.mm7040a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sturgeon JA, Finan PH, Zautra AJ. Affective disturbance in rheumatoid arthritis: psychological and disease-related pathways. Nat Rev Rheumatol. 2016;12(9):532–542. doi: 10.1038/nrrheum.2016.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks PM. Impact of osteoarthritis on individuals and society: how much disability? Social consequences and health economic implications. Curr Opin Rheumatol. 2002;14(5):573. doi: 10.1097/00002281-200209000-00017 [DOI] [PubMed] [Google Scholar]
- 10.van der Woude D, van der Helm-van Mil AHM. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2018;32(2):174–187. doi: 10.1016/j.berh.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 11.Sowers M. Epidemiology of risk factors for osteoarthritis: systemic factors. Curr Opin Rheumatol. 2001;13(5):447. doi: 10.1097/00002281-200109000-00018 [DOI] [PubMed] [Google Scholar]
- 12.Yucesoy B, Charles LE, Baker B, Burchfiel CM. Occupational and genetic risk factors for osteoarthritis: A review. Work. 2015;50(2):261–273. doi: 10.3233/WOR-131739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conigliaro P, Chimenti MS, Triggianese P, Sunzini F, Novelli L, Perricone C, et al. Autoantibodies in inflammatory arthritis. Autoimmun Rev. 2016;15(7):673–683. doi: 10.1016/j.autrev.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 14.Scherer HU, Häupl T, Burmester GR. The etiology of rheumatoid arthritis. J Autoimmun. 2020;110:102400. doi: 10.1016/j.jaut.2019.102400 [DOI] [PubMed] [Google Scholar]
- 15.Spitzer C, Barnow S, Völzke H, John U, Freyberger HJ, Grabe HJ. Trauma, posttraumatic stress disorder, and physical illness: findings from the general population. Psychosom Med. 2009;71(9):1012–1017. doi: 10.1097/PSY.0b013e3181bc76b5 [DOI] [PubMed] [Google Scholar]
- 16.Carette S, Surtees PG, Wainwright NW, Khaw KT, Symmons DP, Silman AJ. The role of life events and childhood experiences in the development of rheumatoid arthritis. J Rheumatol. 2000;27(9):2123–2130. [PubMed] [Google Scholar]
- 17.Spitzer C, Wegert S, Wollenhaupt J, Wingenfeld K, Barnow S, Grabe HJ. Gender-specific association between childhood trauma and rheumatoid arthritis: A case–control study. J Psychosom Res. 2013;74(4):296–300. doi: 10.1016/j.jpsychores.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 18.Baiden P, Panisch LS, Onyeaka HK, LaBrenz CA, Kim Y. Association of childhood physical and sexual abuse with arthritis in adulthood: Findings from a population-based study. Prev Med Rep. 2021;23:101463. doi: 10.1016/j.pmedr.2021.101463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubinstein TB, Bullock DR, Ardalan K, Mowrey WB, Brown NM, Bauman LJ, et al. Adverse Childhood Experiences Are Associated with Childhood-Onset Arthritis in a National Sample of US Youth: An Analysis of the 2016 National Survey of Children’s Health. J Pediatr. 2020;226:243–250.e2. doi: 10.1016/j.jpeds.2020.06.046 [DOI] [PubMed] [Google Scholar]
- 20.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31:285–293. doi: 10.1093/ije/31.2.285 [DOI] [PubMed] [Google Scholar]
- 21.Schnurr PP, Wachen JS, Green BL, Kaltman S. Trauma exposure, PTSD, and physical health. In: Handbook of PTSD: Science and Practice, 3rd Ed. The Guilford Press; 2021:462–479. [Google Scholar]
- 22.Bürgin D, Boonmann C, Schmeck K, Schmid M, Tripp P, Nishimi K, et al. Compounding Stress: Childhood Adversity as a Risk Factor for Adulthood Trauma Exposure in the Health and Retirement Study. J Trauma Stress. 2021;34(1):124–136. doi: 10.1002/jts.22617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fink DS, Galea S. Life course epidemiology of trauma and related psychopathology in civilian populations. Curr Psychiatry Rep. 2015;17(5):31. doi: 10.1007/s11920-015-0566-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben-Shlomo Y, Cooper R, Kuh D. The last two decades of life course epidemiology, and its relevance for research on ageing. Int J Epidemiol. 2016;45(4):973–988. doi: 10.1093/ije/dyw096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16(8):1412–1425. doi: 10.1162/0898929042304796 [DOI] [PubMed] [Google Scholar]
- 26.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. Jama. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754 [DOI] [PubMed] [Google Scholar]
- 27.Dunn EC, Soare TW, Raffeld MR, Busso DS, Crawford KM, Davis KA, et al. What life course theoretical models best explain the relationship between exposure to childhood adversity and psychopathology symptoms: Recency, accumulation, or sensitive periods? Psychol Med. 2018;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen MA, Christian LM, Fagundes CP. Immune and Epigenetic Pathways Linking Childhood Adversity and Health Across the Lifespan. Front Psychol. 2021;12:788351. doi: 10.3389/fpsyg.2021.788351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stebbins RC, Maselko J, Yang YC, Plassman BL, Edwards JK, Aiello AE. Lifecourse Traumatic Events and Cognitive Aging in the Health and Retirement Study. Am J Prev Med. 2022;63(5):818–826. doi: 10.1016/j.amepre.2022.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemp BR, Ferraro KF, Morton PM, Thomas PA, Mustillo SA, Crimmins EM. Do Early-Life Social, Behavioral, and Health Exposures Increase Later-Life Arthritis Incidence? Res Aging. 2022;44(7–8):479–493. doi: 10.1177/01640275211044979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Söderlin MK, Bergsten U, Svensson B, BARFOT Study Group. Patient-reported events preceding the onset of rheumatoid arthritis: possible clues to aetiology. Musculoskeletal Care. 2011;9(1):25–31. doi: 10.1002/msc.193 [DOI] [PubMed] [Google Scholar]
- 32.Hassett AL, Clauw DJ. The role of stress in rheumatic diseases. Arthritis Res Ther. 2010;12(3):123. doi: 10.1186/ar3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bürgin D, Boonmann C, Schmid M, Tripp P, O’Donovan A. Fact or artefact? Childhood adversity and adulthood trauma in the U.S. population-based Health and Retirement Study. Eur J Psychotraumatology. 2020;11(1):1721146. doi: 10.1080/20008198.2020.1721146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sumner JA, Kubzansky LD, Kabrhel C, Roberts AL, Chen Q, Winning A, et al. Associations of Trauma Exposure and Posttraumatic Stress Symptoms With Venous Thromboembolism Over 22 Years in Women. J Am Heart Assoc. 2016;5(5):e003197. doi: 10.1161/JAHA.116.003197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deane KD, Demoruelle MK, Kelmenson LB, Kuhn KA, Norris JM, Holers VM. Genetic and environmental risk factors for rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2017;31(1):3–18. doi: 10.1016/j.berh.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Connor MI. Sex differences in osteoarthritis of the hip and knee. J Am Acad Orthop Surg. 2007;15 Suppl 1:S22–25. [PubMed] [Google Scholar]
- 37.Norman SB, Means-Christensen AJ, Craske MG, Sherbourne CD, Roy-Byrne PP, Stein MB. Associations between psychological trauma and physical illness in primary care. J Trauma Stress. 2006;19(4):461–470. doi: 10.1002/jts.20129 [DOI] [PubMed] [Google Scholar]
- 38.Roberts AL, Gilman SE, Breslau J, Breslau N, Koenen KC. Race/ethnic differences in exposure to traumatic events, development of post-traumatic stress disorder, and treatment-seeking for post-traumatic stress disorder in the United States. Psychol Med. 2011;41(1):71–83. doi: 10.1017/S0033291710000401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolen J, Schieb L, Hootman JM, Helmick CG, Theis K, Murphy LB, et al. Differences in the Prevalence and Impact of Arthritis Among Racial/Ethnic Groups in the United States, National Health Interview Survey, 2002, 2003, and 2006. Prev Chronic Dis. 2010;7(3):A64. [PMC free article] [PubMed] [Google Scholar]
- 40.Krause N, Shaw BA, Cairney J. A descriptive epidemiology of lifetime trauma and the physical health status of older adults. Psychol Aging. 2004;19(4):637–648. doi: 10.1037/0882-7974.19.4.637 [DOI] [PubMed] [Google Scholar]
- 41.Clarke P, Fisher G, House J, Smith J, Weir D. Guide to Content of the HRS Psychosocial Leave-behind Participant Lifestyle Questionnaires: 2004 & 2006. University of Michigan; 2008. Accessed June 15, 2023. http://www-personal.umich.edu/~mkimball/keio/6.%20surveys/HRS2006LBQscale%20copy.pdf [Google Scholar]
- 42.Xiang X, Wang X. Childhood adversity and major depression in later life: A competing-risks regression analysis. Int J Geriatr Psychiatry. 2021;36(1):215–223. doi: 10.1002/gps.5417 [DOI] [PubMed] [Google Scholar]
- 43.Puterman E, Gemmill A, Karasek D, Weir D, Adler NE, Prather AA, et al. Lifespan adversity and later adulthood telomere length in the nationally representative US Health and Retirement Study. Proc Natl Acad Sci. 2016;113(42):E6335–E6342. doi: 10.1073/pnas.1525602113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zivin K, Llewellyn DJ, Lang IA, Vijan S, Kabeto MU, Miller EM, et al. Depression among older adults in the United States and England. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry. 2010;18(11):1036–1044. doi: 10.1097/JGP.0b013e3181dba6d2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika. 1991;78(3):691–692. doi: 10.1093/biomet/78.3.691 [DOI] [Google Scholar]
- 46.Nishimi K, Koenen KC, Coull BA, Chen R, Kubzansky LD. Psychological resilience predicting cardiometabolic conditions in adulthood in the Midlife in the United States Study. Proc Natl Acad Sci. 2021;118(32):e2102619118. doi: 10.1073/pnas.2102619118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55(7):626–632. doi: 10.1001/archpsyc.55.7.626 [DOI] [PubMed] [Google Scholar]
- 48.Creamer M, Parslow R. Trauma Exposure and Posttraumatic Stress Disorder in the Elderly: A Community Prevalence Study. Am J Geriatr Psychiatry. 2008;16(10):853–856. doi: 10.1097/01.JGP.0000310785.36837.85 [DOI] [PubMed] [Google Scholar]
- 49.Theis KA. Prevalence of Arthritis and Arthritis-Attributable Activity Limitation — United States, 2016–2018. MMWR Morb Mortal Wkly Rep. 2021;70. doi: 10.15585/mmwr.mm7040a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cutolo M, Straub RH. Stress as a Risk Factor in the Pathogenesis of Rheumatoid Arthritis. Neuroimmunomodulation. 2007;13(5–6):277–282. doi: 10.1159/000104855 [DOI] [PubMed] [Google Scholar]
- 51.Ong KL, Wu BJ, Cheung BMY, Barter PJ, Rye KA. Arthritis: its prevalence, risk factors, and association with cardiovascular diseases in the United States, 1999 to 2008. Ann Epidemiol. 2013;23(2):80–86. doi: 10.1016/j.annepidem.2012.11.008 [DOI] [PubMed] [Google Scholar]
- 52.Seavey WG, Kurata JH, Cohen RD. Risk factors for incident self-reported arthritis in a 20 year followup of the Alameda County Study Cohort. J Rheumatol. 2003;30(10):2103–2111. [PubMed] [Google Scholar]
- 53.Punzi L, Galozzi P, Luisetto R, Favero M, Ramonda R, Oliviero F, et al. Post-traumatic arthritis: overview on pathogenic mechanisms and role of inflammation. RMD Open. 2016;2(2):e000279. doi: 10.1136/rmdopen-2016-000279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boscarino JA, Forsberg CW, Goldberg J. A twin study of the association between PTSD symptoms and rheumatoid arthritis. Psychosom Med. 2010;72(5):481–486. [DOI] [PubMed] [Google Scholar]
- 55.Lee YC, Agnew-Blais J, Malspeis S, Keyes K, Costenbader K, Kubzansky LD, et al. Post-Traumatic Stress Disorder and Risk for Incident Rheumatoid Arthritis. Arthritis Care Res Hoboken. 2016;68(3):292–298. doi: 10.1002/acr.22683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Donovan A, Cohen BE, Seal KH, Bertenthal D, Margaretten M, Nishimi K, et al. Elevated risk for autoimmune disorders in iraq and afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77(4):365–374. doi: 10.1016/j.biopsych.2014.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frissa S, Hatch SL, Fear NT, Dorrington S, Goodwin L, Hotopf M. Challenges in the retrospective assessment of trauma: comparing a checklist approach to a single item trauma experience screening question. BMC Psychiatry. 2016;16(1):20. doi: 10.1186/s12888-016-0720-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edwards VJ, Anda RF, Nordenberg DF, Felitti VJ, Williamson DF, Wright JA. Bias assessment for child abuse survey: factors affecting probability of response to a survey about childhood abuse. Child Abuse Negl. 2001;25(2):307–312. doi: 10.1016/s0145-2134(00)00238-6 [DOI] [PubMed] [Google Scholar]
- 59.Wasserman A. Rheumatoid Arthritis: Common Questions About Diagnosis and Management. Am Fam Physician. 2018;97(7):455–462. [PubMed] [Google Scholar]
- 60.Neogi T, Zhang Y. Epidemiology of Osteoarthritis. Rheum Dis Clin. 2013;39(1):1–19. doi: 10.1016/j.rdc.2012.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


