Abstract
Intraoperative navigation is a novel technology that can provide real-time feedback to the surgeon during implantation and enhance the accuracy and precision of glenoid component positioning. Applications of intraoperative navigation systems have demonstrated increased precision in baseplate version and inclination, as well as improved baseplate screw placement, with fewer screws used and greater purchase length achieved when compared to standard instrumentation. Early clinical studies have shown favorable results, with significantly improved patient-reported and clinical outcomes and decreased complications. The implementation of intraoperative navigation is associated with a short learning curve and a minimal increase in operative time. Nevertheless, further research is necessary to substantiate the clinical benefit of navigation and evaluate its economic cost-effectiveness and impact on implant survival. Augmented reality and robotic-assisted surgery are additional emerging technologies that, while novel, hold the potential to further advance the field of shoulder arthroplasty.
Keywords: Reverse shoulder arthroplasty, Intraoperative navigation, Preoperative planning, Augmented Reality, Robotic-assisted Surgery
Reverse total shoulder arthroplasty (RSA) has experienced a significant increase in incidence over the past decade, with projections indicating continued exponential growth.1) This increase can be attributed to its evolving surgical techniques, improving implant technology, and well-established success in the treatment of various shoulder pathologies, most commonly rotator cuff tear arthropathy.1,2,3)
Accurate positioning and secure initial fixation of the glenoid component are critical factors for success in RSA.4,5,6) Malposition of the glenoid component can result in scapular notching, which can subsequently cause pain, diminished function, instability, and glenoid loosening.4,6,7,8) Thus, positioning of the baseplate inferiorly on the glenoid without introducing excessive superior tilt is imperative for optimizing impingement-free range of motion (ROM).4,5,7) Optimization of the length and number of peripheral screws and targeting areas characterized by high bone quality to attain the strongest purchase is essential for stable fixation as well.4,9) However, severely pathologic glenoids with bone deformities and/or poor bone stock can present challenges that may compromise fixation and correct positioning of the baseplate.
Advancements in shoulder arthroplasty have been developed in the hopes of improving the accuracy of glenoid component implantation and prolonging implant longevity, particularly for complex glenoid deformities. Intraoperative navigation, also known as computer-assisted surgery, is a modern technology that uses detailed 3-dimensional (3D) preoperative planning to provide the surgeon with real-time feedback during implantation in order to enhance precision, accuracy, and safety. Intraoperative navigation, which has already gained utility in hip and knee arthroplasty and shown to improve the alignment of components,10,11,12,13) has also become increasingly implemented in shoulder arthroplasty with multiple studies exhibiting favorable outcomes.14,15,16,17,18) As such, the purpose of this review is to explore the application of intraoperative navigation in reverse shoulder arthroplasty, evaluate its efficacy in terms of glenoid component placement accuracy, screw selection, clinical outcomes, the learning curve, and notable challenges, and finally delve into the future potential of alternative emerging technologies.
APPLICATION OF INTRAOPERATIVE NAVIGATION
Preoperative Planning and Templating
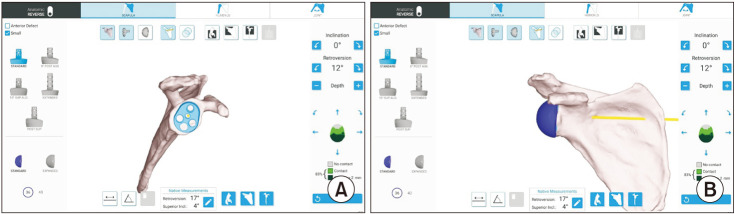
The utility of intraoperative navigation is contingent upon precise preoperative planning and thus involves the acquisition of thin-slice computed tomography (CT) scans of the entire scapula for every patient. The recommended CT slice thickness is 0.625 mm, with a minimum of 0.3 mm and maximum of 1.25 mm.19) Once attained, the CT images are uploaded into a preoperative planning software application in order to render 3D images of the glenoid (Fig. 1).15) Using the software, the operating surgeon begins planning the placement of the glenoid component on the 3D glenoid model to achieve the desired deformity correction and backside contact (Fig. 1).15) This involves the surgeon using their discretion to determine the depth and extent of high-side reaming, whether patient-specific instrumentation, augmentation, or bone grafting is necessary, the desired rotation, version, and inclination of the baseplate, as well as the number, length, and precise placement and trajectory of screws within the scapula. The surgeon is able to place sized-to-scale representations of all baseplate options onto the 3D model and make incremental adjustments of 1 mm and 1° in baseplate positioning across all planes.20) The preoperative plan is then saved and exported to the navigation system before surgery for intraoperative utilization.15) Templating serves as a valuable tool, particularly in fractures, dysplasia, intricate glenoid deformities involving significant bone loss, or revision surgeries.
Fig. 1. A computed tomography scan of the shoulder and scapula is loaded into a 3-dimensional planning software. The implants can then be virtually implanted to ensure adequate placement of the baseplate (A) and glenosphere (B).
Intraoperative Guidance
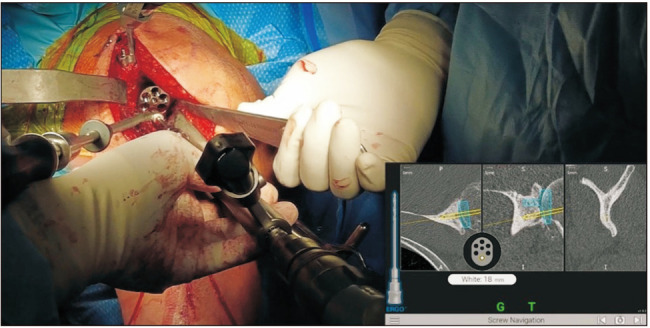
In order to provide the surgeon with real-time visual guidance, a stereotactic tracker is calibrated with the computer monitor and then mounted on the superior surface of the coracoid and fixed with 2 screws (Fig. 2).15) Registration of the orientation of the system is then performed using a handheld sensor stylus to obtain image acquisitions from multiple predetermined bony landmarks on the glenoid and coracoid surfaces (Fig. 2).15,18) Successful registration allows the navigation system to display actual 2-dimensional and 3D CT images of the patient’s glenoid on the computer monitor.15) An additional tracker that corresponds with the computer monitor is mounted onto the shaft of the power instruments that are used for drilling and reaming (Fig. 3).15,20) This tracker allows for the positional information of the tools, with respect to glenoid version and inclination, depth of reaming, and direction of screw insertion, to be captured and displayed on the screen.15) The navigation system guides all stages, including reaming (Fig. 3), central cage or screw drilling (Fig. 4), baseplate insertion and rotation (Fig. 5), and peripheral peg or screw drilling (Fig. 6).18) Thus, every step in the placement of the glenoid baseplate and central and peripheral screws can be visualized in real-time, with accurate feedback on their trajectory in coronal and axial CT slices in comparison to the trajectory determined during the preoperative plan. Intraoperative navigation can allow the surgeon to maximize backside contact and fixation in the bone while preserving glenoid bone stock.18) Once the procedure is completed, an intraoperative navigation report documenting the position, axis, and depth of all the utilized surgical tools is generated.15)
Fig. 2. A tracker is affixed to the coracoid with 2 pins. Registration of the glenoid then takes place with a stylus.
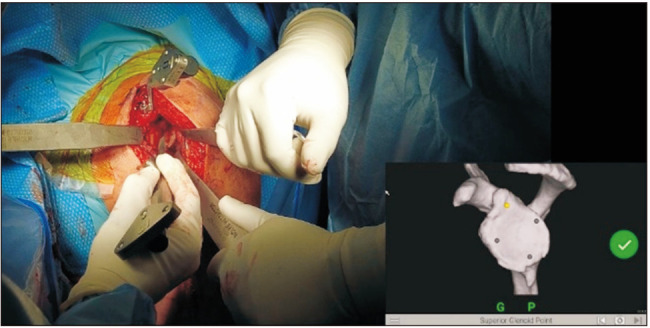
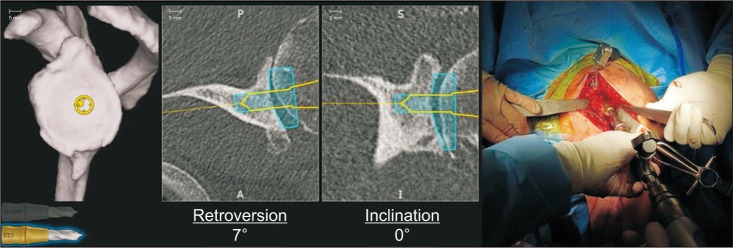
Fig. 3. An additional tracker is mounted onto the shaft of power instruments. The glenoid is then reamed and guided by the preoperative plan to the appropriate orientation and depth.
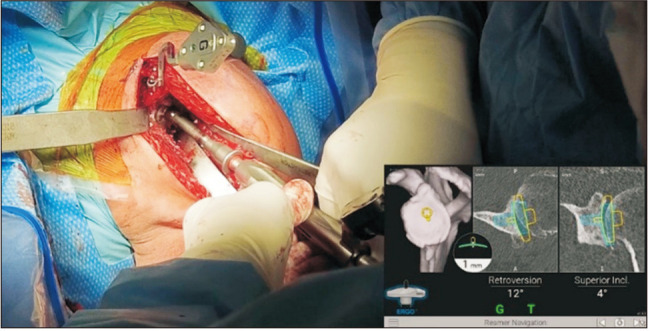
Fig. 4. The central cage is then reamed, which will set the version and inclination of the final implant.
Fig. 5. The placement of the baseplate is also navigated to ensure adequate orientation and rotation.
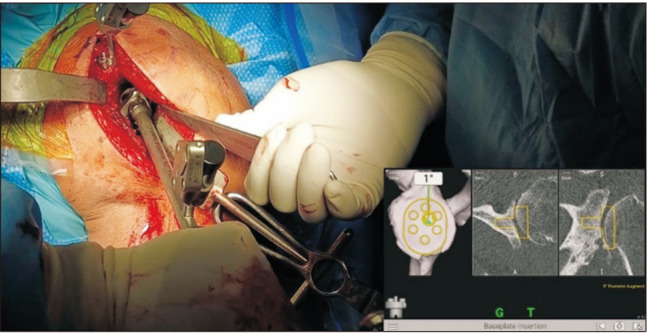
Fig. 6. Once the baseplate is impacted into the glenoid, the screws are then drilled and placed. Navigation is used to ensure good fixation into the native glenoid bone and to avoid placement into the spinoglenoid notch or base of the scapular spine.
INTRAOPERATIVE ACCURACY
The implementation of intraoperative navigation systems in RSA has demonstrated favorable outcomes in improving implantation precision and accuracy.14,15,21,22,23,24) In a recent retrospective review of 16,723 RSAs and anatomic total shoulder arthroplasties (aTSA), Larose et al.15) showed high intraoperative accuracy and precision in the execution of the preoperative plan. The authors found only a minimal deviation in the final intraoperative glenoid implant position relative to the position determined preoperatively, with respect to version (0.6° ± 1.96°), inclination (0.2° ± 2.04°), and distance from starting point (1.90 ± 1.21 mm).15)
The use of intraoperative navigation in RSA has also shown to enhance the positioning of the glenoid component when compared to traditional RSA. One retrospective study by Sasaki et al.21) compared 2 groups of 25 patients who underwent RSA with or without the use of intraoperative navigation. The authors sought to assess the accuracy and precision of component placement, with the intended position being neutral on the glenoid with a 10 degree inferior tilt.21) One-year postoperative CT scans revealed a significantly greater inclination error in the control group (18.3°) compared to the navigation group (4.9°), and while the version error was also greater in the control group (7.3°) relative to the navigation group (5.6°), this difference did not reach statistical significance.21) Another study by Kida et al.23) explored 64 patients who underwent RSA with or without navigation systems and found a significant difference in baseplate alignment between the 2 groups. The conventional RSA group had 1° of retroversion and 2.4° of superior inclination, compared to 0.2° of retroversion and 0.3° of superior inclination in the navigated RSA group, respectively.23) The authors concluded that navigated RSAs provide more precision in baseplate version and inclination when compared to conventional RSAs.23) Finally, Burns et al.14) conducted a systematic review and meta-analysis to explore the utility of surgical navigation systems in the setting of total shoulder arthroplasty. The authors reported that the use of navigation systems led to improved glenoid positioning outcomes when compared to the use of standard instrumentation.14)
SCREW SELECTION
The integration of intraoperative navigation has been shown to enhance the efficacy of screw selection in terms of number and length for RSA. In a retrospective cohort study, Hones et al.25) conducted a comparative analysis of screw selection between 100 RSAs utilizing computer navigation and 100 RSAs without navigation. The authors demonstrated that computer navigation in RSA led to significantly fewer screws being used per case (3.4 vs. 4.1) and a significantly greater average screw length (35.0 mm vs. 32.6 mm).25) The authors reported that a significantly higher proportion of computer navigation cases implanted only 3 screws (61% compared to 1% in non-navigated cases) and used screws > 30 mm in length (84.6% compared to 73.7% in non-navigated cases).25)
A recent systematic review, encompassing 6 trials with a total of 633 RSAs (329 using navigation and 334 using standard instrumentation), was performed to evaluate the effect of intraoperative navigation on the parameters of screw length and number for primary baseplate fixation.16) The authors reported a statistically significant pooled mean difference in screw purchase length (SPL) of 5.839 mm, in favor of navigation.16) The navigation group was also found to use a mean 0.547 fewer screw per case, with a threefold greater proportion of cases fixed with only 2 screws, compared to the standard group.16)
Moreover, Nashikkar et al.26) assessed SPL, screw angulation, and central cage perforation in 27 navigated and 23 standard RSAs using multiplanar CT scans. They reported significantly increased median SPL for anterior (20 mm vs. 15 mm) and posterior (20 mm vs. 13 mm) screws in favor of navigation, with a lower incidence of inadequate screw purchase for the anterior (64.7% vs. 95.2%) and posterior (70.6% vs. 100%) screws as well.26) Both axial and coronal screw angulations were found to differ significantly. Axial screw angulation was significantly more posterior in the navigation group on average for anterior screw (2° vs. −10°) and inferior screw (−6° vs. 0°), and coronal screw angulation was significantly inferiorized for the superior screw (−2° vs. 3°) and superiorized for the posterior screw (2° vs. −4°), in comparison to the standard RSA group.26) The navigation group also had a significantly reduced incidence of central cage perforation (17.7% vs. 52.4%).26)
Intraoperative navigation appears to improve baseplate screw placement in RSA, allowing the use of fewer screws with greater purchase length to achieve primary fixation of the glenoid component. Utilizing fewer screws may conserve glenoid bone stock, prevent the introduction of additional glenoid stress risers, and potentially decrease operative time and cost.16,25) Navigation can provide certainty about the screw trajectory and the ability to achieve greater length safely, improving surgeon confidence in fixation with fewer implants as well.16) However, studies with longer follow-up are needed to assess whether these findings on screw selection will in fact increase the longevity of the prosthesis or influence the clinical outcomes of patients.16)
CLINICAL OUTCOMES
Early clinical studies have demonstrated favorable outcomes and minimal complications with the use of intraoperative navigation in RSA. Holzgrefe et al.17) performed a retrospective cohort study comparing clinical outcomes in 113 patients who underwent navigated primary RSA to 113 patients who underwent non-navigated RSA with a mean follow-up of 32.8 months (minimum 24 months). The authors found that patients who underwent RSA with navigation had significantly better active forward elevation (135° vs. 129°), active external rotation (39° vs. 32°), and Constant scores (71.1 vs. 65.5) at final postoperative follow-up, although both groups had similar rates of improvement in ROM and functional scores.17) While the difference did not reach clinical significance, the navigated group was found to have lower rates of complication (1.8% vs. 5.3%), scapular notching (3.1% vs. 8.0%), and revision (0.9% vs. 3.5%).17)
Similarly, Youderian et al.18) conducted a comparative analysis of clinical outcomes between 533 RSAs with intraoperative navigation and a 2:1 age- and sex-matched cohort of RSAs without intraoperative navigation, with a minimum 2-year follow-up (average, 30.6 months). The navigated RSA group demonstrated significant improvements compared to the non-navigated group in internal rotation, external rotation, maximum lifting weight, the Simple Shoulder Test (SST), Constant score, and Shoulder Arthroplasty Smart (SAS) score.18) The navigated RSA group also showed an absolute risk reduction of 1.7% for postoperative complications and 0.7% for dislocations, although no difference was found in glenoid implant loosening, acromial stress fracture, scapular notching, or revision.18) The authors also performed a sub-analysis of the effect of retroversion and version correction on clinical outcomes of navigated RSA patients.18) While no significant difference was found in outcome scores in patients with greater or less than 10° of retroversion, higher degrees of version correction (> 15°) exhibited improvement in forward elevation, internal rotation, pain, SST, Constant, SAS, American Shoulder and Elbow Surgeons (ASES), University of California-Los Angeles (UCLA), and Shoulder Pain and Disability Index (SPADI) scores.18) A higher percentage of augmented glenoid usage was found when using navigation (72.5%) compared to non-navigated RSA (26.3%); the authors attribute this increase to the increased utilization of preoperative planning.18)
In contrast to the 2 previous studies, Gaj et al.27) reported no statistically significant differences in ROM, satisfaction, SST, ASES, Disabilities of Arm, Shoulder and Hand, and visual analog scale scores between patients receiving intraoperative navigation and standard RSA at mean follow-up of 16 months. However, the study may be limited by its small sample size, comparing only 16 RSAs with navigation to 17 standard RSAs, as well as its shorter follow-up period. The authors did also show that patients from the standard RSA group had significant differences between planned and postoperative inclination, while patients with navigation did not.27) Another case-control study assessing 2 groups of 25 patients who underwent RSA with or without navigation found no significant difference in bleeding and reported no complications related to navigation.21)
While the current available literature on the clinical outcomes of intraoperative navigation is relatively limited, early reports are encouraging and suggest that intraoperative navigation improves patient clinical outcomes and may decrease complications. Nevertheless, longer-term outcomes are required to support the clinical benefit of navigation.
LEARNING CURVE AND OPERATIVE TIME
With the implementation of any new technology, like intraoperative navigation for shoulder arthroplasty, surgeons must consider the learning curve, both for themselves and for the surgical team. The addition of intraoperative navigation to shoulder arthroplasty necessitates a larger surgical incision to expose the coracoid process and anterior glenoid neck for tracker placement and proper anatomic landmark registration, respectively. After surgical exposure and tracker placement, the surgeon probes anatomic landmarks, which synchronize with preoperative imaging to provide intraoperative navigation.26,28) These additional steps may extend the duration of surgery. Wang et al.29) conducted a study of 23 RSAs performed with navigation and reported a downward trend in operative time during the first 8 cases performed, after which a plateau was observed. This suggests a relatively short learning curve for the use of navigation in shoulder arthroplasty, which could be feasibly attained by any surgeon who performs RSA with regularity.
Kircher et al.30) initially reported an increase of approximately 30 minutes with the use of navigation in aTSA; however, their study only included 10 patients and thus scarcely surpassed the learning curve described by Wang et al.29) Subsequent studies have consistently reported lower increases in surgical time with navigation.15,18,20,27,31) Youderian et al.18) evaluated 749 patients undergoing shoulder arthroplasty with navigation, of which 533 were RSAs, and found that the surgical time increased by a mean of 10.4 minutes, comparing individual surgeons’ data with their own non-navigated cases. The authors hypothesized that the operative time may actually reduce with navigation due to the fact that it may allow surgeons to have greater confidence in their baseplate fixation.18) Furthermore, Larose et al.15) showed that the average number of registration attempts performed by a surgeon during the navigation acquisition steps decreased exponentially as more cases were performed. The quality of the registrations was also shown to improve with increased experience, with the percentage of “perfect” registration points improving until reaching a plateau at around 130 cases.15)
CHALLENGES AND CONSIDERATIONS
The adoption of intraoperative navigation systems in shoulder arthroplasty is not without its challenges and limitations. A major barrier to the institutional implementation and use of intraoperative navigation is the cost, and research on its economic cost-effectiveness is lacking in the literature. When considering the use of navigation in shoulder arthroplasty, surgeons should take into account patient-specific factors that may alter its utility. The most common form of navigation employs a tracker fixed to the coracoid process, which communicates with a receiver outside of the surgical field. In certain cases, the coracoid may not be a reliable landmark, such as in those with prior trauma to the area or those who have undergone Latarjet or other coracoid transfer procedures. The bone quality of the coracoid process also warrants consideration. Multiple studies have documented rare instances of intraoperative tracker loosening or coracoid fracture during tracker placement, both of which are more frequently observed in osteopenic patients.15,18,28,29) Larose et al.15) reported the incidence of coracoid fractures to be 0.05% (9/16,723), with 7 cases occurring intraoperatively. As navigation is highly dependent on the appropriate positioning and fixation of the tracker, surgeons may need to continue the procedure non-navigated in these rare situations.
While the use of intraoperative navigation may help surgeons to be more precise and accurate in the placement of implants according to the preoperative plan, surgeons are still confronted with decisions regarding glenoid placement that require careful attention.18) These include factors such as the extent of bone removal during corrective reaming, the optimal positioning of the baseplate on the glenoid, the percentage of backside contact, and baseplate version and inclination.18) Surgeons must also make decisions on the size of the glenosphere and humeral stem, which can strongly impact patient outcomes. Moreover, accurate placement and version of the humeral component, which is essential for stability, deltoid efficiency, and joint loading,32) remains an area where intraoperative navigation systems have yet to be implemented in clinical practice.
AUGMENTED REALITY
Augmented reality (AR) is a novel addition to the repertoire of navigation tools in RSA. AR creates an interactive experience whereby a 3D virtual model of the glenoid is displayed overlaying the surgical environment through smart glasses.33) The aim of creating this virtual model is to provide guidance in both preoperative planning and intraoperative stages as well as improve the accuracy of glenoid component placement, which can be limited by the challenging field of view and the surgeon’s experience.33)
Several studies have evaluated the use of AR in RSA. Kriechling et al.34) performed a cadaveric study to assess the accuracy of guidewire placement in RSA baseplate positioning using AR 3D scapula images superimposed over the cadaver’s shoulder. The mean deviations of the entry point and trajectory were found to be 3.5 mm ± 1.7 mm and 3.8° ± 1.7°, respectively, showing a high accuracy.34) By virtually exposing the scapula and the entire glenoid, this technology may assist surgeons performing RSA and help improve glenoid component positioning. Similar studies have reported lower error margins for entry point, such as 2.4 ± 0.7 mm and 2.3 ± 1.1 mm, as well as for trajectory, such as 2.7° ± 1.3° and 3.9° ± 2.4°.35,36) It is important to note that these studies implemented markerless. AR. Rojas et al.37) evaluated marker-based AR and demonstrated even lower error margins for distance and angular measurements of 0.6 mm ± 0.5 mm and 0.6° ± 0.4°, respectively. This may suggest that the use of markers can further enhance the accuracy and robustness of the obtained model. AR holds potential for diverse applications as well, such as recreating the pre-morbid glenoid to determine the necessity of a graft or the choice of implant, glenoid reaming following K-wire placement, or placing the glenosphere or an augmented glenoid baseplate.38,39)
Although this technology may improve accuracy, it is not without its restraints. These limitations include the cost-effectiveness of AR and the inherent limitations of smart glasses, which were not originally designed for surgical applications.33,40) Another shortcoming experienced by Schlueter-Brust et al.35) was the importance of minimizing head-movements while placing the Kirschner wire to optimize superimposition accuracy, which was uncomfortable to the operating surgeon. While this can be improved by using tracker-based AR,33,37) the use of trackers has its own pitfalls. These include additional costs, theoretical risk of tracker-induced fractures, and transmission of false information if the tracker position changes intraoperatively due to loose placement.37,39) While an exciting and innovative technology, further research is needed to properly assess the utility of AR and how it compares to standard instrumentation.
ROBOTIC-ASSISTED SURGERY
Advancements in shoulder arthroplasty have brought patient-specific instrumentation and computer navigation to the field. However, unlike in hip and knee arthroplasty where the utility of robotic technology has been demonstrated to assist in joint replacement surgery,41,42,43) robot-assisted surgery has not achieved widespread adoption in shoulder arthroplasty.
The literature on applications of robotic-assisted surgery in the shoulder is limited. Darwood et al.44) developed a robotic platform with bespoke software designed for intraoperative production of patient-specific guides for precise guide pin placement in the glenoid. After the initial exposure of the glenoid, the surgeon creates a mold to capture the anatomical structure. The mold is then inserted into the robot and optically scanned for the anatomic surface to be registered in correlation with the preoperative CT scan. The robot proceeds to drill a hole on the solidified mold, which is then placed back onto the patient’s glenoid. This pilot hole serves as a patient-specific guide for the precise placement of the initial guide pin. In their study involving 24 cadavers, the average positional error of the guide pin was 1.13–1.19 mm, with a mean angular deviation error of 1.85°–2.16°.44) The authors reported that the entire guide production time averaged less than 4 minutes.44) Although this technology addresses the human error implicit in glenoid component placement, it does not employ the type of robotic assistance seen in hip and knee arthroplasty.
Smith et al.45) developed a robotic system that employs on force-space navigation, complete with an attached burring tool designed to map and prepare the glenoid for aTSA. They compared the performance of the robotic system against that of a single surgeon in glenoid preparation for both standard and augmented components, using Sawbone model glenoids. Following glenoid preparation, all components were placed and cemented by the surgeon. When preparing the glenoid model for a standard component, the robotic system outperformed the surgeon in both medial-lateral positioning and face rotation, while the surgeon performed better in superior-inferior positioning.45) For augmented glenoid components, the robot yielded better medial-lateral positioning of the glenoid component compared to the surgeon.45) This study serves as a compelling proof of concept, showcasing the potential for future success of robotic-assisted shoulder arthroplasty.
While rare in the scientific literature, Han et al.46) reported on a case where an RSA was performed using a 3D image-guided robotic arm (TiRobot II) in China. It is important to note that this robotic arm is only capable of glenoid position planning and does not assist in glenoid reaming or the humeral osteotomy. The operation was completed in 2 hours, with the authors reporting satisfactory postoperative radiographs and ROM.46) However, the authors did not comment on the indication for using the robotic arm in this patient’s particular case, nor did they report long-term outcome data for the patient.46)
CONCLUSION
The application of intraoperative navigation in RSA represents a promising advancement in the field. Navigation can provide real-time feedback to the surgeon during implantation and enhance the accuracy and precision of the position of the glenoid component. Its utilization improves baseplate screw placement with fewer screws used and greater purchase length achieved. While a novel technology, early clinical studies have been highly encouraging showing significantly improved patient-reported and clinical outcomes and decreased complications, as well as an acceptable learning curve and operative time. Nevertheless, further research with longer-term outcomes is necessary to substantiate the clinical benefit of navigation, evaluate its impact on the longevity and survival of implants, and assess its cost-effectiveness. The future of shoulder arthroplasty holds potential and is anticipated to continue to grow with the addition of emerging technologies like AR and robotic-assisted surgery.
Footnotes
CONFLICT OF INTEREST: Joseph A. Abboud would like to disclose royalties from a company or supplier, DJO Global, Zimmer-Biomet, Smith and Nephew, Stryker, Globus Medical, Inc.; research support from a company or supplier as a PI from Lima Corporation-Italy, Orthofix, Arthrex, OREF; royalties, financial or material support from Wolters Kluwer; and board member/committee appointments for a society, American Shoulder and Elbow Society, Pacira.
Lawrence V. Gulotta would like to disclose royalties from a company or supplier, Exactech, Inc.; personal fees from a company or supplier, Zimmer-Biomet, Smith and Nephew, Inc.; stock or share ownership, Imagen, Responsive Arthroscopy, Inc.
No other potential conflict of interest relevant to this article was reported.
References
- 1.Wagner ER, Farley KX, Higgins I, Wilson JM, Daly CA, Gottschalk MB. The incidence of shoulder arthroplasty: rise and future projections compared with hip and knee arthroplasty. J Shoulder Elbow Surg. 2020;29(12):2601–2609. doi: 10.1016/j.jse.2020.03.049. [DOI] [PubMed] [Google Scholar]
- 2.Galvin JW, Kim R, Ment A, et al. Outcomes and complications of primary reverse shoulder arthroplasty with minimum of 2 years’ follow-up: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2022;31(11):e534–e544. doi: 10.1016/j.jse.2022.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Heifner JJ, Kumar AD, Wagner ER. Glenohumeral osteoarthritis with intact rotator cuff treated with reverse shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2021;30(12):2895–2903. doi: 10.1016/j.jse.2021.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Verborgt O, Vanhees M, Heylen S, Hardy P, Declercq G, Bicknell R. Computer navigation and patient-specific instrumentation in shoulder arthroplasty. Sports Med Arthrosc Rev. 2014;22(4):e42–e49. doi: 10.1097/JSA.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 5.Boileau P, Gauci MO, Wagner ER, et al. The reverse shoulder arthroplasty angle: a new measurement of glenoid inclination for reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2019;28(7):1281–1290. doi: 10.1016/j.jse.2018.11.074. [DOI] [PubMed] [Google Scholar]
- 6.Codsi MJ, Iannotti JP. The effect of screw position on the initial fixation of a reverse total shoulder prosthesis in a glenoid with a cavitary bone defect. J Shoulder Elbow Surg. 2008;17(3):479–486. doi: 10.1016/j.jse.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Collotte P, Bercik M, Vieira TD, Walch G. Long-term reverse total shoulder arthroplasty outcomes: the effect of the inferior shifting of glenoid component fixation. Clin Orthop Surg. 2021;13(4):505–512. doi: 10.4055/cios20245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res. 2011;469(9):2512–2520. doi: 10.1007/s11999-010-1695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chebli C, Huber P, Watling J, Bertelsen A, Bicknell RT, Matsen F., 3rd Factors affecting fixation of the glenoid component of a reverse total shoulder prothesis. J Shoulder Elbow Surg. 2008;17(2):323–327. doi: 10.1016/j.jse.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Singh V, Realyvasquez J, Simcox T, Rozell JC, Schwarzkopf R, Davidovitch RI. Robotics versus navigation versus conventional total hip arthroplasty: does the use of technology yield superior outcomes? J Arthroplasty. 2021;36(8):2801–2807. doi: 10.1016/j.arth.2021.02.074. [DOI] [PubMed] [Google Scholar]
- 11.Snijders T, van Gaalen SM, de Gast A. Precision and accuracy of imageless navigation versus freehand implantation of total hip arthroplasty: a systematic review and meta-analysis. Int J Med Robot. 2017;13(4):e1843. doi: 10.1002/rcs.1843. [DOI] [PubMed] [Google Scholar]
- 12.van der List JP, Chawla H, Joskowicz L, Pearle AD. Current state of computer navigation and robotics in unicompartmental and total knee arthroplasty: a systematic review with meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2016;24(11):3482–3495. doi: 10.1007/s00167-016-4305-9. [DOI] [PubMed] [Google Scholar]
- 13.Matziolis G, Krocker D, Weiss U, Tohtz S, Perka C. A prospective, randomized study of computer-assisted and conventional total knee arthroplasty: three-dimensional evaluation of implant alignment and rotation. J Bone Joint Surg Am. 2007;89(2):236–243. doi: 10.2106/JBJS.F.00386. [DOI] [PubMed] [Google Scholar]
- 14.Burns DM, Frank T, Whyne CM, Henry PD. Glenoid component positioning and guidance techniques in anatomic and reverse total shoulder arthroplasty: a systematic review and meta-analysis. Shoulder Elbow. 2019;11(2 Suppl):16–28. doi: 10.1177/1758573218806252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larose G, Greene AT, Jung A, et al. High intraoperative accuracy and low complication rate of computer-assisted navigation of the glenoid in total shoulder arthroplasty. J Shoulder Elbow Surg. 2023;32(6S):S39–S45. doi: 10.1016/j.jse.2022.12.021. [DOI] [PubMed] [Google Scholar]
- 16.Velasquez Garcia A, Abdo G. Does computer-assisted navigation improve baseplate screw configuration in reverse shoulder arthroplasty?: a systematic review and meta-analysis of comparative studies. J Orthop. 2022;36:29–35. doi: 10.1016/j.jor.2022.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holzgrefe RE, Hao KA, Panther EJ, et al. Early clinical outcomes following navigation-assisted baseplate fixation in reverse total shoulder arthroplasty: a matched cohort study. J Shoulder Elbow Surg. 2023;32(2):302–309. doi: 10.1016/j.jse.2022.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Youderian AR, Greene AT, Polakovic SV, et al. Two-year clinical outcomes and complication rates in anatomic and reverse shoulder arthroplasty implanted with Exactech GPS intraoperative navigation. J Shoulder Elbow Surg. 2023;32(12):2519–2532. doi: 10.1016/j.jse.2023.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Moreschini F, Colasanti GB, Cataldi C, Mannelli L, Mondanelli N, Giannotti S. Pre-operative CT-based planning integrated with intra-operative navigation in reverse shoulder arthroplasty: data acquisition and analysis protocol, and preliminary results of navigated versus conventional surgery. Dose Response. 2020;18(4):1559325820970832. doi: 10.1177/1559325820970832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sprowls GR, Wilson CD, Stewart W, et al. Intraoperative navigation and preoperative templating software are associated with increased glenoid baseplate screw length and use of augmented baseplates in reverse total shoulder arthroplasty. JSES Int. 2020;5(1):102–108. doi: 10.1016/j.jseint.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasaki Y, Ochiai N, Kotani T, et al. Clinical application of intraoperative O-arm navigation in reverse shoulder arthroplasty. J Orthop Sci. 2020;25(5):836–842. doi: 10.1016/j.jos.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Leafblad N, Asghar E, Tashjian RZ. Innovations in shoulder arthroplasty. J Clin Med. 2022;11(10):2799. doi: 10.3390/jcm11102799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kida H, Urita A, Momma D, et al. Implications of navigation system use for glenoid component placement in reverse shoulder arthroplasty. Sci Rep. 2022;12(1):21190. doi: 10.1038/s41598-022-25833-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarallo L, Giorgini A, Micheloni G, Montanari M, Porcellini G, Catani F. Navigation in reverse shoulder arthroplasty: how the lateralization of glenosphere can affect the clinical outcome. Arch Orthop Trauma Surg. 2023;143(9):5649–5656. doi: 10.1007/s00402-023-04879-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hones KM, King JJ, Schoch BS, Struk AM, Farmer KW, Wright TW. The in vivo impact of computer navigation on screw number and length in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2021;30(10):e629–e635. doi: 10.1016/j.jse.2021.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Nashikkar PS, Scholes CJ, Haber MD. Role of intraoperative navigation in the fixation of the glenoid component in reverse total shoulder arthroplasty: a clinical case-control study. J Shoulder Elbow Surg. 2019;28(9):1685–1691. doi: 10.1016/j.jse.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Gaj E, Pagnotta SM, Berlinberg EJ, et al. Intraoperative navigation system use increases accuracy of glenoid component inclination but not functional outcomes in reverse total shoulder arthroplasty: a prospective comparative study. Arch Orthop Trauma Surg. 2024;144(1):91–102. doi: 10.1007/s00402-023-05038-y. [DOI] [PubMed] [Google Scholar]
- 28.Nashikkar PS, Scholes CJ, Haber MD. Computer navigation re-creates planned glenoid placement and reduces correction variability in total shoulder arthroplasty: an in vivo case-control study. J Shoulder Elbow Surg. 2019;28(12):e398–e409. doi: 10.1016/j.jse.2019.04.037. [DOI] [PubMed] [Google Scholar]
- 29.Wang AW, Hayes A, Gibbons R, Mackie KE. Computer navigation of the glenoid component in reverse total shoulder arthroplasty: a clinical trial to evaluate the learning curve. J Shoulder Elbow Surg. 2020;29(3):617–623. doi: 10.1016/j.jse.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Kircher J, Wiedemann M, Magosch P, Lichtenberg S, Habermeyer P. Improved accuracy of glenoid positioning in total shoulder arthroplasty with intraoperative navigation: a prospective-randomized clinical study. J Shoulder Elbow Surg. 2009;18(4):515–520. doi: 10.1016/j.jse.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Rosenthal Y, Rettig SA, Virk MS, Zuckerman JD. Impact of preoperative 3-dimensional planning and intraoperative navigation of shoulder arthroplasty on implant selection and operative time: a single surgeon’s experience. J Shoulder Elbow Surg. 2020;29(12):2564–2570. doi: 10.1016/j.jse.2020.03.041. [DOI] [PubMed] [Google Scholar]
- 32.Eng K, Eyre-Brook A, Shields DW. A systematic review of the utility of intraoperative navigation during total shoulder arthroplasty. Cureus. 2022;14(12):e33087. doi: 10.7759/cureus.33087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daher M, Ghanimeh J, Otayek J, Ghoul A, Bizdikian AJ, El Abiad R. Augmented reality and shoulder replacement: a state-of-the-art review article. JSES Rev Rep Tech. 2023;3(3):274–278. doi: 10.1016/j.xrrt.2023.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kriechling P, Loucas R, Loucas M, Casari F, Furnstahl P, Wieser K. Augmented reality through head-mounted display for navigation of baseplate component placement in reverse total shoulder arthroplasty: a cadaveric study. Arch Orthop Trauma Surg. 2023;143(1):169–175. doi: 10.1007/s00402-021-04025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlueter-Brust K, Henckel J, Katinakis F, et al. Augmented-reality-assisted K-wire placement for glenoid component positioning in reversed shoulder arthroplasty: a proof-of-concept study. J Pers Med. 2021;11(8):777. doi: 10.3390/jpm11080777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kriechling P, Roner S, Liebmann F, Casari F, Fürnstahl P, Wieser K. Augmented reality for base plate component placement in reverse total shoulder arthroplasty: a feasibility study. Arch Orthop Trauma Surg. 2021;141(9):1447–1453. doi: 10.1007/s00402-020-03542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rojas JT, Jost B, Zipeto C, Budassi P, Zumstein MA. Glenoid component placement in reverse shoulder arthroplasty assisted with augmented reality through a head-mounted display leads to low deviation between planned and postoperative parameters. J Shoulder Elbow Surg. 2023;32(12):e587–e596. doi: 10.1016/j.jse.2023.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Berhouet J, Gulotta LV, Dines DM, et al. Preoperative planning for accurate glenoid component positioning in reverse shoulder arthroplasty. Orthop Traumatol Surg Res. 2017;103(3):407–413. doi: 10.1016/j.otsr.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 39.Rojas JT, Ladermann A, Ho SW, Rashid MS, Zumstein MA. Glenoid component placement assisted by augmented reality through a head-mounted display during reverse shoulder arthroplasty. Arthrosc Tech. 2022;11(5):e863–e874. doi: 10.1016/j.eats.2021.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong J, Min SW, Lee B. Integral floating display systems for augmented reality. Appl Opt. 2012;51(18):4201–4209. doi: 10.1364/AO.51.004201. [DOI] [PubMed] [Google Scholar]
- 41.Khlopas A, Sodhi N, Sultan AA, Chughtai M, Molloy RM, Mont MA. Robotic arm-assisted total knee arthroplasty. J Arthroplasty. 2018;33(7):2002–2006. doi: 10.1016/j.arth.2018.01.060. [DOI] [PubMed] [Google Scholar]
- 42.Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31(10):2353–2363. doi: 10.1016/j.arth.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 43.Liow MH, Xia Z, Wong MK, Tay KJ, Yeo SJ, Chin PL. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis: a prospective randomised study. J Arthroplasty. 2014;29(12):2373–2377. doi: 10.1016/j.arth.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Darwood A, Hurst SA, Villatte G, et al. Novel robotic technology for the rapid intraoperative manufacture of patient-specific instrumentation allowing for improved glenoid component accuracy in shoulder arthroplasty: a cadaveric study. J Shoulder Elbow Surg. 2022;31(3):561–570. doi: 10.1016/j.jse.2021.08.035. [DOI] [PubMed] [Google Scholar]
- 45.Smith CD, Athwal GS, Ferreira LM. Early experience with force-space navigated robotics for glenoid implantation during total shoulder arthroplasty. Ann Robot Autom. 2021;5(1):1–10. [Google Scholar]
- 46.Han W, Jia Z, Zhang T, Wang J, Shen A, Jiang X. A case report of the first successful use of 3D image-guided robot-assisted reverse shoulder arthroplasty. Intell Surg. 2023;6:50–53. [Google Scholar]