Abstract
Companion dogs have served an important role in cancer immunotherapy research. Sharing similar environments and diets with humans, dogs naturally develop many of the same cancers. These shared exposures, coupled with dogs’ diverse genetic makeup, makes them ideal subjects for studying cancer therapies. Tumors like osteosarcoma (cOSA), hemangiosarcoma (cHSA), soft-tissue sarcoma (cSTS), and non-Hodgkin lymphoma (cnHL) occur with greater frequency than their counterpart disease in humans. Canine brain tumors allow study of therapy strategies with imaging, surgery, and radiotherapy equipment in veterinary patients with near-human geometry. Non-specific immunostimulants, autologous and allogeneic vaccines, immune checkpoint inhibitors, and cellular therapies used treating canine cancers have been tested in veterinary clinical trials. These treatments have not only improved outcomes for dogs but have also provided valuable insights for human cancer treatment. Advancements in radiation technology and the development of tools to characterize canine immune responses have further facilitated the ability to translate veterinary clinical trial results to human applications. Advancements in immunotherapy of canine tumors have directly supported translation to human clinical trials leading to approved therapies for cancer patients around the world. The study of immunotherapy in dogs has been and will continue to be a promising avenue for advancing human cancer treatment.
INTRODUCTION
Companion dogs share the same environment, breathe the same air, and often eat the same food as the humans in their lives. Both species naturally develop many of the same forms of cancers in the context of a cancer-conditioned immune system. Dogs are an outbred population of individuals with some inbred characteristics of breed leading to disease-specific risks. Canine tumors like osteosarcoma (cOSA), hemangiosarcoma (cHSA), soft-tissue sarcoma (cSTS), and non-Hodgkin lymphoma (cnHL) occur with greater frequency than the counterpart disease in humans. Rigid standards of care are not defined in veterinary patients, allowing more rapid clinical trial accrual of patients receiving earlier line therapy. Brain tumors occur naturally in companion dogs providing an opportunity to study therapy strategies in clinical veterinary patients with near-human geometry using human imaging, surgical, and radiotherapy equipment. As in human oncology, immunotherapy has been of great interest in veterinary oncology with many studies providing foundational data for translation to the human clinic. While it is impossible to adequately cover the entire breadth of cancer immunotherapy study in companion dogs, this manuscript will discuss many of the lines of research that have contributed directly to translation, complemented studies in human patients, or offer future hope of defining critical mechanisms of success or failure of immunotherapy to improve patient selection, agent development, and rates of response.
Companion dogs have benefitted from and contributed to the understanding of many advances in cancer immunotherapy. Non-specific immunostimulants have shown benefit in veterinary clinical trials alone and in combination with cytotoxic chemotherapy (1,2). Autologous and allogeneic vaccines have been trialed helping to define the underlying mechanisms of efficacy and failure (3–7). Vaccine strategies using DNA and peptide vaccines have improved outcomes for companion dogs with cancer and may help define similar strategies for human cancers (3–6). Drugs that inhibit the immune checkpoint axes are now available for companion dogs both experimentally and commercially, allowing veterinary clinical trials to test hypotheses that have been difficult to pursue in human patients (8–10). Cellular therapies have been tested and are available commercially for companion dogs that have enabled human clinical trials and may transform the cost and availability of highly active cellular immunotherapy (11,12). Radiation delivery technology is now identical between human and veterinary clinical care, allowing veterinary clinical trials that advance human application. Reagents and technologies to adequately characterize canine immune responses are maturing and facilitate translation of veterinary clinical trial results (13–15). We will discuss these areas of immunotherapy research in companion dogs and present new data that support the past success and future promise of canine immunotherapy for human translation.
Evaluating success in cancer immunotherapy clinical trials can be challenging, particularly in comparison to cytotoxic chemotherapy or radiation therapy clinical trials. Tumor response assessment must be modified for the possibility of pseudoprogression following tumor inflammation using iRECIST parameters modified for dogs (16). Trial design can introduce bias into evaluation of progression free survival, disease free intervals and overall survival when patients must be deemed in a clinical remission following an initial standard therapy before starting the immune intervention. Such patients that have maintained remission for months following initial definitive therapy may be a population sub-selected for better survival by having already survived a substantial time without disease progression. Thus, control patients must be sub-selected in the same manner to make meaningful comparisons. Euthanasia can also introduce bias into overall survival analysis as families elect to relieve suffering in a dog or delay such a decision in the hope that the intervention will result in disease remission even as the dog’s clinical condition deteriorates. Measured immune endpoints such as antibody generation, cell cytotoxicity assays, or evidence of clonal expansion can support the immune mechanism of an intervention and provide evidence of potential translational efficacy. The cancer-affected outbred companion dog with its attendant tumor genomic heterogeneity in the context of a cancer-conditioned immune system is likely the most realistic test of novel immunotherapy interventions in the translational pipeline. Decades of veterinary clinical research in companion dogs has facilitated mechanistic understanding and clinical translation in a variety of immunotherapy strategies.
Non-specific immunostimulants
Non-specific immune stimulation is an inexpensive means of breaking immune tolerance to tumor cells that can complement cytotoxic therapy. The synthetic molecule MDP activates monocyte/macrophage lineage cells to a cytotoxic state in rodent models of cancer (1). A pegylated liposomal encapsulation of muramyl tripeptide (L-MTP-PE) derivative of MDP activates both human and canine monocytes, enhancing tumoricidal effects. A veterinary study administered L-MTP-PE to dogs with cOSA following amputation twice weekly for 8 weeks and compared it to a control group of dogs undergoing amputation with infusion of empty liposomes (1) (Table 1). All control dogs developed metastatic cOSA at a median of 58 days with a median overall survival (OS) of 77 days (1). Two dogs in the L-MTP-PE group were metastasis-free two years following amputation with the group developing metastasis at a median of 168 days (P=0.002) and a median OS of 222 days (P<0.002) (1). The same group subsequently tested the chemoimmunotherapy combination of cisplatin and L-MTP-PE in dogs with cOSA free of pulmonary metastasis (2). Dogs received four doses of cisplatin chemotherapy with an initial cohort receiving L-MTP-PE or empty liposomes by blinded randomization if the dogs were metastasis-free following chemotherapy (2). Subsequently, the cohorts were expanded into a multi-institutional phase with dogs receiving cisplatin concurrently with L-MTP-PE or empty liposomes (2). Forty dogs were enrolled in the first phase with 13 being lost to development of metastasis and two dying of other causes prior to randomizing 25 dogs (2). Dogs receiving sequential cisplatin and L-MTP-PE had a significant extension of disease-free interval (DFI; P<0.035) and OS (P<0.01) compared to dogs receiving placebo (2). Dogs receiving simultaneous cisplatin and L-MTP-PE saw no improvement in DFI or OS compared to placebo (see Figure 1) (2). The authors concluded that cisplatin may abrogate monocyte/macrophage activation by L-MTP-PE when given concurrently (2). The benefit of L-MTP-PE was recapitulated in a series of dogs with cHSA with evidence of increased plasma tumor necrosis factor alpha (TNFα) and interleukin-6 (IL-6) following drug administration (17). L-MTP-PE resulted in a survival benefit in dogs with early stage canine mucosal melanoma, but not those with advanced stage disease (18). For dogs with canine mammary carcinoma the drug did not produce any improvement in DFI or OS (19). Subsequent to the studies in dogs, L-MTP-PE was trialed in patients, first with relapsed OSA and then in conjunction with chemotherapy as first-line treatment (20,21). L-MTP-PE extended survival in both disease-free and relapsed settings (20,21) and chemotherapy did not reduce tumoricidal properties or cytokine secretion of circulating monocytes in patients (22–24). The drug has been approved for chemoimmunotherapy in Europe and South America, but remains an orphan drug for OSA in the United States.
Table 1:
Summary of Veterinary Clinical Trials of Immunotherapy in Dogs with Osteosarcoma
| Cancer type | Treatment | Treated | Control | CR | PR | SD | PD | DFI | OS | Significant? | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| cHSA, Stage I and II | Splenectomy followed by L-MTP-PE or placebo | 16 dogs splenectomy + L-MTP-PE | 16 dogs splenectomy + placebo | - | - | - | - | 188 d vs. 127 d. | 277 d vs. 143 d | Y | 17 |
| cHSA, Stage I-III | Splenectomy followed by allogeneic cultured cHSA cell vaccine combined with cationic liposome and plasmic DNA complexes | 28 dogs (13 with splenic stage II + doxorubicin) received 8 vaccines over 22 weeks with KLH as a control peptide to monitor immune response following surgery | 24 historical control dogs with stage II cHSA treated with splenectomy and doxorubicin | - | - | - | - | 146 d vs. 126 d | 182 d vs. 133 d | Y for OS | 32 |
| cnHL | Dogs with stage IV or V B cell cnHL received modified CHOP chemotherapy with autologous activated T cell infusion X 1–3 following completion of chemotherapy | 8 dogs (7 stage IV, 1 stage V) received modified CHOP followed by autologous activated T cell therapy (1 X 1, 1 X 2, 6 X 3) | 12 dogs with stage IV or V B cell cnHL received modified CHOP | 7 (88%) | 1 (12%) | 0 | 0 | 338 d vs. 71 d | 392 d vs. 167 d | Y | 53 |
| cOSA, first-line | L-MTP-PE +/− amputation | 14 dogs with amputation + L-MTP-PE | 13 dogs amputation + placebo | - | - | - | - | 168 d vs. 58d | 222d vs. 77d | Y | 1 |
| cOSA, first-line | Amputation followed by sequential cisplatin X 4 + L-MTP-PE or placebo | 11 dogs cisplatin + L-MTP-PE | 14 dogs cisplatin + placebo | - | - | - | - | 11.2 m vs. 7.6 m | 9.8m vs. 14.4m | Y | 2 |
| Amputation followed by concurrent cisplatin X 4 + L-MTP-PE or placebo | 21 dogs received cisplaitin + twice weekly L-MTP-PE, 21 dogs received cisplatin + once weekly L-MTP-PE | 22 dogs received cisplatin + placebo | - | - | - | - | 7.5 m, 6.3 m vs. 5.8 m | 10.3 m, 10.5 m vs. 7.6 m | N | 2 | |
| cOSA, first-line | TALL-104 cells infused following amputation or limb-sparing surgery and cisplatin X 1–4 cycles | 23 dogs (20 amputated, 3 limb-spare) received infusion of TALL-104 cytotoxic cells if in clinical remission following chemotherapy | None | - | - | - | - | 9.8 m | 11.5 m | N | 51 |
| cOSA, first-line | Radiation therapy to advanced, non-metastatic cOSA resions were treated also with 2 intralesional injections of expanded autologous NK cells | 10 dogs with advanced, non-metastatic cOSA received 9 Gy X 3 palliative RT followed by autologous expanded NK cell intralesional injections without chemotherapy | None | - | - | - | - | Not reached | - | N | 54 |
| cOSA, first-line | Amputation followed by autologous vaccination X 3, then leukapheresis for activated T cell therapy and low-dose IL-2 X 5 | 14 dogs with amputation, activated T cell therapy and low-dose IL-2 X 5 of which 10 dogs received the entire protocol | None | - | - | - | - | 213 d | 415 d | N | 11 |
| cOSA, first-line | Amputation with carboplatin X 4 then peptide vaccine derived from autologous or allogeneic tumor cells stressed by Salmonella enterica Ty21a | 20 dogs with amputation + peptide tumor vaccine from autologous or allogeneic tumor cells | 34 dogs with amputation and chemotherapy, free of metastatic disease at end of chemotherapy | 308 d vs. 240 d | 621 d vs. 278 d | Y | 5 | ||||
| cOSA, first-line | Neoadjuvant vesicular stomatitis virus (VSV-IFNβ-NIS) administered prior to amputation then followed by carboplatin X 6 | 28 dogs receiving VSV-IFNβ-NIS prior to amputation and then chemotherapy, 15 open-label and 7 in a blinded randomization with placebo | Contemporaneous cohorts of 57 dogs from University of Minnesota and 157 dogs from NCI-COTC | - | - | - | - | No difference from contemporaneous cohorts | Longest-survived quartile was larger than comparator studies | N | 48 |
| cOSA, metastatic Canine Melanoma | Dogs with metastatic to lung cOSA or melanoma received escalating dosese of inhaled rhIL-15 by nebulization twice daily for 14 days with evaluation at day 28 | 18 (8 cOSA and 10 melanoma) dogs reached day 28 for evaluation by RECIST criteria | None | 1 (6%) | 1 (6%) | 5 (27%) | 11 (61%) | - | 82.5 d cOSA and 113.5 d melanoma | N | 46 |
| cOSA, metastatic | Dogs with metastatic to lung cOSA were treated with toceranib in combination with losartan at 1 mg/kg and 10 mg/kg daily | 24 dogs were evaluable (8 in 1 mg/kg cohort and 16 in 10 mg/kg cohort) for response and survival | 22 dogs receiving toceranib alone for pulmonary metastatic cOSA in a separate single-agent trial | 0 | 0 at 1 mg/kg and 4 (25%) at 10 mg/kg | 3 (38%) at 1 mg/kg and 4 (25%) at 10 mg/kg | 5 (63%) at 1 mg/kg and 8 (50%) at 10 mg/kg | 61 d for 1 mg/kg and 111 d for 10 mg/kg vs. 57 d | 109 d for 1 mg/kg and 148 d for 10 mg/kg vs. 89 d | N | 47 |
| Canine Glioma | Dogs following resection of glioma were treated intradermally with tumor cell lysates and CD200AL compared to a historical tumor lysate vaccine control | 20 dogs underwent resection of glioma followed by autologous tumor cell lysate vaccine preparation with CD200AL | Historical control of 15 dogs treated with autologous tumor cell lysate vaccination following resection | 0 | 5/20 reported | - | - | - | 12.9 m vs. 6.83 m | Y | 7 |
| Canine Mammary Carcinoma | Following surgical resection, dogs were randomized to receive L-MTP-PE or placebo | 13 dogs received L-MTP-PE twice weekly for 8 weeks | 14 dogs received placebo twice weekly for 8 weeks | - | - | - | - | 165 d vs. 133 d | 222 d vs. 182 d | N | 19 |
| Canine Melanoma | By stage, dogs were randomized to receive L-MTP-PE or placebo following surgery | 25 dogs received surgery followed by L-MTP-PE once weekly | 25 dogs received surgery followed by placebo once weekly | - | - | - | - | 346 d vs. 174 d | 504 d vs. 271 d | N | 18 |
| By stage, dogs were randomized to receive rcGM-CSG or placebo then L-MTP-PE following surgery | 24 dogs received rcGM-CSF followed by L-MTP-PE either once or twice weekly | 24 dogs received saline followed by L-MTP-PE either once or twice weekly | - | - | - | - | 212 d vs. 290 d | 498 d vs. 501 d | N | 18 | |
| Canine Melanoma | Following local therapy, dogs received 17M98 melanoma cells tranfected with hgp100 | 34 dogs with stage II-IV melanoma received up to 8 vaccines and monitored for response | None | 1 (3%) | 5 (15%) | 6 (18%) | 22 (64%) | Responders (12 dogs) 170 d | Responders vs. non-responders 337 d vs. 95d | Y | 30 |
| Canine Melanoma | Following control of local disease, dogs received xenogeneic tyrosinase DNA vaccine X 4 then q. 6 months | 58 dogs with Stage II or III locoregionally controlled melanoma received xenogeneic DNA vaccine | 53 historical control dogs locoregionally controlled | - | - | - | - | - | Median not reached vs. 324 d | Y | 3 |
| Canine Melanoma | 15 dogs with stage IV melanoma among a series of dogs with a variety of aggressive cancers received a chimeric or caninized anti-PD1 mAb | 15 dogs with stage IV melanoma received surgery and/or radiation therapy for local disease plus anti-PD1 mAb | 23 historical control dogs with stage IV melanoma treated with surgery, radiation therapy, or chemotherapy | 2 (13%) | 2 (13%) | 2 (13%) | 9 (60%) | 72 d | 166 d vs. 55 d | Y | 44 |
| Canine Melanoma | 35 dogs with advanced non-oral melanoma (29 with measurable disease) received caninized anti-PD1 mAb q. 2 weeks until progression | 35 dogs received caninized anti-PD1 mAb q. 2 weeks with 29 having measurable (7 not evaluable) and 6 having non-measurable disease | No control group | 1 (3%) | 1 (3%) | 6 (21%) | 14 (48 %) | 70 d | 215 d | N/A | 45 |
| Canine Meningioma | Dogs following resection of meningioma were treated with autologous tumor cell lysate vaccine combined with CpG or imiquimod | 5 dogs received autologous tumor cell lysate with CpG and 6 received tumor cell lysate with imiquimod following resection | 12 dogs in a historical control cohort treated with surgery alone | - | - | - | - | - | 645 d vs. 222 d | Y | 25 |
Figure 1:
Kaplan-Meier curves for DFI and OS of dogs receiving L-MTP-PE to dogs in clinical remission following cisplatin chemotherapy showing benefit to the sequential administration (panels A and B). When L-MTP-PE was administered concurrently with cisplatin no benefit in DFI or OS was observed (panels C and D). This may have been due to cisplatin abrogating the immune mechanism of L-MTP-PE (unlikely) or possibly that dogs in remission at the end of chemotherapy are more likely to benefit from immunotherapy. (Images reprinted from Clinical Cancer Research; ref. 2; with permission.)
Autologous vaccination
Autologous vaccination strategies have met with limited success in companion dogs, as in human patients. Experimental and now commercial vaccines are common in the literature for cancer immunotherapy in dogs. Surgically resected canine meningiomas were used to prepare autologous lysate vaccines for 11 dogs receiving six doses each, either combined with CpG (five dogs) or at sites treated with imiquimod cream (six dogs) (25). Vaccines induced immune reactions including circulating anti-tumor antibodies measured by Western blot as well as antibody-dependent cellular cytotoxicity measured by in vitro assay (25). At necropsy peritumoral brain of some vaccinates showed an increased mononuclear and lymphocyte infiltrate; two dogs also had clinical sequelae that were severe with brain-reactive antibodies identified on necropsy (25). Collectively, the data support the generation of an anti-meningioma immune response but also the potential on-target, off-tumor effect of cell lysate vaccination in brain tissue (25). The same group reported an autologous canine meningioma vaccine augmented with a CD200 activating ligand (CD200AL) to enhance antigen presenting cell function (7). Survival time was doubled in the recipient dogs compared to unvaccinated dogs and collected lymphocytes showed downregulation of checkpoint molecules (7). An autologous B cell canine non-Hodgkin lymphoma (cnHL) vaccine adjuvanted with hydroxyapatite and heat shock proteins to treat companion dogs also receiving chemotherapy may increase cnHL-specific survival with minimal increase in toxicosis (26,27). Autologous vaccines are being commercially produced with one company suggesting signal of efficacy in high-stage cHSA with the ability to upregulate MHC II and CD80 in a cultured canine monocyte line and another company reporting tolerability and elevated serum cytokines after vaccination (28,29). Autologous vaccines are relatively inexpensive to produce, contain patient-specific mutations, and may translate as technology advances, but must be evaluated in larger controlled trials.
Allogeneic vaccination
Allogeneic vaccines offer the potential benefits of low cost of production, storage for future use, and “off-the-shelf” simplicity for cancer immunotherapy. A canine melanoma cell line 17CM98 transfected with human glycoprotein 100 (hgp100) was administered to companion dogs with melanoma (30). The surface molecule gp100 was selected as it stimulates MHC I-restricted cytotoxic T cell responses (30). Thirty-four companion dogs with stage II-IV canine oral melanoma were vaccinated with 2 X 107 irradiated cells up to an intended eight vaccinations (30). Dogs were monitored with intradermal skin testing (12 dogs) for anti-tumor immune response and primary and metastatic lesions were followed using modified RECIST criteria (30,31). Objective responses were seen in 6/34 dogs including one complete response (CR) with six additional dogs experiencing stable disease (SD) for at least six weeks following initiation of vaccination (30). Responding dogs and those experiencing SD had a longer OS than non-responders (337 days vs. 95 days; P=0.001) (30).
Cultured cHSA cell lines were used to create a vaccine with cationic liposome and plasmid DNA complexes (LDC) administered with or without chemotherapy for cHSA in companion dogs (32). Lysates from two cHSA cell lines were combined with LDC and keyhole limpet hemocyanin (KLH) as a control peptide (32). Twenty-eight companion dogs with stage I-III cHSA received up to eight doses of vaccine over 22 weeks (32). Six dogs assessed developed a titer to KLH (P=0.03) and flow cytometric increase in reactivity to a cHSA cell line (P=0.05); control non-cHSA tumor cells showed no reactivity (32). A small median survival benefit was seen in a subset of dogs with stage II cHSA compared to historic controls (182 days vs. 133 days) and no P value was reported (32). To date, in spite of some promise in eliciting immune reactions, no commercial allogeneic vaccines exist.
Bacteria have been used with cells and alone as vaccines for cancer immunotherapy. Salmonella enterica serovar typhi Ty21a was used to stress autologous tumor cells releasing immunogenic peptides to treat 20 dogs with cOSA following amputation with four doses of carboplatin (5). Compared to similarly treated control dogs without vaccination, the vaccinates had a significantly longer DFI (308 vs. 240 days) and OS (621 vs. 278 days) and exhibited humoral and cellular responses ex vivo (5). A cryopreserved, attenuated Listeria transfected with the HER2 gene was administered following amputation and four doses of carboplatin (33). Survival data have not been published for this vaccine but four dogs cultured positive for the administered Listeria organism (33). These strategies are potentially inexpensive and immunogenic if safety concerns can be mitigated.
DNA, peptide vaccination
Vaccination for tumor-associated antigens is one of the least expensive, off-the-shelf strategies for active tumor immunotherapy. In companion dogs, two recent examples stand out. In canine melanoma, the United States Department of Agriculture has approved a DNA vaccine against a tumor xenoantigen, human tyrosinase (3). The pivotal trial enrolled 58 dogs with stage II or III locoregionally controlled canine oral melanoma and vaccinated four times with the xenogeneic DNA vaccine compared to a cohort of 53 historical controls of the same disease stage and locoregional control (3). The control group had a median survival of 324 days and the median was not reached for the vaccinates, suggesting clinical benefit (3). Another approach using a peptide vaccination against an extracellular epitope of the epidermal growth factor receptor (EGFR) has generated antibody responses to EGFR and HER2, induced IgG deposition on tumor cells and CD8 T cell invasion of tumors, and caused remission of metastatic cOSA lesions post-vaccination in companion dogs (4). The 12 month survival among the reported cohort of dogs receiving amputation, carboplatin chemotherapy (4–6 doses) and EGFR vaccine was 65% (95% CI 53–75%) without a reported control (4). Both approaches offer an inexpensive and potentially beneficial adjunct to standard of care therapy.
Immune checkpoint inhibitors and strategies for breaking tolerance
Human immunotherapy has been revolutionized by checkpoint inhibition and agonists of activating molecules. Critical immune checkpoint molecules PD-L1, B7H3, and HVEM have been identified on many canine tumors and checkpoint PD-1 identified on immune cells (34–43). Antibodies against canine PD-1 and PD-L1 have been synthesized in chimeric and fully caninized forms showing initial efficacy against several cancers including oral melanoma, undifferentiated oral sarcoma, mammary gland carcinoma, squamous cell carcinoma, and lymphoma with most efficacy seen in melanoma (8–10,42,44,45). In the early veterinary clinical trials, immune-related adverse effects have been seen including sterile nodular panniculitis, myasthenia gravis, and hypothyroidism (45). The existence and future availability of these clinical reagents will expand the potential to test novel combinations of therapy as well as assess mechanisms of response through more opportunities for serial biopsy than available in human studies.
Other strategies to break tolerance have been employed, some of which could be combined with checkpoint inhibition to augment their effect. Nebulized human interleukin-15 (IL-15) at 50 սg administered twice daily for 14 days showed benefit in dogs with pulmonary metastatic cOSA (46). High-dose losartan and toceranib have been shown to suppress cOSA pulmonary metastatic disease in 25% of cases through inhibition of the CCL2-CCR2 axis reducing monocyte migration (47). Tumor-tropic viruses including vesicular stomatitis virus (VSV-IFNβ-NIS) have been administered prior to amputation and chemotherapy in dogs with cOSA improving OS over controls from the same institution as well as patient cohort from the Comparative Oncology Trials Consortium (bioRxiv 2023.04.16.533664) (48). Adenoviruses including a conditionally replicative adenovirus bearing a CAV2 gene (OC-CAVE1) injected into dogs following tumor resection induce antibody titers to CAV2 in all patients but no increase in OS (49). The immunogenicity of viruses in cancer cells can unmask them and break immune tolerance.
Cellular therapies
Cellular therapies are among the most active of any anti-tumor strategy. One of the earliest clinically employed in companion dogs was the xenogeneic cell line TALL-104, a human MHC non-restricted cytotoxic T cell line. In vitro studies demonstrated cytotoxicity by 51Cr release assay (50). Nineteen dogs with a variety of tumors (sarcomas, carcinomas, cnHL) that were all pre-treated received infusions of irradiated cells on two schedules (51). The xenogeneic cells were well tolerated and seven of the dogs showed at least transient clinical benefit including one canine malignant histiocytosis experiencing a CR (51). When companion dogs with cOSA received TALL-104 cells following cisplatin, both humoral and cellular xenogeneic immune responses were detectable in the recipient dogs, and the xenogeneic cells were eliminated from circulation 24 hours after administration (50). Nonetheless, the authors concluded that OS in the studied population was longer than typical following cisplatin chemotherapy (50). As with other immunotherapy strategies, the subselection of dogs without metastatic disease at the end of chemotherapy makes the median DFI and OS difficult to interpret; however, the extended survival of several of the dogs suggests that the immunotherapy had some impact on outcome (50). The lack of a control group makes evaluation difficult.
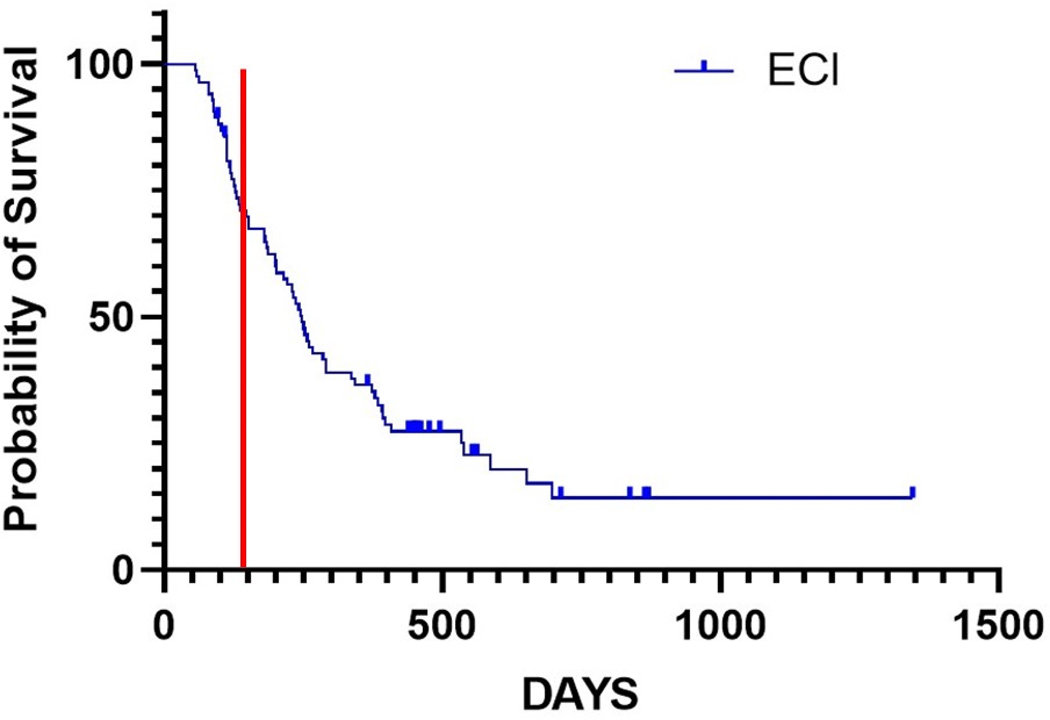
Activated autologous T cells have been employed as adjuvant therapy for cancer in companion dogs in several contexts. Fourteen companion dogs with cOSA received a vaccine-enhanced activated T cell approach without chemotherapy following amputation for adjuvant therapy of cOSA (11). Autologous tumor tissue was used to create a vaccine administered weekly three times (11). Dogs underwent leukapheresis to harvest peripheral blood mononuclear cells (PBMCs) and yield a non-specifically activated T cell product (11). The activated product was transfused into the dogs and followed by low-dose IL-2 five times following on an every other day basis (11). The resulting median DFI was 213 days and OS was 415 days with five dogs surviving beyond two years (11). The lack of chemotherapy administration convincingly suggests an immune mechanism extending survival in these dogs. Further, one dog underwent spontaneous remission of a cytologically confirmed cutaneous cOSA metastasis, further supporting immune activation (11). No control group receiving only amputation or adjuvant chemotherapy was present in this study requiring cautious interpretation of the outcome. The company ELIAS Animal Health has offered the vaccine-enhanced activated T cell therapy commercially under experimental label since the pilot study was complete. A similar long-term survival group has been seen (Figure 2) in those companion dogs with cOSA. The data from the canine study was presented to the Food and Drug Administration leading to Fast Track designation for a clinical trial in human glioblastoma patients by the parent company TVAX Biomedical. The results of the ELIAS immunotherapy ECI-OSA-04 pivotal study were accepted by the USDA-CVB for full approval in January 2024.
Figure 2:
Kaplan-Meier curve of dogs receiving the commercial administration of vaccine-enhanced adoptive T cell therapy (ECI) following amputation for OSA at multiple sites across the United States. A subset of dogs (~30%) survive longer than two years with a median overall survival of 247 days in this group. A vertical line has been included in red at 134 days, the median survival of 162 dogs treated with amputation alone (75). If the treatment approach taken to the dogs represented in this figure were no better than amputation, the median would be expected to be much closer to that identified previously for only amputation. (Data are courtesy of Tammie Wahaus and Noe Reyes, DVM of ELIAS Animal Health.)
B cell cnHL consolidation therapy has been attempted with activated autologous T cells following multi-agent chemotherapy (52). Peripheral blood mononuclear cells were harvested and expanded on γ-irradiated antigen presenting cells (APCs) in the presence of rhIL-2 and rhIL-21 (52). The combination of cytokines enhanced non-specific T cell expansion in culture on the APCs (52). T cells were treated with vital dye ex vivo prior to transfusion (52). Cells were tracked following transfusion by monitoring CD4+/CD8+ lymphocyte ratios in the peripheral blood and through lymph node biopsy identifying dye-positive cells in the nodes (52). Compared to a historical control of dogs with B cell cnHL receiving multi-agent chemotherapy alone, the DFI and OS was improved in the eight dogs receiving the treatment (52). Seven of the eight dogs were in complete remission at the end of the multi-agent chemotherapy, which was statistically different from the control dogs presented in the Kaplan-Meier curves in the manuscript (52). As several of the control dogs apparently lost remission at approximately the time of the first T cell infusion, characteristic of being in remission at the end of chemotherapy may have independently selected a better performing cohort of dogs in the immunotherapy group and may not represent clear benefit of the activated T cells (52).
Natural killer (NK) cells mediate the “missing self” response and protect against cancer cells displaying reduced MHC I expression. Recent characterization of canine NK characteristics and function make therapeutic application feasible. The molecule Ly49 on the surface of NK cells has been predicted in silico to dock with MHC I (15). Bulk and single-cell RNAseq detected the message for Ly49 exclusively in NK cell populations (15). These data suggest that Ly49 is the functional interaction between NK cells and MHC I in dogs (15). Similarities of dog NK to human and murine NK cells was recently reviewed (14). Companion dogs with cOSA received radiation therapy to primary bone lesions with subsequent intratumoral injections of ex vivo expanded NK cells (53). Homing of the NK cells was confirmed by presence in tumor biopsies and absence in peripheral blood by flow cytometry (53). Five of ten dogs reached the six month endpoint of the study without evidence of metastasis (53). One dog developed an apparent pulmonary metastasis at three months that had resolved on computed tomography scan at the six month point (53). Another group investigated the use of allogeneic invariant NK (iNK) cells carrying the invariant T cell receptor α (iTCRα) chain with specific reactivity to α-glycolipid (α-GC) antigens on the tumor cells (54). The investigators expanded canine lymphocytes in the presence of IL-2 and α-GC to selectively expand iNK cells (54). Once expanded, these cells clustered with well characterized human iNK cells and away from other lymphocyte populations by single-cell sequencing (SCseq), confirming their identity (54). The cells were confirmed to have restricted reactivity to CD1d/αGC similar to human iNK cells (54). Optimized iNK cells were infused into MHC-mismatched healthy recipients with minimal preconditioning (low dose cyclophosphamide +/− 2 Gy total body irradiation) and iNK cells were detectable to 78 days (54). These studies demonstrate the utility of cancer-bearing companion dogs in cellular therapy research and the development of necessary assay reagents makes the studies feasible.
Genetically modified chimeric antigen receptor T (CAR-T) cells are highly specific and effective for B cell cnHL/leukemia and multiple myeloma. Six CAR-T products are currently FDA approved with over 1,400 CAR-T or CAR-T-related clinical trials in lymphoma/leukemia and solid tumors enrolling (55). Engineered T cells are in their infancy for application in companion dogs. Lentiviral-transduced second generation CAR-T cells have been administered to dogs with B cell cnHL and tracked in their function showing selection pressure on CD20+ B cells leading to CD20- lymphoma clones dominating (56). Further optimized cell transduction protocols have been published along with a case report of cytokine release syndrome in a treated companion dog with CD20+ B cell cnHL (12,57). A second group has done preclinical work in optimizing culture conditions to generate anti-CD20 CAR-T cells that become memory T cells (58). Application of CAR-T technology to solid tumors has been developed in B7-H3 targeted cells that killed cOSA cells in culture and were tolerable following cyclophosphamide/fludarabine conditioning in normal laboratory dogs (59). The company LifEngine Laboratories is advancing genetic engineering principles in xenogeneic CAR-T cells targeting canine CD20 which may improve the production process and facilitate off-the-shelf cells for humans in the future (see Figure 3). The approach both inserts a CAR construct for anti-CD20 targeting and inactivates 𝛃2 microglobulin to downregulate MHCI. Genetic engineering avoids the introduction of viral vectors into the process which can be a failure point in production as well as immune responses in patients resulting in false positive HIV testing (55,60). The clinical studies to date have been minimal, but the potential for application in companion dog models to improve outcomes, particularly in solid tumors, for dogs and people is great.
Figure 3:
Generating precision enforced CAR-T cells with dual GeneWeld (dGW). CRISPR/Cas9 nuclease targets precision double-strand DNA breaks in the genome at TRAC and B2M simultaneously. Additionally, two double-stranded breaks in each plasmid donor DNA liberate cargo DNA containing the CAR or Transgene (X) to activate homology-directed integration at TRAC and B2M on human chromosomes 14 and 15, respectively. To achieve precision integration into the genome, Cas9 generates site-specific double stranded DNA breaks at which the ends are processed and resected, leading to homology-mediated DNA repair and the insertion of the CAR or (X) transgenes at the endogenous TRAC or B2M locus with base-pair resolution. Insertion of the CAR or transgene (X), either as secreted and/or cell-intrinsic factors, into precise genomic loci results in the ablation of TCR and MHC-I surface expression. (Figure courtesy of Wesley A. Wierson and Alex M. Abel, LifEngine Animal Health Laboratories Incorporated and created in part with BioRender.com.)
Radiation therapy strategies
Radiation therapy is well-established in both companion animals and humans as a means to achieve local tumor control. Increasingly, however, the systemic effects of local radiotherapy have demonstrated changes to tumor microenvironment and immune effectors at distant, unirradiated sites (61). A complex relationship exists between radiation and immune response, impacted by radiation dose, fraction size, location, and disease (62). Better elucidation of the relationship between radiation and the immune system could optimize radiation techniques to avoid immunosuppressive effects and maximize immunostimulatory effects following radiotherapy. One such investigation compared preclinical murine and Phase 1 clinical human data with dogs receiving Stereotactic Body Radiation Therapy (SBRT) for nasopharyngeal carcinoma. Investigators found that delivering elective radiation to the regional lymph nodes caused decreased expression of genes associated with antigen presentation (MHC I/II) as well as decreased activation of effector T cells (GZMB, IFNG, CXCL10), and increased expression of genes associated with immunosuppression (FOXP3, IL10RA, IL17RB) (63).
Spatially fractionated radiation therapy (SFRT), where radiation dose is delivered heterogeneously across a treatment volume, was initially developed as a method of sparing radiation side effects, but has more recently been investigated as a means of activating an anti-tumor immune response (see Figure 4) (64). Six dogs receiving GRID therapy for large cSTS demonstrated tolerability and a decrease in secretory sphingomyelinase activity and TNFa after treatment (65). Lattice radiation therapy places high dose regions in a 3-dimensional lattice within a low dose volume. Dogs with STS were randomized to receive either lattice SBRT or conventional palliative radiation therapy (pRT). Dogs receiving lattice SBRT had an upregulation in immunostimulatory pathways (4.4-fold change in IFNA7 and 2.3-fold change in complement-related genes) within tumor biopsy samples that were not present in the pRT arm (unpublished preliminary data using Nanostring Canine IO panel).
Figure 4:
Sagittal view of a spatially fractionated photon radiation therapy plan in a dog with a STS of the distal forelimb. In this case, 20 Gy was delivered to the GTV margin (red outline) while 66.7 Gy was delivered to latticed subvolumes (orange contours) within the GTV. The colorwash isodose lines are provided in the legend above. The plan was delivered in 5 fractions via simultaneous integrated boost.
Radiation has been delivered in conjunction with immunotherapy in attempts to improve treatment outcomes. Canine-specific OX40 agonists can upregulate anti-tumor responses, primarily by activating effector T lymphocytes (60). Combination of OX40 and TLR 3/9 agonists with SBRT resulted in depletion and decreased expression of T-regulatory cells and an increase in the circulating levels of IL-2, IL-7, IL-15, and IL-18 in dogs with canine melanoma, carcinoma, and sarcoma (66). Dogs with metastatic cOSA or canine melanoma received external beam radiotherapy to the primary tumor site with intratumoral IL-2 fusion cytokines, which was followed by targeted radionuclide therapy using a novel alkylphosphocholine (86Y/90Y-NM600) delivering 2 Gy to all metastatic lesions to break immune tolerance in the immunosuppressive microenvironment (67). Dogs with pulmonary metastatic melanoma receiving Anti-PD-L1 therapy (c4G12 antibody) had improved metastasis control and longer survival if they received prior hypofractionated radiotherapy than no or concurrent radiotherapy (68).
Immunomonitoring
To progress immunotherapy trials in companion dogs, predicting which dogs are likely to respond and monitoring those responses will require new tools and biomarkers of disease and response. Flow cytometry reagents and protocols are described to capture lymphocyte phenotypes and macrophage polarization in canine samples (69,70). Bulk RNA sequencing (RNAseq) along with cytokine measurement has been used to compare the human and canine T cell responses to activating stimuli (71). Many pathways of activation are shared between species, and many are not (71). Human T cells exhibit greater transcriptional change and higher IFNγ production than canine T cells, but canine macrophages are more reactive to lower concentrations of IFNγ (71). Nanostring analysis of RNA in tumors can be used for immune profiling requiring relatively small samples and providing reasonable resolution of the immune landscape in the tumor microenvironment (72). Bulk RNAseq has also been used to develop a representation of the T cell receptor (TCRseq) beta chain to assess overall clonality of TCR in a dog with cancer (73). This bulk sequencing approach is cost effective and can be used to monitor changes in clone-specific immune responses (73). Single-cell sequencing has been used to produce an atlas of leukocytes of dogs bearing cOSA compared to healthy controls (74). Subpopulations of polymorphonuclear and monocytic myeloid-derived suppressor cell populations were increased in tumor-bearing dogs compared to healthy controls (74). A protocol and reagents for single-cell TCRseq have been described analyzing samples from a group of dogs participating in an oral melanoma immunotherapy trial (13). This protocol permits the evaluation of the TCR α and β chains at the single-cell level, offering the deepest look yet at the array of clonality in dogs (see Figure 5).
Figure 5:
T cell clonality represented by single-cell TCRseq from samples collected from healthy dog lymph nodes, healthy dog peripheral blood mononuclear cells (PBMCs), PBMCs collected from melanoma-bearing dogs in an immunotherapy clinical trial, and cells collected from a lymph node of a dog effaced by aggressive peripheral T cell lymphoma. Gray dots represent clones of TCR α/β identified in only a single cell and red dots represent increasing numbers of cells sharing the same clone of TCR α/β. The increasing oligoclonality in the immunotherapy-treated cancer patients and the near complete clonality of the T cell lymphoma sample are clearly evident. (Figure courtesy of Joshua F. McMichael and Obi L. Griffith, Washington University in St. Louis.)
CONCLUSIONS
Clinical veterinary studies have generated data underpinning the application of immunotherapy to human cancer patients. The signal of efficacy of L-MTP-PE in cOSA in companion dogs led to the clinical application and eventual regulatory approval in much of the world for human OSA patients. Inexpensive to produce autologous, allogeneic, DNA, and peptide vaccines have had a positive impact on survival in dogs with various cancers; as the technologies in these areas improve, translation to the human clinic may become feasible. The increased prevalence of sarcomas, particularly cOSA and cSTS, in dogs makes possible more rapidly accruing studies in CAR-T therapy for solid tumors; rapid accrual can facilitate more rapid iteration of the technology to optimize outcome. The ability to trial novel radiotherapy concepts in companion dogs allows development of preliminary data in heterogeneous tumors with complex vasculature to inform human trial design. Finally, the quality, specificity, and resolution of immune reagents has progressed in dogs to a level that facilitates translational studies and allows highly detailed immune monitoring. Companion dogs offer an unrivaled opportunity to trial novel immunotherapy in complex, heterogeneous tumors in the context of a cancer-conditioned immune system with human scale and geometry equipment.
Funding acknowledgment:
Dr. Bryan receives funding from the National Cancer Institute (3R37CA266344–02S1, SJ Kenderian PI) in collaboration with LifEngine Animal Health Laboratories and Mayo Clinic for xenogeneic CAR-T research.
Footnotes
Conflicts of Interest:
Dr. Bryan serves on the Scientific Advisory Board of ELIAS Animal Health and receives an honorarium for serving but owns no part of the company and receives no royalties from the company.
Dr. Maitz serves as a veterinary consultant for Elekta, Inc. There was a no-cost collaboration of lattice radiotherapy technique with Elekta employees.
References
- 1.MacEwen EG, Kurzman ID, Rosenthal RC, Smith BW, Manley PA, Roush JK, et al. Therapy for osteosarcoma in dogs with intravenous injection of liposome-encapsulated muramyl tripeptide. J Natl Cancer Inst. 1989;81:935–8. [DOI] [PubMed] [Google Scholar]
- 2.Kurzman ID, MacEwen EG, Rosenthal RC, Fox LE, Keller ET, Helfand SC, et al. Adjuvant therapy for osteosarcoma in dogs: results of randomized clinical trials using combined liposome-encapsulated muramyl tripeptide and cisplatin. Clin Cancer Res. 1995;1:1595–601. [PubMed] [Google Scholar]
- 3.Grosenbaugh DA, Leard AT, Bergman PJ, Klein MK, Meleo K, Susaneck S, et al. Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. Am J Vet Res. 2011;72:1631–8. [DOI] [PubMed] [Google Scholar]
- 4.Doyle HA, Gee RJ, Masters TD, Gee CR, Booth CJ, Peterson-Roth E, et al. Vaccine-induced ErbB (EGFR/HER2)-specific immunity in spontaneous canine cancer. Transl Oncol. 2021;14:101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marconato L, Melacarne A, Aralla M, Sabattini S, Tiraboschi L, Ferrari V, et al. A Target Animal Effectiveness Study on Adjuvant Peptide-Based Vaccination in Dogs with Non-Metastatic Appendicular Osteosarcoma Undergoing Amputation and Chemotherapy. Cancers [Internet]. 2022;14. Available from: 10.3390/cancers14051347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarone L, Giacobino D, Camerino M, Maniscalco L, Iussich S, Parisi L, et al. A chimeric human/dog-DNA vaccine against CSPG4 induces immunity with therapeutic potential in comparative preclinical models of osteosarcoma. Mol Ther. 2023;31:2342–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olin MR, Ampudia-Mesias E, Pennell CA, Sarver A, Chen CC, Moertel CL, et al. Treatment Combining CD200 Immune Checkpoint Inhibitor and Tumor-Lysate Vaccination after Surgery for Pet Dogs with High-Grade Glioma. Cancers [Internet]. 2019;11. Available from: 10.3390/cancers11020137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maekawa N, Konnai S, Takagi S, Kagawa Y, Okagawa T, Nishimori A, et al. A canine chimeric monoclonal antibody targeting PD-L1 and its clinical efficacy in canine oral malignant melanoma or undifferentiated sarcoma. Sci Rep. 2017;7:8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Igase M, Inanaga S, Tani K, Nakaichi M, Sakai Y, Sakurai M, et al. Long-term survival of dogs with stage 4 oral malignant melanoma treated with anti-canine PD-1 therapeutic antibody: A follow-up case report. Vet Comp Oncol. 2022;20:901–5. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimoto S, Chester N, Xiong A, Radaelli E, Wang H, Brillantes M, et al. Development and pharmacokinetic assessment of a fully canine anti-PD-1 monoclonal antibody for comparative translational research in dogs with spontaneous tumors. MAbs. 2023;15:2287250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flesner BK, Wood GW, Gayheart-Walsten P, Sonderegger FL, Henry CJ, Tate DJ, et al. Autologous cancer cell vaccination, adoptive T-cell transfer, and interleukin-2 administration results in long-term survival for companion dogs with osteosarcoma. J Vet Intern Med. 2020;34:2056–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atherton MJ, Rotolo A, Haran KP, Mason NJ. Case Report: Clinical and Serological Hallmarks of Cytokine Release Syndrome in a Canine B Cell Lymphoma Patient Treated With Autologous CAR-T Cells. Front Vet Sci. 2022;9:824982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoang MH, Skidmore ZL, Rindt H, Chu S, Fisk B, Foltz JA, et al. Single-cell T-cell receptor repertoire profiling in dogs. Commun Biol. 2024;7:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razmara AM, Gingrich AA, Toedebusch CM, Rebhun RB, Murphy WJ, Kent MS, et al. Improved characterization and translation of NK cells for canine immunotherapy. Front Vet Sci. 2024;11:1336158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gingrich AA, Razmara AM, Gingrich PW, Rebhun RB, Murphy WJ, Kent MS, et al. Missing a “Missing Self” Mechanism: Modeling and Detection of Ly49 Expression in Canine NK Cells. Immunohorizons. 2023;7:760–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lampreht Tratar U, Milevoj N, Cemazar M, Znidar K, Ursic Valentinuzzi K, Brozic A, et al. Treatment of spontaneous canine mast cell tumors by electrochemotherapy combined with IL-12 gene electrotransfer: Comparison of intratumoral and peritumoral application of IL-12. Int Immunopharmacol. 2023;120:110274. [DOI] [PubMed] [Google Scholar]
- 17.Vail DM, MacEwen EG, Kurzman ID, Dubielzig RR, Helfand SC, Kisseberth WC, et al. Liposome-encapsulated muramyl tripeptide phosphatidylethanolamine adjuvant immunotherapy for splenic hemangiosarcoma in the dog: a randomized multi-institutional clinical trial. Clin Cancer Res. 1995;1:1165–70. [PubMed] [Google Scholar]
- 18.MacEwen EG, Kurzman ID, Vail DM, Dubielzig RR, Everlith K, Madewell BR, et al. Adjuvant therapy for melanoma in dogs: results of randomized clinical trials using surgery, liposome-encapsulated muramyl tripeptide, and granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 1999;5:4249–58. [PubMed] [Google Scholar]
- 19.Teske E, Rutteman GR, vd Ingh TS, van Noort R, Misdorp W. Liposome-encapsulated muramyl tripeptide phosphatidylethanolamine (L-MTP-PE): a randomized clinical trial in dogs with mammary carcinoma. Anticancer Res. 1998;18:1015–9. [PubMed] [Google Scholar]
- 20.Kleinerman ES, Meyers PA, Raymond AK, Gano JB, Jia SF, Jaffe N. Combination therapy with ifosfamide and liposome-encapsulated muramyl tripeptide: tolerability, toxicity, and immune stimulation. J Immunother Emphasis Tumor Immunol. 1995;17:181–93. [DOI] [PubMed] [Google Scholar]
- 21.Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children’s Oncology Group. J Clin Oncol. 2008;26:633–8. [DOI] [PubMed] [Google Scholar]
- 22.Hudson MM, Snyder JS, Jaffe N, Kleinerman ES. In vitro and in vivo effect of adriamycin therapy on monocyte activation by liposome-encapsulated immunomodulators. Cancer Res. 1988;48:5256–63. [PubMed] [Google Scholar]
- 23.Asano T, Fujimaki W, McWatters A, An T, Matsushima K, Kleinerman ES. Effect of Adriamycin on liposomal muramyl tripeptide’s ability to up-regulate monocyte cytokine expression. Cancer Immunol Immunother. 1993;37:408–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleinerman ES, Snyder JS, Jaffe N. Influence of chemotherapy administration on monocyte activation by liposomal muramyl tripeptide phosphatidylethanolamine in children with osteosarcoma. J Clin Oncol. 1991;9:259–67. [DOI] [PubMed] [Google Scholar]
- 25.Andersen BM, Pluhar GE, Seiler CE, Goulart MR, SantaCruz KS, Schutten MM, et al. Vaccination for invasive canine meningioma induces in situ production of antibodies capable of antibody-dependent cell-mediated cytotoxicity. Cancer Res. 2013;73:2987–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marconato L, Frayssinet P, Rouquet N, Comazzi S, Leone VF, Laganga P, et al. Randomized, placebo-controlled, double-blinded chemoimmunotherapy clinical trial in a pet dog model of diffuse large B-cell lymphoma. Clin Cancer Res. 2014;20:668–77. [DOI] [PubMed] [Google Scholar]
- 27.Marconato L, Stefanello D, Sabattini S, Comazzi S, Riondato F, Laganga P, et al. Enhanced therapeutic effect of APAVAC immunotherapy in combination with dose-intense chemotherapy in dogs with advanced indolent B-cell lymphoma. Vaccine. 2015;33:5080–6. [DOI] [PubMed] [Google Scholar]
- 28.Goodrich RP, Weston J, Hartson L, Griffin L, Guth A. Pilot Acute Safety Evaluation of Innocell™ Cancer Immunotherapy in Canine Subjects. J Immunol Res. 2020;2020:7142375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucroy MD, Clauson RM, Suckow MA, El-Tayyeb F, Kalinauskas A. Evaluation of an autologous cancer vaccine for the treatment of metastatic canine hemangiosarcoma: a preliminary study. BMC Vet Res. 2020;16:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexander AN, Huelsmeyer MK, Mitzey A, Dubielzig RR, Kurzman ID, Macewen EG, et al. Development of an allogeneic whole-cell tumor vaccine expressing xenogeneic gp100 and its implementation in a phase II clinical trial in canine patients with malignant melanoma. Cancer Immunol Immunother. 2006;55:433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen SM, Thamm DH, Vail DM, London CA. Response evaluation criteria for solid tumours in dogs (v1.0): a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol. 2015;13:176–83. [DOI] [PubMed] [Google Scholar]
- 32.U’Ren LW, Biller BJ, Elmslie RE, Thamm DH, Dow SW. Evaluation of a novel tumor vaccine in dogs with hemangiosarcoma. J Vet Intern Med. 2007;21:113–20. [DOI] [PubMed] [Google Scholar]
- 33.Musser ML, Berger EP, Tripp CD, Clifford CA, Bergman PJ, Johannes CM. Safety evaluation of the canine osteosarcoma vaccine, live Listeria vector. Vet Comp Oncol. 2021;19:92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cascio MJ, Whitley EM, Sahay B, Cortes-Hinojosa G, Chang L-J, Cowart J, et al. Canine osteosarcoma checkpoint expression correlates with metastasis and T-cell infiltrate. Vet Immunol Immunopathol. 2021;232:110169. [DOI] [PubMed] [Google Scholar]
- 35.Maekawa N, Konnai S, Ikebuchi R, Okagawa T, Adachi M, Takagi S, et al. Expression of PD-L1 on canine tumor cells and enhancement of IFN-γ production from tumor-infiltrating cells by PD-L1 blockade. PLoS One. 2014;9:e98415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shosu K, Sakurai M, Inoue K, Nakagawa T, Sakai H, Morimoto M, et al. Programmed Cell Death Ligand 1 Expression in Canine Cancer. In Vivo. 2016;30:195–204. [PubMed] [Google Scholar]
- 37.Maekawa N, Konnai S, Okagawa T, Nishimori A, Ikebuchi R, Izumi Y, et al. Immunohistochemical Analysis of PD-L1 Expression in Canine Malignant Cancers and PD-1 Expression on Lymphocytes in Canine Oral Melanoma. PLoS One. 2016;11:e0157176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coy J, Caldwell A, Chow L, Guth A, Dow S. PD-1 expression by canine T cells and functional effects of PD-1 blockade. Vet Comp Oncol. 2017;15:1487–502. [DOI] [PubMed] [Google Scholar]
- 39.Filley A, Henriquez M, Bhowmik T, Tewari BN, Rao X, Wan J, et al. Immunologic and gene expression profiles of spontaneous canine oligodendrogliomas. J Neurooncol. 2018;137:469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartley G, Elmslie R, Dow S, Guth A. Checkpoint molecule expression by B and T cell lymphomas in dogs. Vet Comp Oncol. 2018;16:352–60. [DOI] [PubMed] [Google Scholar]
- 41.Ganbaatar O, Konnai S, Okagawa T, Nojima Y, Maekawa N, Minato E, et al. PD-L1 expression in equine malignant melanoma and functional effects of PD-L1 blockade. PLoS One. 2020;15:e0234218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maekawa N, Konnai S, Nishimura M, Kagawa Y, Takagi S, Hosoya K, et al. PD-L1 immunohistochemistry for canine cancers and clinical benefit of anti-PD-L1 antibody in dogs with pulmonary metastatic oral malignant melanoma. NPJ Precis Oncol. 2021;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinard CJ, Hocker SE, Poon AC, Inkol JM, Matsuyama A, Wood RD, et al. Evaluation of PD-1 and PD-L1 expression in canine urothelial carcinoma cell lines. Vet Immunol Immunopathol. 2022;243:110367. [DOI] [PubMed] [Google Scholar]
- 44.Igase M, Nemoto Y, Itamoto K, Tani K, Nakaichi M, Sakurai M, et al. A pilot clinical study of the therapeutic antibody against canine PD-1 for advanced spontaneous cancers in dogs. Sci Rep. 2020;10:18311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Igase M, Inanaga S, Nishibori S, Itamoto K, Sunahara H, Nemoto Y, et al. Proof-of-concept study of the caninized anti-canine programmed death 1 antibody in dogs with advanced non-oral malignant melanoma solid tumors. J Vet Sci. 2024;25:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rebhun RB, York D, Cruz SM, Judge SJ, Razmara AM, Farley LE, et al. Inhaled recombinant human IL-15 in dogs with naturally occurring pulmonary metastases from osteosarcoma or melanoma: a phase 1 study of clinical activity and correlates of response. J Immunother Cancer [Internet]. 2022;10. Available from: 10.1136/jitc-2022-004493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regan DP, Chow L, Das S, Haines L, Palmer E, Kurihara JN, et al. Losartan Blocks Osteosarcoma-Elicited Monocyte Recruitment, and Combined With the Kinase Inhibitor Toceranib, Exerts Significant Clinical Benefit in Canine Metastatic Osteosarcoma. Clin Cancer Res. 2022;28:662–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LeBlanc AK, Mazcko CN, Cherukuri A, Berger EP, Kisseberth WC, Brown ME, et al. Adjuvant Sirolimus Does Not Improve Outcome in Pet Dogs Receiving Standard-of-Care Therapy for Appendicular Osteosarcoma: A Prospective, Randomized Trial of 324 Dogs. Clin Cancer Res. 2021;27:3005–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agarwal P, Gammon EA, Sandey M, Lindley SS, Koehler JW, Matz BM, et al. Evaluation of tumor immunity after administration of conditionally replicative adenoviral vector in canine osteosarcoma patients. Heliyon. 2021;7:e06210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Visonneau S, Cesano A, Jeglum KA, Santoli D. Adjuvant treatment of canine osteosarcoma with the human cytotoxic T-cell line TALL-104. Clin Cancer Res. 1999;5:1868–75. [PubMed] [Google Scholar]
- 51.Cesano A, Visonneau S, Jeglum KA, Owen J, Wilkinson K, Carner K, et al. Phase I clinical trial with a human major histocompatibility complex nonrestricted cytotoxic T-cell line (TALL-104) in dogs with advanced tumors. Cancer Res. 1996;56:3021–9. [PubMed] [Google Scholar]
- 52.O’Connor CM, Sheppard S, Hartline CA, Huls H, Johnson M, Palla SL, et al. Adoptive T-cell therapy improves treatment of canine non-Hodgkin lymphoma post chemotherapy. Sci Rep. 2012;2:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Canter RJ, Grossenbacher SK, Foltz JA, Sturgill IR, Park JS, Luna JI, et al. Radiotherapy enhances natural killer cell cytotoxicity and localization in pre-clinical canine sarcomas and first-in-dog clinical trial. J Immunother Cancer. 2017;5:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rotolo A, Whelan EC, Atherton MJ, Kulikovskaya I, Jarocha D, Fraietta JA, et al. Unedited allogeneic iNKT cells show extended persistence in MHC-mismatched canine recipients. Cell Rep Med. 2023;4:101241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michels KR, Sheih A, Hernandez SA, Brandes AH, Parrilla D, Irwin B, et al. Preclinical proof of concept for VivoVec, a lentiviral-based platform for in vivo CAR T-cell engineering. J Immunother Cancer [Internet]. 2023;11. Available from: 10.1136/jitc-2022-006292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panjwani MK, Atherton MJ, MaloneyHuss MA, Haran KP, Xiong A, Gupta M, et al. Establishing a model system for evaluating CAR T cell therapy using dogs with spontaneous diffuse large B cell lymphoma. Oncoimmunology. 2020;9:1676615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rotolo A, Atherton MJ, Kasper BT, Haran KP, Mason NJ. Genetic re-direction of canine primary T cells for clinical trial use in pet dogs with spontaneous cancer. STAR Protoc. 2021;2:100905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakai O, Yamamoto H, Igase M, Mizuno T. Optimization of Culture Conditions for the Generation of Canine CD20-CAR-T Cells for Adoptive Immunotherapy. In Vivo. 2022;36:764–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang S, Black RG, Kohli K, Hayes BJ, Miller C, Koehne A, et al. B7-H3 Specific CAR T Cells for the Naturally Occurring, Spontaneous Canine Sarcoma Model. Mol Cancer Ther. 2022;21:999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hauser JR, Hong H, Babady NE, Papanicolaou GA, Tang Y-W. False-Positive Results for Human Immunodeficiency Virus Type 1 Nucleic Acid Amplification Testing in Chimeric Antigen Receptor T Cell Therapy. J Clin Microbiol [Internet]. 2019;58. Available from: 10.1128/JCM.01420-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10:718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Demaria S, Guha C, Schoenfeld J, Morris Z, Monjazeb A, Sikora A, et al. Radiation dose and fraction in immunotherapy: one-size regimen does not fit all settings, so how does one choose? J Immunother Cancer [Internet]. 2021;9. Available from: 10.1136/jitc-2020-002038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Darragh LB, Gadwa J, Pham TT, Van Court B, Neupert B, Olimpo NA, et al. Elective nodal irradiation mitigates local and systemic immunity generated by combination radiation and immunotherapy in head and neck tumors. Nat Commun. 2022;13:7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnsrud AJ, Jenkins SV, Jamshidi-Parsian A, Quick CM, Galhardo EP, Dings RPM, et al. Evidence for Early Stage Anti-Tumor Immunity Elicited by Spatially Fractionated Radiotherapy-Immunotherapy Combinations. Radiat Res. 2020;194:688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nolan MW, Gieger TL, Karakashian AA, Nikolova-Karakashian MN, Posner LP, Roback DM, et al. Outcomes of Spatially Fractionated Radiotherapy (GRID) for Bulky Soft Tissue Sarcomas in a Large Animal Model. Technol Cancer Res Treat. 2017;16:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boss M-K, Watts R, Harrison LG, Hopkins S, Chow L, Trageser E, et al. Immunologic Effects of Stereotactic Body Radiotherapy in Dogs with Spontaneous Tumors and the Impact of Intratumoral OX40/TLR Agonist Immunotherapy. Int J Mol Sci [Internet]. Multidisciplinary Digital Publishing Institute (MDPI); 2022. [cited 2024 Feb 27];23. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8775899/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Magee K, Marsh IR, Turek MM, Grudzinski J, Aluicio-Sarduy E, Engle JW, et al. Safety and feasibility of an in situ vaccination and immunomodulatory targeted radionuclide combination immuno-radiotherapy approach in a comparative (companion dog) setting. PLoS One. 2021;16:e0255798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deguchi T, Maekawa N, Konnai S, Owaki R, Hosoya K, Morishita K, et al. Enhanced Systemic Antitumour Immunity by Hypofractionated Radiotherapy and Anti-PD-L1 Therapy in Dogs with Pulmonary Metastatic Oral Malignant Melanoma. Cancers [Internet]. 2023;15. Available from: 10.3390/cancers15113013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lyu Q, Veldhuizen EJA, Ludwig IS, Rutten VPMG, van Eden W, Sijts AJAM, et al. Characterization of polarization states of canine monocyte derived macrophages. PLoS One. 2023;18:e0292757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilkerson MJ, Dolce K, Koopman T, Shuman W, Chun R, Garrett L, et al. Lineage differentiation of canine lymphoma/leukemias and aberrant expression of CD molecules. Vet Immunol Immunopathol. 2005;106:179–96. [DOI] [PubMed] [Google Scholar]
- 71.Chow L, Wheat W, Ramirez D, Impastato R, Dow S. Direct comparison of canine and human immune responses using transcriptomic and functional analyses. Sci Rep. 2024;14:2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vanhaezebrouck IF, Bakhle KM, Mendez-Valenzuela CR, Lyle LT, Konradt K, Scarpelli ML. Single institution study of the immune landscape for canine oral melanoma based on transcriptome analysis of the primary tumor. Front Vet Sci. 2023;10:1285909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zuleger CL, Schwartz RW, Ong IM, Newton MA, Vail DM, Albertini MR. Development of a next-generation sequencing protocol for the canine T cell receptor beta chain repertoire. Vet Immunol Immunopathol. 2024;268:110702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ammons DT, Harris RA, Hopkins LS, Kurihara J, Weishaar K, Dow S. A single-cell RNA sequencing atlas of circulating leukocytes from healthy and osteosarcoma affected dogs. Front Immunol. 2023;14:1162700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spodnick GJ, Berg J, Rand WM, Schelling SH, Couto G, Harvey HJ, et al. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978–1988). J Am Vet Med Assoc. 1992;200:995–9. [PubMed] [Google Scholar]