Abstract
It has now been about a century since a flurry of discoveries identified first the pituitary, then more specifically the anterior pituitary and soon thereafter the central nervous system as components regulating gonadal and downstream reproductive functions. This was an era of ablation/replacement designs using at first rudimentary and then increasingly pure preparations of gonadal and pituitary ‘activities’ or transplanting actual glands, whole or homogenized, among subjects. There was, of course, controversy as is typical of lively and productive scientific debates to this day. The goals of this commentary are to briefly review the history of this work and how the terms referring to interactions among the components of the hypothalamo (as the central neural component was soon associated with)-pituitary-gonadal (HPG) axis evolved, and then to question if the current terms used have kept up with our understanding of the system. The focus in this review will be the actions of estradiol primarily upon the hypothalamus. Important actions of progesterone on the hypothalamus as well as both steroids on the pituitary response to hypothalamic factors are both acknowledged and largely ignored in this document, as are any sex differences as we focus on females.
Early history of the HPG axis
Pflüger wrote in 1877 that “Accommodations of living beings are always teleological, are subject to the law of causality.”1 Defining the postulated interactions among HPG components in a teleologic manner, that is the purpose they serve vs the cause by which they arise2, has remained a consistent theme. Among the first hints of a humoral relationship between the pituitary and the gonads were observations that the former were enlarged upon removal of the latter3,4. This was due to an increase in size and vacuolization of some basophilic cells, now known to be the source of gonadotropins, after castration5. Feeding or intraperitoneal injections of pituitary extracts to rats produced mixed results, with both activation and inhibition of the reproductive system reported in each sex6–8. The advent of transsphenoidal pituitary ablation in rodents in 1926 by Smith9 in combination with pituitary replacement built consensus around an activating effect of the anterior pituitary on the gonads. Ablation of the whole gland, but not just the posterior pituitary, reduced the size of reproductive organs, which could be partially restored by daily intramuscular injections of fresh anterior pituitary. These studies confirmed reports of a lack of effect of posterior pituitary hormones on the gonads in mammals. Together with earlier ablation studies of the various subdivisions of the pituitary of Rana pipiens10, this work supported the postulate that each part of the pituitary served different physiological functions and that the anterior pituitary was the major influencer of reproductive function in mammals.
In studies of the reproductive tracts, pituitary ablation/replacement along with castration soon demonstrated the requirement of intact gonads for pituitary replacement to have trophic effects upon the reproductive tract in both sexes11. Treating immature female rodents with pituitaries from castrated animals was more effective in advancing vaginal opening and increasing ovarian mass than treating with pituitaries from gonad-intact animals, suggesting functional changes in the pituitary upon castration12. Activation of immature ovaries upon their transplantation into adults further solidified the relationship between the anterior pituitary and gonads, and led to early speculation that the release of the gonad-activating substance of the pituitary was periodic and perhaps linked to vaginal cycle stages11. As these discoveries progressed, the nature of the relationship appeared to clarify. Work in freemartin cattle by Lillie13 and rodents by Steinach14 led to the hypothesis that the gonadal hormones of the sexes antagonized one another, leading to damage to the gonadal and reproductive systems of the opposite sex. The gonadal antagonism hypothesis had sustained popularity through this period.
A new concept of hormone interactions
Other groups were working to identify the active substances, or hormones as Starling first referred to them15, that produced these responses. Studies of urine from women in different reproductive states16 suggested the pituitary likely contained at least two substances with different gonadal functions, soon identified as a luteinizing hormone (LH) and a follicle-stimulating hormone (FSH)17. The first crystallization of the follicle-derived hormone theelin, or estrone was published in 193018, followed by estriol19 and eventually estradiol20. These biochemical advances helped standardize extract preparation and enabled increased rigor and reproducibility for the physiologic studies.
Pivotal in the evolution of thought on the interrelationships between the pituitary and gonad was the elegant reasoning21 and experimental work22 of Moore and Price, who proposed “A new conception of hormone interactions” in their 1932 publication22. These are so simply stated that an attempt to paraphrase would not do them justice, and they are quoted below in full:
“1. Gonad hormones stimulate homologous reproductive accessories, but are without effect upon heterologous accessories.
2. Secretions produced by the hypophysis stimulate the gonads to function both in germ cell production and in hormone secretion.
3. Gonad hormones have no direct effect on the gonads of either the same, or the opposite, sex.
4. Gonad hormones, of either sex, exert a depressing effect upon the hypophysis which results in a diminished amount of the sex-stimulating factor available to the organism.”
While point 3 is now known to be incorrect for both sexes, these statements explained a great deal of existing data regarding the interactions between the anterior pituitary and gonad, and quickly supplanted the gonadal antagonism hypothesis.
Two additional aspects of this interaction were co-emerging. First, Hohlweg and Junkmann added the central nervous system to the Moore and Price model, proposing that gonadal hormones acted centrally to control pituitary output23. Although they incorrectly bypassed the pituitary as a recipient of gonadal hormone action, this added the central neural component of the hypothalamo-pituitary-gonadal (HPG) axis. Central involvement in reproduction had been hinted at in prior studies that intentionally damaged the tuber cinereum of the hypothalamus, this left the pituitary intact but nonetheless reduced reproductive organ function9,11. Second, Hohlweg made what he termed a surprising discovery when larger doses of Progynon, an early estrogen preparation, was observed to induce both pituitary enlargement (with a different underlying histological changes than caused by castration) and luteinization of the follicles24. He speculated the formation of corpora lutea was caused by a central-neural-mediated increase in release of a separate luteinizing hormone from the pituitary. This was contrary to the inhibitory actions on the gonads observed in most studies using lower doses of steroid. Earlier work had suggested a similar dose dependence for what we now know as estrogenic responses with higher concentrations needed to induce estrous behavior than to induce vaginal cornification25. Fevold et al., showed evidence for an initial stimulation and subsequent suppression of ovarian mass in response to high doses of oestrin26; the timing of the observations and use of ovarian mass and structures as the bioassay caused the initial suppression upon estrogen treatment to be missed. The concept of biphasic estrogen action on the gonads and a requirement for the pituitary in these actions was thus emerging and increasingly associated with cyclic changes, including in humans27–32.
Estradiol and feedback
It is likely that any discussion of the HPG axis amongst scientists will before long mention the phenomena of negative and positive feedback actions of estradiol. Interestingly, none of the early work cited above use the term feedback. Instead, phrases such as: the reaction of the various glands, the reciprocal interaction between the pituitary and gonad, reciprocity, complex interrelationships, interplay, influence, suppression, depression, activation, stimulation are used. Feedback is not only missing with regard to steroids, but entirely from searchable PDFs of the cited work. While not an all-encompassing review, the above is a reasonable sample of studies defining this axis. The lack of use of feedback extends to some classics of the field including Harris33, Everett and Sawyer34, and Greep and Jones’ report at the Laurentian Conference in 195035. Even Dorothy Price’s further work did not use the term feedback36. But on page 6 of his iconic 1955 book Neural Control of the Pituitary Gland, Geoffrey Harris used feedback37, a noted change from his 1948 review with the same title33.
What brought about this change? A likely suspect is the 1952 Ramon Guiteras lecture by Charles Huggins at the annual meeting of the American Urological Association in Atlantic City37. Huggins, who shared the Nobel Prize in Physiology and Medicine in 1966 with Peyton Rous for his work on cancer cell biology, suggested in his address that “Feedback circuits are now extensively utilized by engineers and the feedback concept is useful in visualizing endocrine physiologic control devices of the body.” Huggins melded physiology with emerging concepts from engineering, such as Black’s38 invention of the negative feedback amplifier to produce more stable signals, and Norbert Wiener’s theory of Cybernetics39, “a new field that combined the study of what in a human context is sometimes loosely described as thinking and in engineering is known as control and communication.”40 Huggins specifically refers to using the words of “communications experts” when describing feedback. Without examining every publication prior to the Huggins article, it is impossible to know if this was indeed the first use of the term feedback for endocrine systems. The scholarly combination of concepts across disciplines is notable and intentional, and the word feedback increasingly appears in endocrine publications after this date41–46.
Feedback and the female reproductive cycle-tonic and surge LH release
Coevolving with the adoption of the term feedback was information on the pattern of LH during the female cycle. Early bioassays47 revealed LH concentrations were fairly steady in urine and blood samples obtained as often as daily, except for a sharp peak at mid cycle; this peak was preceded by increased estrogens and followed by ovulation and increased progesterone32,48–53. Involvement of a neural component in this process was supported by the discovery of a 24h rhythm in the LH peak in rodents34,54. Barraclough and Gorski55 used “tonic” to describe the steady, non-peak LH release that caused estrogen synthesis but not ovulation and “surge” to refer to the ovulation-inducing peak. These authors presciently hypothesized that these release modes were controlled by the medial basal and more rostral hypothalamus, respectively, a concept supported by the work of Hálasz56
In this tonic-surge understanding of the cycle, the terms negative and positive feedback work fairly well. A feedback loop is formed in a control system when an output is fed into its input, creating a closed loop. In negative feedback, the output has an inhibitory effect on the system, promoting stability of the output within a variable range; this is common in physiology due to the need for stability of the internal environment. While negative feedback is often equated with homeostasis, the term “homeostatic” applied to estradiol negative feedback has likely been misused, including by the authors57. Homeostasis strictly requires a regulated variable that is sensed (e.g., blood pressure by baroreceptors) as well as non-regulated variables that are controlled by effectors (e.g., heart rate), but are themselves not directly sensed58. An essential element of homeostasis is minimizing error between the set point of a sensed variable and its current value. In the HPG axis, different concentrations of hormones are naturally produced and have varied effects (e.g., tonic vs surge LH pattern), but specific set points have not been described nor have formal sensors that measure hormones vs a desired set point; rather there are receptive cells that change function in response to hormones58. In a tonic-surge cycle, negative feedback maintains gonadotropins to promote folliculogenesis and steroidogenesis. In positive feedback, the output has a stimulatory effect on the system, leading to increases in output towards the limit of the system or until something disrupts the positive feedback loop. Physiologic positive feedback systems are less common but are found in contexts that demand deviation from a steady state. In the tonic-surge cycle, positive feedback arises following exposure to sustained high concentrations of estradiol from the dominant follicle (s), resulting in both an increase in output and a marked shift in pattern of gonadotropin release to a surge mode59–67.
Discoveries in the HPG axis complicate things
As understanding of the hormonal changes of the HPG axis became more detailed, inconsistencies appeared in this straightforward view of estradiol negative and positive feedback. A major shift in understanding was the discovery that tonic LH concentrations were not tonic at all, rather circulating LH concentrations varied in an episodic, or pulsatile, fashion68. The hypothalamic factor controlling pituitary gonadotropins, gonadotropin-releasing hormone (GnRH), was sequenced69 and LH pulses were shown to be driven by GnRH pulses in pituitary portal blood70,71. The pituitary was found to need episodic GnRH input to maintain ‘tonic’ gonadotropin concentrations72, and circulating gonadotropin concentrations were GnRH-pulse frequency dependent, with higher frequencies favoring LH and lower frequencies favoring FSH73. Natural variations in LH pulse frequency, the best available assay for GnRH release in most mammals74, were observed during the primate menstrual cycle75–80. Specifically, lower LH-pulse frequencies were observed during the early follicular phase when FSH is relatively higher, and higher LH-pulse frequencies during the later follicular phase when the FSH:LH ratio had decreased. Together with the FSH-specific suppression by inhibin81, changes in GnRH-pulse frequency helped explain how one hypothalamic releasing factor could induce the differential release of LH and FSH needed for proper folliculogenesis. Beyond pulses, estrogens were found to have a similar biphasic effect upon GnRH release mode as they had on LH82,83. The estrogen receptor needed for these responses, ERα84, was not, however, typically detected in GnRH neurons85,86 indicating a need for estrogen-sensitive GnRH-neuron afferents87. The rostral and medial basal hypothalamic regions postulated by Barraclough and Gorski in the early 1960s55 do express ERα86,88,89, and in 2003, kisspeptin-producing, ERα-expressing afferents of GnRH neurons were identified in these two regions in rodents, specifically within the anteroventral periventricular (AVPV) area rostrally and arcuate within the medial basal hypothalamus90–93. From this rash of discoveries, the complications of the episodic mode of release and multiple sites for estradiol action to control GnRH release are considered below.
Pulses
The discovery of pulses complicated the simple idea that estradiol negative feedback controls LH through the follicular phase. Variables including frequency, amplitude, nadir, total and mean LH concentrations have been reported. The term negative feedback came from the clear reduction in mean/total LH concentrations in response to estrogens94,95. But in experiments conducted during the follicular phase or with physiologic estradiol concentrations and with a sufficiently high blood sampling frequency, divergent effects were observed on LH-pulse amplitude vs frequency. As mentioned above, LH-pulse frequency increases during the primate follicular phase, in particular when early vs late follicular phases are compared as the peak frequency appears to be achieved within the first week and frequency then plateaus. In sheep, estradiol reduced LH-pulse amplitude96, but increased LH-pulse frequency after removal of progesterone97 or when compared to ovariectomized animals98, indicating a clear stimulation of frequency. Direct measurements of GnRH release in sheep tell a similar story. Increased GnRH-pulse frequency was observed concomitant with increased estradiol concentrations from the early to late follicular phase64. Evans, Karsch and co-investigators undertook a series of studies to quantify the effects of estradiol upon GnRH release in the sheep model. Comparing no, basal and peak estradiol during an artificial follicular phase, total GnRH in portal blood was markedly decreased by peak estradiol compared to no estradiol; this was due to reduced GnRH-pulse amplitude but was accompanied by increased GnRH-pulse frequency99. Frequent sampling (1-min intervals) of pituitary portal blood during both artificial and natural follicular phases revealed a progressive change from a strictly episodic GnRH release pattern, to pulses imposed on a rising baseline, to continuously elevated GnRH concentrations in which clear pulses were not evident100; this latter observation confirmed studies in which samples taken as frequently as every 30 seconds during the estradiol-induced surge failed to reveal a pulsatile pattern101. Finally, a careful comparison of individual GnRH-pulse characteristics among ewes with no, basal or peak estradiol treatment confirmed estradiol suppressed total GnRH and GnRH-pulse amplitude and increased GnRH-pulse frequency, and further revealed a progressive decrease in pulse duration and increase in basal GnRH concentrations between pulses with increasing estradiol concentrations102.
GnRH is the hormonal output from the brain to the pituitary and in most cases LH pulses are a good surrogate74. There are also biophysical measurements that have good correspondence with LH pulses and are thought to reflect the operation of the neural component of a GnRH-pulse generator. These have primarily been made in the medial basal hypothalamus and provide an opportunity for long-term monitoring. Volleys of multiunit neural activity (MUA) in the infundibular region of monkeys were first associated with LH pulses in 1984103. LH-associated MUA volleys increase in frequency during the early follicular phase in both monkeys and goats104,105. The increase in goats and most of the increase in monkeys precedes increases in circulating estradiol suggesting removal of progesterone inhibition is the primary cause. Of interest, however, subsequent increases in estradiol do not suppress the frequency of MUA volleys or LH pulses. Also of note, clear resolution of LH pulses can become more difficult when underlying GnRH pulse frequency increases, leading to a potential underestimation of LH pulse frequency74. In mice, peaks in intracellular calcium produced by GCaMP6 targeted to arcuate (medial basal hypothalamic) kisspeptin neurons were monitored by fiber photometry over the estrous cycle106. The nadir in frequency of these events is on estrus following the LH surge. Event frequency is greater on all other cycle days with no statistical differences detected between them. There is, however, a trend for increased frequency from metestrus to diestrus to proestrus. Further, examining the raw data in the example shown for proestrus reveals more “baseline activity” reminiscent of that observed for GnRH release in sheep receiving peak estradiol treatment. An interesting question to contemplate regarding these calcium data is if the biology of the HPG axis is more sensitive than statistical tests as a pulse frequency detector.
A persistent question is whether the episodic mode of release is interrupted/replaced by the surge mode or if it persists throughout. As mentioned, clear pulses have not been detected during the GnRH surge64,100. The GnRH surge lasts hours longer than the LH surge and well beyond the peak in estradiol that induces it64, because the LH surge reduces further estradiol synthesis by the follicle107. In fact, removal of estradiol near the start of the surge does not block it, although the duration is somewhat reduced (to about 20 h from 30 in persistent E-treated ewes)108. Similarly, blocking neural activity with general anesthesia is only effective in blocking the LH surge that day if given at the right time before surge onset, after which the surge proceeds and later treatments are ineffective54. In this ‘all or none’ nature, the GnRH surge in some respects resembles an action potential: it is induced after a threshold concentration of estradiol is reached and then runs its course well beyond the loss of the induction signal. Unfortunately, the long duration of the GnRH surge and the experimental focus on its onset and generation has resulted is a near absence of data as to what happens to the pattern of release after the surge. There is a single observation in a synchronized ovine follicular phase in which sampling continued through the surge (ewe 1S in64); GnRH concentrations in portal blood appear to be at the level of detection for 3–4 hours before small pulses are again observed. In monkeys, hypothalamic MUA is not evident during the estradiol-induced or preovulatory LH surge, suggesting either a cessation of episodic activity or, alternatively, reduced coordination among cells that would preclude detection of bouts of multiunit activity104,109. MUA frequency declines but does not disappear during the preovulatory LH surge in goats105. In apparent contrast, the frequency of intracellular calcium events in murine arcuate kisspeptin neurons is maintained for hours into the proestrous LH surge106. Individual bouts of GnRH release into the median eminence were resolved using electrochemical methods in mouse brain slices during the early estradiol-induced LH surge110; these bouts were higher frequency than during the presurge period but whether this shift reflects the in vivo situation remains to be tested.
So, is estradiol feedback on pulsatile release negative (mean, amplitude) or positive (frequency, increased baseline)? To answer this question, we need to know what effect the LH pattern has at the ovary. Unfortunately, the effects of LH-pulse parameters on follicular maturation have not been determined. Interpretation of work that has been done is tricky because of the two-cell, two-gonadotropin mode of ovarian steroidogenesis111, the presence/absence of FSH, which induces expression of aromatase in granulosa cells112, and the use of estradiol, which is dependent upon all of these variables, as an output measure. Nonetheless, in in vitro studies, pulsatile LH administration was moderately more effective at inducing estradiol synthesis and reducing follicle atresia than continuous administration of the same mass of LH113–115. Reducing LH pulse amplitude with a GnRH receptor antagonist increased the rate of follicular atresia, but this treatment also reduced total LH thus a specific effect of LH-pulse amplitude is difficult to identify116. LH increases steroidogenesis through induction of both steroidogenic acute regulatory protein and cholesterol side-chain cleavage enzyme (CYP11A), which carry out the rate-limiting transport and enzymatic steps of steroidogenesis, respectively117. The effect of LH-pulse parameters on expression of these genes does not appear to have been studied. Two indirect examples provide support for a role of LH-pulse frequency. First, when women with hypothalamic amenorrhea were treated with the same mass of GnRH per day; more robust LH surges were induced in women receiving GnRH at one pulse per hour than one pulse per two hours even though the amplitude in the former was half and GnRH pulse characteristics were likely reflected in LH pulse patterns produced118. Second, an indirect relationship between LH-pulse frequency and steroidogenesis can be inferred from studies in which a stress-mimicking concentrations of cortisol reduced LH-pulse frequency and delayed the estradiol rise in follicular phase ewes119. This suggests lower LH-pulse frequency may be less effective at inducing steroidogenesis, however an inhibitory effect of glucocorticoids on estradiol production by granulosa cells, independent of LH, has also been reported120.
If one considers GnRH pulse frequency the primary signal from the hypothalamus, an argument can be made that the substantive effects of estradiol on the hypothalamus to control pulsatile GnRH and LH release are positive or neutral, not negative. LH effects on steroidogenesis are primarily mediated by PKA-dependent pathways, which are induced by relatively low LH concentrations121 thus higher-frequency low-amplitude LH pulses may effectively produce a sustained increase in estradiol production. In this model, rather than estradiol action switching from negative to positive feedback at surge onset, a second mode of estradiol action is initiated. This mode requires higher concentrations of estradiol and triggers the GnRH and subsequent LH surges in what has classically been referred to as positive feedback. The signal for the second mode is terminated because the LH surge inhibits, rather than stimulates, further production of estradiol because surge LH concentrations engage additional signaling pathways at the ovary and begin the process of luteinization (Figure 1)107,121.
Figure 1.
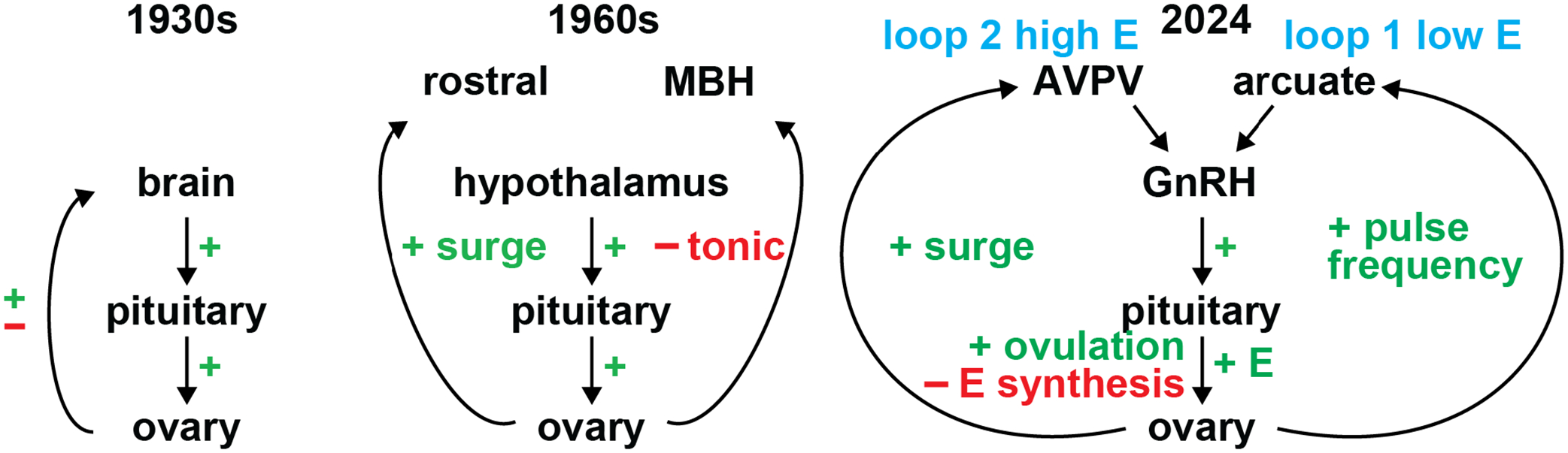
Evolution of estradiol action in the HPG axis of females. Left, early HPG axis at the time of Moore and Price and Hohlweg. Middle, refinements to the axis, including the hypothalamus as the main central neural player and the postulated regions of control for tonic and surge gonadotropin release by Barraclough and Gorski. Right, contemporary view with two interacting loops. Loop 1 produces episodic GnRH/LH output and it shows stimulation of pulse frequency by estradiol. Loop 1 cycles for hours, days or week depending on the species to accumulate sufficient estradiol to activate loop 2 to induce the surge. The high concentration of LH during the surge initiate multiple processes at the ovary to trigger ovulation but also terminate the activating estradiol signal for loop 2 by beginning the process of luteinization of the follicle cells. Progesterone from the corpus luteum, not pictured, provides the primary inhibitory input for pulse frequency.
Hypothalamic sites of estradiol action
The dual control of pulse and surge modes of LH release postulated by Barraclough55 was advanced by the discovery of two kisspeptin populations in the arcuate and anteroventral periventricular (AVPV) regions. Their roles in the HPG axis have been extensively studied and recently reviewed57,93,122–126. The concept that arcuate kisspeptin neurons mediate negative feedback, whereas AVPV kisspeptin neurons mediate positive feedback arose from and is supported by work in rodents124,127–129. Other species exhibit this dichotomy to different extents but more recent work suggests positive regulation of the more rostral (e.g., AVPV) population by estradiol may be a more common feature among species including humans than previously recognized130.
Two points are of note for the consideration of the concepts of estradiol negative and positive feedback as popularly used. First, these hypothalamic neural populations are differentially regulated by estradiol in terms of kisspeptin mRNA expression129, and firing activity128,131,132, with estradiol suppressing or having no effect on these parameters in the arcuate and increasing them in the AVPV. This point calls into question the blanket use of the adjectives negative or positive because both negative (inhibition in the arcuate) and positive (activation in the AVPV) actions can occur simultaneously in the same subject in response to the same estradiol signal. Second, this anatomical division of pulse and surge control of GnRH release violates the requirement that feedback is directed at the source of the original output58. Using the arcuate as an example, episodic activity of these neurons controls GnRH pulses and thereby drives the estradiol rise. By strict definition, negative feedback would involve high estradiol regulating arcuate activity to restore baseline estradiol concentration. During the cycle, however, the return towards baseline estradiol concentration is instead brought about by the LH surge, which is initiated by estradiol action on the AVPV to change the mode of GnRH release. Estradiol action thus incorporates feed-forward elements that may be thought of as second ‘feedback’ loops (Figure 1), and the dichotomous framing of the arcuate and AVPV populations as negative vs positive estradiol feedback arms may be oversimplified.
Concluding thoughts
The actions of estradiol within the hypothalamus to regulate reproductive neuroendocrine output are many. These actions, where they occur, the neurobiological processes affected and the direction of change can all vary throughout the female reproductive cycle. In considering the actions of estradiol during the follicular phase as primarily positive, we are giving primacy to the effects of estradiol to increase pulse frequency and induce the surge. This ignores the marked reduction of GnRH- and LH-pulse amplitude by estradiol. While it is intuitive that pulse amplitude is an inverse function of pulse frequency, several points argue that amplitude can be independently regulated. First, electrical stimulation of GnRH neurons in brain slices from mice elicits a greater GnRH secretory response when the slices are from castrated vs intact mice. This suggests increased excitation-secretion efficacy may underlie higher GnRH pulse amplitude observed in castrates in vivo133. Second, estradiol can both reduce and enhance the pituitary response to GnRH96,134. Third, LH pulse frequency and amplitude are simultaneously elevated in women with polycystic ovary syndrome (PCOS)135,136. It is possible that the reduced LH-pulse amplitude through the typical follicular phase plays important physiologic roles in selection of the dominant follicle116 and/or guarding against premature ovulation by selectively activating only certain signaling pathways121. It must be pointed out that while several studies show a lack of estradiol effects to inhibit the frequency of various parameters associated with GnRH output, the best evidence for the ability of estradiol to increase GnRH and LH pulse frequency comes from a single species, the sheep. This could be a specialization of this species. Or it could reflect the ability to measure directly GnRH in a reliable manner, which led to this species dominating research in the area137. Regardless, the consideration of the primary effects of estradiol as positive emphasizes the critical nature of progesterone negative feedback to reduce GnRH pulse frequency64,96 allowing for the early follicular phase FSH rise needed for folliculogenesis. Loss of efficacy of progesterone negative feedback is seen in women with PCOS and the resulting persistent high frequency of GnRH/LH release contributes to disruption of cycles138.
It might seem revolutionary to propose we reconsider the terms negative, positive and feedback as used to describe hormonal interactions in the HPG axis. The goal of this review was to stimulate thought about the operation of this axis, not necessarily incite a revolution. We are hopeful that such thought will inspire experiments that address gaps in our understanding of the axis, or that test more firmly established principles in new arenas. For example, direct measurement of GnRH in multiple species would test gonadal-hypothalamic feedback concepts that were established primarily in sheep. These experiments could also reveal if direct measurement of GnRH is indeed clarifying, or if surrogate LH measurements are mostly sufficient for monitoring GnRH release. The applicability of negative/positive feedback terms depends partly on the effects of LH pulse parameters on ovarian estradiol synthesis, and there are few data available on this topic. In vivo studies of the effects of LH frequency and amplitude on ovarian function could either add support to the postulate that frequency is the most critical variable, or force our thinking in new directions. A factor that remains a mystery is why, in common laboratory and domestic species, there is a multi-hour delay from the achievement of peak estradiol levels in the circulation to the switch in estradiol action to induce a surge67,139–147. Understanding the mechanisms underlying this delay might help explain how the two loops proposed in Figure 1 are connected.
There is a stark difference between the pulse and surge modes of GnRH secretion, and control of these patterns has been mapped primarily in rodents to different hypothalamic kisspeptin neuron populations. The separation of the arcuate and AVPV into purely negative/episodic and positive feedback/surge systems may, however, be premature as it deemphasizes the feed-forward hormonal interplay between these systems as well as possible interactions between these and other central systems.
Adoption of the engineering term of ‘feedback’ to describe hormone interactions in the reproductive and other endocrine systems in the middle of the last century was an appropriate advance in thinking. In light of a better understanding of the elements of the HPG axis, however, a more scrupulous way to refer to estradiol actions in this system may be to revert to the earlier terminology of stimulation and inhibition as appropriate for each individual mechanism being discussed, to better reflect the complex nature of the biology. This could lead to new interpretations of data as “If you change the way you look at things, the things you look at change.” (Wayne Dyer).
Acknowledgements:
Deepl was used to translate some portable document format (PDF)s from the original German to English https://www.deepl.com/en/translator/files
Grant Support:
National Institute of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant R01HD034860, R01HD041469, R01HD104345 (SMM). Support for JRS was provided F31HD097830.
Footnotes
Conflict of interest: The authors declare no competing financial interests.
Literature Cited
- 1.Pflüger E Die teleologische Mechanik der lebendigen Natur. Archiv für die gesamte Physiologie des Menschen und der Tiere 15, 57–103 (1877). https://doi.org: 10.1007/BF01628340 [DOI] [Google Scholar]
- 2.Oxford English Dictionary, <https://www.oed.com/?tl=true> (2023).
- 3.Fichéra G Sur l’hypertrophie de la gland pituitaire consécutive à la castration. Arch Ital d Biol 43, 405–426 (1905). [Google Scholar]
- 4.Tandler J & Grosz S Ueber den Einfluss der kastration auf den Organismus. Klin. Wochenschr 20, 1596–1597 (1907). [Google Scholar]
- 5.Addison WH The cell‐changes in the hypophysis of the albino rat, after castration. J Comp Neurol 28, 441–463 (1917). [Google Scholar]
- 6.Marinus CJ The effect of feeding pars tuberalis and pars anterior proprior of bovine pituitary glands upon the early development of the white rat. Am J Physiol-Legacy Content 49, 238–247 (1919). [Google Scholar]
- 7.Evans HM & Long JA The effect of feedting the anterior lobe of the hypophysis on the oestrous cycle of the rat. Anat Rec 21, 62 (1921). [Google Scholar]
- 8.Evans HM & Long JA The effect of the anterior lobe administered intraperitoneally upon growth, maturity, and oestrous cycles of the rat. Anat Rec 21, 62–63 (1921). [Google Scholar]
- 9.Smith PE Ablation and transplantation of the hypophysis in the rat. Anat. Rec 32, 221 (1926). [Google Scholar]
- 10.Allen BM Experiments in the Transplantation of the Hypophysis of Adult Rana pipiens to Tadpoles. Science 52, 274–276 (1920). https://doi.org: 10.1126/science.52.1342.274 [DOI] [PubMed] [Google Scholar]
- 11.Smith PE & Engle ET Experimental evidence regarding the role of the anterior pituitary in the development and regulation of the genital system. Am J Anat 40, 159–217 (1927). [Google Scholar]
- 12.Engle ET The effect of daily transplants of the anterior lobe from gonadectomized rats on immature test animals. Am J Physiol 88, 101–106 (1929). [Google Scholar]
- 13.Lillie FR The Theory of the Free-Martin. Science 43, 611–613 (1916). https://doi.org: 10.1126/science.43.1113.611 [DOI] [PubMed] [Google Scholar]
- 14.Steinach E & Kun H Antagonistishe Wirkungen der Keimdrüsen-Hormone. Biologia Generalis 2, 815–834 (1926). [Google Scholar]
- 15.Starling EH The Croonian Lectures ON THE CHEMICAL CORRELATION OF THE FUNCTIONS OF THE BODY. The Lancet 166, 339–341 (1905). https://doi.org: 10.1016/S0140-6736(01)11877-5 [DOI] [Google Scholar]
- 16.Aschheim S & Zondek B Hypophysenvorderlappenhormon und Ovarialhormon im Harn von Schwangeren. Klin. Wochensch 6, 1322–1322 (1927). https://doi.org: 10.1007/BF01728562 [DOI] [Google Scholar]
- 17.Fevold HL The follicle stimulating and luteinizing hormones of the anterior pituitary. 2 edn, (The Williams & Wilkins Company, 1930). [Google Scholar]
- 18.Doisy EA, Thayer S & Veler CD The Crystals of the Follicular Ovarian Hormone. Proc Soc Exp Biol Med 27, 417–419 (1930). https://doi.org: 10.3181/00379727-27-4791 [DOI] [Google Scholar]
- 19.Thayer SA, Levin L & Doisy EA Characterization of Theelol. The Journal of biological chemistry 91, 655–665 (1931). https://doi.org: 10.1016/S0021-9258(18)76577-0 [DOI] [Google Scholar]
- 20.Huffman MN, Thayer SA & Doisy EA The isolation of α-dihydrotheelin from human placenta. The Journal of biological chemistry 133, 567–571 (1940). https://doi.org: 10.1016/S0021-9258(18)73338-3 [DOI] [Google Scholar]
- 21.Moore CR & Price D The question of sex hormone antagonism. Proc Soc Exp Biol Med 28, 38–40 (1930). [Google Scholar]
- 22.Moore CR & Price D Gonad hormone functions, and the reciprocal influence between gonads and hypophsis with its bearing on the problem of sex hormone antagonism. Am J Anat 50, 13–67 (1932). [Google Scholar]
- 23.Hohlweg W & Junkmann K Die Hormonal-Nervöse regulierung der Funktion des Hypophysenvorderlappens und der Keimdrüsen. Klin Wochensch 11, 321–323 (1932). [Google Scholar]
- 24.Hohlweg W Veränderungen des Hypophsenforderlappens und des Ovariums nach behandlung mit grossen Dosen von Follikelhormon. Klin Wochensch 13, 92–95 (1934). [Google Scholar]
- 25.Marrian GF & Parkes AS The relative amounts of œstrin required to produce the various phenomena of œstrus. J Physiol 69, 372–376 (1930). https://doi.org:doi: 10.1113/jphysiol.1930.sp002656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fevold HL, Hisaw FL & Greep R Effect of oestrin on the activity of the anterior lobe of the pituitary. Am J Physiol 114, 508–513 (1935). https://doi.org: 10.1152/ajplegacy.1935.114.2.508 [DOI] [Google Scholar]
- 27.Lane CE Some influences of oestrin on the hypophyseal-gonad complex of the immature female rat. Am J Physiol-Legacy Content 110, 681–685 (1934). https://doi.org: 10.1152/ajplegacy.1934.110.3.681 [DOI] [Google Scholar]
- 28.Meyer RK, Leonard SL, Hisaw FL & Martin SJ The influece of oestrin on the gonad-stimulating complex of the anterior pituitary of castrated male and female rats. Endocrinology 16, 655–665 (1932). https://doi.org: 10.1210/endo-16-6-655 [DOI] [Google Scholar]
- 29.Selye H, Collip JB & Thomson DL Effect of Oestrin on Ovaries and Adrenals. Pro Soc Exp Biol Med 32, 1377–1381 (1935). https://doi.org: 10.3181/00379727-32-8100C [DOI] [Google Scholar]
- 30.Ellison ET & Burch JC The effect of estrogenic substances upon the pituitary, adrenals and ovaries. Endocrinology 20, 746–752 (1936). https://doi.org: 10.1210/endo-20-6-746 [DOI] [Google Scholar]
- 31.Mazer H & Israel SL Studies on the optimal dosage of estrogens: an experimental and clinical evaluation. JAMA 108, 163–169 (1937). https://doi.org: 10.1001/jama.1937.02780030001001 [DOI] [Google Scholar]
- 32.D’Amour FE Further Studies on Hormone Excretion During the Menstrual Cycle. Am J Ob Gyn 40, 958–965 (1940). https://doi.org: 10.1016/S0002-9378(15)31447-2 [DOI] [Google Scholar]
- 33.Harris GW Neural control of the pituitary gland. Physiological reviews 28, 139–179 (1948). [DOI] [PubMed] [Google Scholar]
- 34.Everett JW & Sawyer CHA 24-hour periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology 47, 198–218 (1950). [DOI] [PubMed] [Google Scholar]
- 35.Greep RO & Jones IC Steroid control of pituitary function. Omnia Med 28, 461–462 (1950). [PubMed] [Google Scholar]
- 36.Price D & Ortiz E The relation of age to reactivity in the reproductive system of the rat. Endocrinology 34, 215–239 (1944). https://doi.org: 10.1210/endo-34-4-215 [DOI] [Google Scholar]
- 37.Harris GW Neural control of the pituitary gland. (Edward Arnold, 1955). [Google Scholar]
- 38.Black HS Stabilized feedback amplifiers. The Bell System Technical Journal 13, 1–18 (1934). https://doi.org: 10.1002/j.1538-7305.1934.tb00652.x [DOI] [Google Scholar]
- 39.Wiener N Cybernetics. Sci Am 179, 14–19 (1948). [DOI] [PubMed] [Google Scholar]
- 40.Wiener N Cybernetics: or Control and Communication in the Animal and the Machine. (John Wiley & Sons, Inc., 1948). [Google Scholar]
- 41.Hammond J in Vitamins & Hormones Vol. 12 (eds Harris Robert S., Marrian GF, & Thimann Kenneth V.) 157–206 (Academic Press, 1954).14374257 [Google Scholar]
- 42.Schapiro S, Marmorston J & Sobel H The Steroid Feedback Mechanism. Am J Physiol-Legacy Content 192, 58–62 (1957). https://doi.org: 10.1152/ajplegacy.1957.192.1.58 [DOI] [PubMed] [Google Scholar]
- 43.Valk William L & Owens Robert H Endocrine Inhibition as Related to Carcinoma of the Prostate. Journal of Urology 72, 516–524 (1954). https://doi.org: 10.1016/S0022-5347(17)67617-1 [DOI] [PubMed] [Google Scholar]
- 44.Dorfman RI Biosynthesis of adrenocortical steroids. Cancer 10, 741–745 (1957). https://doi.org: [DOI] [PubMed] [Google Scholar]
- 45.Bogdanove EM Direct Gonad-Pituitary Feedback: An Analysis of Effects of Intracranial Estrogenic Depots on Gonadotrophin Secretion. Endocrinology 73, 696–712 (1963). https://doi.org: 10.1210/endo-73-6-696 [DOI] [PubMed] [Google Scholar]
- 46.Ramirez VD, Abrams RM & McCann SM Effect of Estradiol Implants in the Hypothalamo-Hypophysial Region of the Rat on the Secretion of Luteinizing Hormone. Endocrinology 75, 243–248 (1964). https://doi.org: 10.1210/endo-75-2-243 [DOI] [PubMed] [Google Scholar]
- 47.Parlow AF in Human Pituitary Gonadotropins (eds Albert A & Thomas CC) 300 (1961). [Google Scholar]
- 48.D’Amour FE, Funk D & Liverman H Daily gonadotropic hormone tests during fifty complete menstrual cycles. Am J Ob Gyn 37, 940–946 (1939). https://doi.org: 10.1016/S0002-9378(39)90233-4 [DOI] [Google Scholar]
- 49.Levin L Quantitative precipitation of the urinary gonadotropin of normal men and women. Endocrinology 28, 378–387 (1941). https://doi.org: 10.1210/endo-28-3-378 [DOI] [Google Scholar]
- 50.McArthur JW, Worcester J & Inghrsoll FM The urinary excretion of interstitial cell and follicle-stimulating hormone activity during the normal menstrual cycle. J Clin Endocrinol Metab 18, 1186–1201 (1958). https://doi.org: 10.1210/jcem-18-11-1186 [DOI] [PubMed] [Google Scholar]
- 51.Neill JD, Johansson ED, Datta JK & Knobil E Relationship between the plasma levels of luteinizing hormone and progesterone during the normal menstrual cycle. J Clin Endocrinol Metab 27, 1167–1173 (1967). [DOI] [PubMed] [Google Scholar]
- 52.Schwartz NB in Recent Progress in Hormone Research: Proceedings of the 1968 Laurentian Hormone Conference. 1–55 (Academic Press, 1969). [Google Scholar]
- 53.Corker CS, Naftolin F & Exley D Interrelationship between Plasma Luteinizing Hormone and Oestradiol in the Human Menstrual Cycle. Nature 222, 1063–1063 (1969). https://doi.org: 10.1038/2221063a0 [DOI] [PubMed] [Google Scholar]
- 54.Sawyer CH, Everett JW & Markee JE A neural factor in the mechanism by which estrogen induces the release of luteinizing hormone in the rat. Endocrinology 44, 218–233 (1949). https://doi.org: 10.1210/endo-44-3-218 [DOI] [PubMed] [Google Scholar]
- 55.Barraclough CA & Gorski RA Evidence that the hypothalamus is responsible for androgen-induced sterility in the female rat. Endocrinology 68, 68–79 (1961). https://doi.org: 10.1210/endo-68-1-68 [DOI] [PubMed] [Google Scholar]
- 56.Halász B & Pupp L Hormone Secretion of the Anterior Pituitary Gland After Physical Interruption of All Nervous Pathways to the Hypophysiotrophic Area. Endocrinology 77, 553–562 (1965). https://doi.org: 10.1210/endo-77-3-553 [DOI] [PubMed] [Google Scholar]
- 57.Starrett JR & Moenter SM Hypothalamic kisspeptin neurons as potential mediators of estradiol negative and positive feedback. Peptides 163, 170963 (2023). https://doi.org: 10.1016/j.peptides.2023.170963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Modell H et al. A physiologist’s view of homeostasis. Adv Physiol Educ 39, 259–266 (2015). https://doi.org: 10.1152/advan.00107.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Docke F & Dorner G The mechanism of the induction of ovulation by oestrogens. J Endocrinol 33, 491–499. (1965). [DOI] [PubMed] [Google Scholar]
- 60.Xia L, Van Vugt D, Alston EJ, luckhaus J, Ferin M A surge of gonadotropin-releasing hormone accompanies the estradiol-induced gonadotropin surge in the rhesus monkey. Endocrinology 131, 2812–2820 (1992). [DOI] [PubMed] [Google Scholar]
- 61.Sarkar DK, Chiappa SA, Fink G & Sherwood NM Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature 264, 461–463 (1976). [DOI] [PubMed] [Google Scholar]
- 62.Levine JE & Ramirez VD Luteinizing hormone-releasing hormone release during the rat estrous cycle and after ovariectomy, as estimated with push-pull cannulae. Endocrinology 111, 1439–1448 (1982). [DOI] [PubMed] [Google Scholar]
- 63.Clarke IJ, Thomas GB, Yao B & Cummins JT GnRH secretion throughout the ovine estrous cycle. Neuroendocrinology 46, 82–88 (1987). [DOI] [PubMed] [Google Scholar]
- 64.Moenter SM, Caraty A, Locatelli A & Karsch FJ Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology 129, 1175–1182 (1991). [DOI] [PubMed] [Google Scholar]
- 65.Clarke IJ, Cummins JT, Crowder ME & Nett TM Pituitary receptors for gonadotropin-releasing hormone in relation to changes in pituitary and plasma gonadotropins in ovariectomized hypothalamo/pituitary-disconnected ewes. II. A marked rise in receptor number during the acute feedback effects of estradiol. Biol Reprod 39, 349–354 (1988). [DOI] [PubMed] [Google Scholar]
- 66.Yamaji T et al. Estrogen induction of LH release in the rhesus monkey. Endocrinology 89, 1034–1041 (1971). [DOI] [PubMed] [Google Scholar]
- 67.Goodman RL, Legan SJ, Ryan KD, Foster DL & Karsch FJ Importance of variations in behavioural and feedback actions of oestradiol to the control of seasonal breeding in the ewe. J Endocrinol 89, 229–240 (1981). [DOI] [PubMed] [Google Scholar]
- 68.Dierschke DJ, Bhattacharya AN, Atkinson LE & Knobil E Circhoral oscillations of plasma LH levels in the ovariectomized rhesus monkey. Endocrinology 87, 850–853 (1970). [DOI] [PubMed] [Google Scholar]
- 69.Matsuo H, Baba Y, Nair RMG, Arimura A & Schally AV Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem Biophys Res Comm 43, 1334–1339 (1971). https://doi.org: 10.1016/S0006-291X(71)80019-0 [DOI] [PubMed] [Google Scholar]
- 70.Clarke IJ & Cummins JT The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology 111, 1737–1739 (1982). [DOI] [PubMed] [Google Scholar]
- 71.Moenter SM, Brand RM, Midgley AR & Karsch FJ Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology 130, 503–510 (1992). [DOI] [PubMed] [Google Scholar]
- 72.Belchetz P, Plant TM, Nakai Y, Keogh EJ & Knobil E Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science 202, 631–633 (1978). [DOI] [PubMed] [Google Scholar]
- 73.Wildt L et al. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 109, 376–385 (1981). https://doi.org: 10.1210/endo-109-2-376 [DOI] [PubMed] [Google Scholar]
- 74.Moenter SM Leap of Faith: Does Serum Luteinizing Hormone Always Accurately Reflect Central Reproductive Neuroendocrine Activity? Neuroendocrinology 102, 256–266 (2015). https://doi.org: 10.1159/000438790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reame N, Sauder SE, Kelch RP & Marshall JC Pulsatile gonadotropin secretion during the human menstrual cycle: evidence for altered frequency of gonadotropin-releasing hormone secretion. J Clin Endocrinol Metab 59, 328–337 (1984). [DOI] [PubMed] [Google Scholar]
- 76.Rossmanith WG et al. Relative changes in LH pulsatility during the menstrual cycle: using data from hypogonadal women as a reference point. Clin Endocrinol 32, 647–660 (1990). https://doi.org: 10.1111/j.1365-2265.1990.tb00909.x [DOI] [PubMed] [Google Scholar]
- 77.Veldhuis JD, Beitins IZ, Johnson ML, Serabian MA & Dufau ML Biologically active luteinizing hormone is secreted in episodic pulsations that vary in relation to stage of the menstrual cycle. J Clin Endocrinol Metab 58, 1050–1058 (1984). https://doi.org: 10.1210/jcem-58-6-1050 [DOI] [PubMed] [Google Scholar]
- 78.Sollenberger MJ, Carlsen EC, Johnson ML, Veldhuis JD & Evans WS Specific physiological regulation of luteinizing hormone secretory events throughout the human menstrual cycle: new insights into the pulsatile mode of gonadotropin release. J Neuroendocrinol 2, 845–852 (1990). https://doi.org: 10.1111/j.1365-2826.1990.tb00650.x [DOI] [PubMed] [Google Scholar]
- 79.Crowley WF Jr. & McArthur JW Simulation of the normal menstrual cycle in Kallman’s syndrome by pulsatile administration of luteinizing hormone-releasing hormone (LHRH). J Clin Endocrinol Metab 51, 173–175 (1980). [DOI] [PubMed] [Google Scholar]
- 80.Norman RL et al. Pulsatile secretion of luteinizing hormone during the menstrual cycle of rhesus macaques. Endocrinology 115, 261–266 (1984). [DOI] [PubMed] [Google Scholar]
- 81.McCullagh DR Dual endocrine activity of the testes. Science 76, 19–20 (1932). [DOI] [PubMed] [Google Scholar]
- 82.Caraty A, Locatelli A & Martin GB Biphasic response in the secretion of gonadotrophin-releasing hormone in ovariectomized ewes injected with oestradiol. J ENdocrinol 123, 375–382 (1989). [DOI] [PubMed] [Google Scholar]
- 83.Moenter SM, Caraty A & Karsch FJ The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology 127, 1375–1384 (1990). [DOI] [PubMed] [Google Scholar]
- 84.Couse JF et al. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estorgen receptor gene. Mol Endocrinol 9, 1441–1454 (1995). [DOI] [PubMed] [Google Scholar]
- 85.Herbison AE & Theodosis DT Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience 50, 283–298 (1992). [DOI] [PubMed] [Google Scholar]
- 86.Lehman MN, Ebling FJ, Moenter SM & Karsch FJ Distribution of estrogen receptor-immunoreactive cells in the sheep brain. Endocrinology 133, 876–886 (1993). [DOI] [PubMed] [Google Scholar]
- 87.Wintermantel TM et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52, 271–280 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cintra A On the cellular localization and distribution of estrogen receptors in the rat tel-and diencephalon using monoclonal antibodies to human estrogen receptor. Neurochemistry international 8, 587–595 (1986). [DOI] [PubMed] [Google Scholar]
- 89.Blurton-Jones MM, Roberts JA & Tuszynski MH Estrogen receptor immunoreactivity in the adult primate brain: Neuronal distribution and association with p75, trkA, and choline acetyltransferase. J Comp Neurol 405, 529–542 (1999). https://doi.org: [DOI] [PubMed] [Google Scholar]
- 90.Seminara SB et al. The GPR54 gene as a regulator of puberty.[see comment]. New Eng J Med 349, 1614–1627 (2003). [DOI] [PubMed] [Google Scholar]
- 91.de Roux N et al. Hypogonadotropic hypogonadism due to loss of function of the Kiss1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A 100, 10972–10976 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oakley AE, Clifton DK & Steiner RA Kisspeptin signaling in the brain. Endocr Rev 30, 713–743 (2009). https://doi.org:er.2009–0005 [pii] 10.1210/er.2009-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goodman RL, Herbison AE, Lehman MN & Navarro VM Neuroendocrine control of gonadotropin-releasing hormone: Pulsatile and surge modes of secretion. J Neuroendocrinol 34, e13094 (2022). https://doi.org: 10.1111/jne.13094doi.org/10.1111/jne.13094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goodman RL, Legan SJ, Ryan KD, Foster DL & Karsch FJ Two effects of estradiol that normally contribute to the control of tonic LH secretion in the ewe. Biol Reprod 23, 415–422 (1980). [DOI] [PubMed] [Google Scholar]
- 95.Leipheimer RE, Bona-Gallo A & Gallo RV Ovarian steroid regulation of pulsatile luteinizing hormone release during the interval between the mornings of diestrus 2 and proestrus in the rat. Neuroendocrinology 41, 252–257 (1985). [DOI] [PubMed] [Google Scholar]
- 96.Goodman RL & Karsch FJ Pulsatile secretion of luteinizing hormone: differential suppression by ovarian steroids. Endocrinology 107, 1286–1290 (1980). [DOI] [PubMed] [Google Scholar]
- 97.Karsch FJ, Foster DL, Bittman EL & Goodman RL A role for estradiol in enhancing luteinizing hormone pulse frequency during the follicular phase of the estrous cycle of sheep. Endocrinology 113, 1333–1339 (1983). [DOI] [PubMed] [Google Scholar]
- 98.Kaynard AH, Follett BK & Karsch FJ Feedback regulation of pulsatile LH secretion in the ewe: stimulation of frequency by estradiol. Neuroendocrinology 48, 81–86 (1988). [DOI] [PubMed] [Google Scholar]
- 99.Evans NP, Dahl GE, Glover BH & Karsch FJ Central Regulation of Pulsatile Gonadotropin-Releasing Hormone (GnRH) Secretion by Estradiol during the Period Leading up to the Preovulatory GnRH Surge in the Ewe. Endocrinology 134, 1806–1811 (1994). https://doi.org: 10.1210/en.134.4.1806 [DOI] [PubMed] [Google Scholar]
- 100.Evans NP et al. Does estradiol induce the preovulatory gonadotropin-releasing hormone (GnRH) surge in the ewe by inducing a progressive change in the mode of operation of the GnRH neurosecretory system. Endocrinology 136, 5511–5519 (1995). [DOI] [PubMed] [Google Scholar]
- 101.Moenter SM, Brand RC & Karsch FJ Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: insights into the mechanism of GnRH surge induction. Endocrinology 130, 2978–2984 (1992). https://doi.org: 10.1210/endo.130.5.1572305 [DOI] [PubMed] [Google Scholar]
- 102.Evans NP, Dahl GE, Mauger D & Karsch FJ Estradiol induces both qualitative and quantitative changes in the pattern of gonadotropin-releasing hormone secretion during the presurge period in the ewe. Endocrinology 136, 1603–1609 (1995). [DOI] [PubMed] [Google Scholar]
- 103.Wilson RC et al. Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology 39, 256–260 (1984). [DOI] [PubMed] [Google Scholar]
- 104.O’Byrne KT et al. Radiotelemetric monitoring of hypothalamic gonadotropin-releasing hormone pulse generator activity throughout the menstrual cycle of the rhesus monkey. Endocrinology 129, 1207–1214 (1991). [DOI] [PubMed] [Google Scholar]
- 105.Tanaka T, Ozawa T, Hoshino K & Mori Y Changes in the gonadotropin-releasing hormone pulse generator activity during the estrous cycle in the goat. Neuroendocrinology 62, 553–561 (1995). [DOI] [PubMed] [Google Scholar]
- 106.McQuillan HJ, Han SY, Cheong I & Herbison AE GnRH Pulse Generator Activity Across the Estrous Cycle of Female Mice. Endocrinology 160, 1480–1491 (2019). https://doi.org: 10.1210/en.2019-00193 [DOI] [PubMed] [Google Scholar]
- 107.Richards JS & Hedin L Molecular Aspects of Hormone Action in Ovarian Follicular Development, Ovulation, and Luteinization. Ann Rev Physiol 50, 441–463 (1988). https://doi.org: 10.1146/annurev.ph.50.030188.002301 [DOI] [PubMed] [Google Scholar]
- 108.Evans NP, Dahl GE, Padmanabhan V, Thrun LA & Karsch FJ Estradiol requirements for induction and maintenance of the gonadotropin-releasing hormone surge: implications for neuroendocrine processing of the estradiol signal. Endocrinology 138, 5408–5414 (1997). [DOI] [PubMed] [Google Scholar]
- 109.Kesner JS et al. Unexpected responses of the hypothalamic gonadotropin-releasing hormone “pulse generator” to physiological estradiol inputs in the absence of the ovary. Proc Natl Acad Sci USA 84, 8745–8749 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Glanowska KM, Venton BJ & Moenter SM Fast scan cyclic voltammetry as a novel method for detection of real-time gonadotropin-releasing hormone release in mouse brain slices. J Neurosci 32, 14664–14669 (2012). https://doi.org: 10.1523/JNEUROSCI.1303-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hillier SG, Whitelaw PF & Smyth CD Follicular oestrogen synthesis: the ‘two-cell, two-gonadotrophin’ model revisited. Mol Cell Endocrinol 100, 51–54 (1994). https://doi.org: 10.1016/0303-7207(94)90278-X [DOI] [PubMed] [Google Scholar]
- 112.Dorrington JH, Moon YS & Armstrong DT Estradiol-17β biosynthesis in cultured granulosa cells from hypophysectomized immature rats: stimulation by follicle-stimulating hormone. Endocrinology 97, 1328–1331 (1975). https://doi.org: 10.1210/endo-97-5-1328 [DOI] [PubMed] [Google Scholar]
- 113.Peluso J, Downey M & Gruenberg M Role of LH pulse amplitude in controlling rat ovarian oestradiol-17β secretion in vitro. Reproduction 71, 107–112 (1984). [DOI] [PubMed] [Google Scholar]
- 114.Peluso J, Durkee T & Gruenberg M The effect of an LH pulse on 3 H-thymidine incorporation into cultured ovaries of metestrous rats. Cell Tiss Res 238, 159–163 (1984). [DOI] [PubMed] [Google Scholar]
- 115.Peluso JJ, Gruenberg ML & Steger RW Regulation of ovarian follicular growth and steroidogenesis by low-amplitude LH pulses. Am J Physiol Reg Integr Comp Physiol 246, R184–R189 (1984). https://doi.org: 10.1152/ajpregu.1984.246.2.R184 [DOI] [PubMed] [Google Scholar]
- 116.Devorshak-Harvey E, Peluso JJ, Bona-Gallo A & Gallo RV Effect of alterations in pulsatile luteinizing hormone release on ovarian follicular atresia and steroid secretion on diestrus 1 in the rat estrous cycle. Biol Reprod 33, 103–111 (1985). [DOI] [PubMed] [Google Scholar]
- 117.Selvaraj V, Stocco DM & Clark BJ Current knowledge on the acute regulation of steroidogenesis†. Biol Reprod 99, 13–26 (2018). https://doi.org: 10.1093/biolre/ioy102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Filicori M et al. Evidence for a specific role of GnRH pulse frequency in the control of the human menstrual cycle. Am J Physiol Endocrinol Metab 257, E930–E936 (1989). [DOI] [PubMed] [Google Scholar]
- 119.Breen KM, Billings HJ, Wagenmaker ER, Wessinger EW & Karsch FJ Endocrine basis for disruptive effects of cortisol on preovulatory events. Endocrinology 146, 2107–2115 (2005). https://doi.org: 10.1210/en.2004-1457 [DOI] [PubMed] [Google Scholar]
- 120.Hsueh AJW & Erickson GF Glucocorticoid inhibition of fsh-induced estrogen production in cultured rat granulosa cells. Steroids 32, 639–648 (1978). https://doi.org: 10.1016/0039-128X(78)90074-0 [DOI] [PubMed] [Google Scholar]
- 121.Richards JS & Ascoli M Endocrine, Paracrine, and Autocrine Signaling Pathways That Regulate Ovulation. Tr Endocrinol Metab 29, 313–325 (2018). https://doi.org: 10.1016/j.tem.2018.02.012 [DOI] [PubMed] [Google Scholar]
- 122.Ivanova D & O’Byrne K New methods to investigate the GnRH pulse generator. J Mol Endocrinol 1 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Uenoyama Y & Tsukamura H KNDy neurones and GnRH/LH pulse generation: current understanding and future aspects. J Neuroendocrinol, e13285 (2023). [DOI] [PubMed] [Google Scholar]
- 124.Wang L et al. Genetic dissection of the different roles of hypothalamic kisspeptin neurons in regulating female reproduction. eLife 8 (2019). https://doi.org: 10.7554/eLife.43999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Piet R Circadian and kisspeptin regulation of the preovulatory surge. Peptides, 170981 (2023). [DOI] [PubMed] [Google Scholar]
- 126.Nestor CC, Merkley CM, Lehman MN, Hileman SM & Goodman RL KNDy neurons as the GnRH pulse generator: Recent studies in ruminants. Peptides 164, 171005 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Porteous R & Herbison AE Genetic Deletion of Esr1 in the Mouse Preoptic Area Disrupts the LH Surge and Estrous Cyclicity. Endocrinology 160, 1821–1829 (2019). https://doi.org: 10.1210/en.2019-00284 [DOI] [PubMed] [Google Scholar]
- 128.Wang L & Moenter SM Differential Roles of Hypothalamic AVPV and Arcuate Kisspeptin Neurons in Estradiol Feedback Regulation of Female Reproduction. Neuroendocrinology 110, 172–184 (2020). https://doi.org: 10.1159/000503006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Smith JT, Cunningham MJ, Rissman EF, Clifton DK & Steiner RA Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146, 3686–3692 (2005). https://doi.org:en.2005–0488 [pii] 10.1210/en.2005-0488 [DOI] [PubMed] [Google Scholar]
- 130.Hrabovszky E, Takács S, Rumpler É & Skrapits K in Handbook of Clinical Neurology Vol. 180 (eds Swaab Dick F. et al.) 275–296 (Elsevier, 2021). [DOI] [PubMed] [Google Scholar]
- 131.Wang L, Burger LL, Greenwald-Yarnell ML, Myers MG Jr. & Moenter SM Glutamatergic Transmission to Hypothalamic Kisspeptin Neurons Is Differentially Regulated by Estradiol through Estrogen Receptor alpha in Adult Female Mice. J Neurosci 38, 1061–1072 (2018). https://doi.org: 10.1523/JNEUROSCI.2428-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Phumsatitpong C, De Guzman RM, Zuloaga DG & Moenter SM A CRH Receptor Type 1 Agonist Increases GABA Transmission to GnRH Neurons in a Circulating-Estradiol-Dependent Manner. Endocrinology 161 (2020). https://doi.org: 10.1210/endocr/bqaa140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen X & Moenter SM Gonadal Feedback Alters the Relationship between Action Potentials and Hormone Release in Gonadotropin-Releasing Hormone Neurons in Male Mice. Journal of Neuroscience 43, 6717–6730 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Clarke IJ & Cummins JT Direct pituitary effects of estrogen and progesterone on gonadotropin secretion in the ovariectomized ewe. Neuroendocrinology 39, 267–274 (1984). [DOI] [PubMed] [Google Scholar]
- 135.Cheung AP, Lu JK & Chang RJ Pulsatile gonadotrophin secretion in women with polycystic ovary syndrome after gonadotrophin-releasing hormone agonist treatment. Human Reproduction 12, 1156–1164 (1997). https://doi.org: 10.1093/humrep/12.6.1156 [DOI] [PubMed] [Google Scholar]
- 136.Morales AJ et al. Insulin, somatotropic, and luteinizing hormone axes in lean and obese women with polycystic ovary syndrome: common and distinct features. The Journal of Clinical Endocrinology & Metabolism 81, 2854–2864 (1996). https://doi.org: 10.1210/jcem.81.8.8768842 [DOI] [PubMed] [Google Scholar]
- 137.Moenter SM & Evans NP Gonadotropin-releasing hormone (GnRH) measurements in pituitary portal blood: A history. J Neuroendocrinol 34, e13065 (2022). https://doi.org: 10.1111/jne.13065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McCartney CR, Campbell RE, Marshall JC & Moenter SM The role of gonadotropin-releasing hormone neurons in polycystic ovary syndrome. J Neuroendocrinol 34, e13093 (2022). https://doi.org: 10.1111/jne.13093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nanda AS, Ward WR & Dobson H Effect of endogenous and exogenous progesterone on the oestradiol-induced LH surge in dairy cows. Reproduction 84, 367–371 (1988). https://doi.org: 10.1530/jrf.0.0840367 [DOI] [PubMed] [Google Scholar]
- 140.Britt JH, Esbenshade KL & Ziecik AJ Roles of Estradiol and Gonadotropin-Releasing Hormone in Controlling Negative and Positive Feedback Associated with the Luteinizing Hormone Surge in Ovariectomized Pigs1. Biology of Reproduction 45, 478–485 (1991). https://doi.org: 10.1095/biolreprod45.3.478 [DOI] [PubMed] [Google Scholar]
- 141.Kim S, Tanaka T & Kamomae H Different Effects of Subnormal Levels of Progesterone on the Pulsatile and Surge Mode Secretion of Luteinizing Hormone in Ovariectomized Goats1. Biology of Reproduction 69, 141–145 (2003). https://doi.org: 10.1095/biolreprod.102.013532 [DOI] [PubMed] [Google Scholar]
- 142.Yen F-P et al. Estradiol-17β Triggers Luteinizing Hormone Release in the Protandrous Black Porgy (Acanthopagrus schlegeli Bleeker) Through Multiple Interactions with Gonadotropin-Releasing Hormone Control1. Biology of Reproduction 66, 251–257 (2002). https://doi.org: 10.1095/biolreprod66.1.251 [DOI] [PubMed] [Google Scholar]
- 143.Wagner EJ, Ronnekleiv OK, Bosch MA & Kelly MJ Estrogen biphasically modifies hypothalamic GABAergic function concomitantly with negative and positive control of luteinizing hormone release. Journal of Neuroscience 21, 2085–2093 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Legan SJ & Karsch FJ A daily signal for the LH surge in the rat. Endocrinology 96, 57–62 (1975). [DOI] [PubMed] [Google Scholar]
- 145.Norman RL & Spies HG Neural control of the estrogen-dependent twenty-four-hour periodicity of LH release in the golden hamster. Endocrinology 95, 1367–1372 (1974). [DOI] [PubMed] [Google Scholar]
- 146.Christian CA, Mobley JL & Moenter SM Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA 102, 15682–15687 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bronson FH & Vom Saal FS Control of the preovulatory release of luteinizing hormone by steroids in the mouse. Endocrinology 104, 1247–1255 (1979). [DOI] [PubMed] [Google Scholar]


