Abstract
Human papillomavirus type 16 (HPV-16) E6 activates telomerase specifically in epithelial cells. The oncogene c-myc has also been shown to activate telomerase in several cell types. Here we show that while both HPV-16 E6 and c-myc require intact E boxes to transactivate the hTERT promoter, E6 does not induce hTERT transcription simply by inducing expression of c-myc. Moreover, hTERT transactivation by HPV-16 E6 correlates with its ability to bind the cellular E6-associated protein (E6AP), suggesting that E6 and E6AP may target a regulator of hTERT expression.
Activation of telomerase is a critical step in cellular transformation (7, 12). Telomerase activity is primarily regulated at the level of expression of the hTERT gene, encoding the catalytic subunit of telomerase (4, 24, 26, 33). Ectopic expression of hTERT in a number of different telomerase-negative cell types has been shown to confer immortality (2, 4, 18, 30). Therefore, much research is now focused on determining the transcriptional regulators of hTERT.
The hTERT promoter contains a number of putative transcription factor binding sites. Several studies have defined the minimal core promoter as the proximal 200 bp upstream of the transcription start site (15, 29). This core promoter contains numerous SP1 binding sites and two canonical E boxes (Myc-Max binding sites) (3, 15, 29, 34). Previous in vitro studies have shown that Myc-Max heterodimers can bind these E boxes in the context of the hTERT promoter and can activate hTERT reporter constructs (15, 29, 35). Myc expression has also been shown to induce telomerase activity in post M0 human mammary epithelial cells (HMECs), the fibroblast lines IMR90 and WI38 (32), and Epstein-Barr virus-immortalized B cells (35). These studies implicate c-Myc as an important transactivator of hTERT.
The human papillomavirus type 16 (HPV-16) E6 oncoprotein can also induce telomerase expression, specifically in epithelial cell types (20). Expression of HPV-16 E6 in either human foreskin keratinocytes (HFKs) or HMECs induces telomerase activity. Another well-established function of HPV-16 E6 is its association with the cellular E6-associated protein (E6AP) to form a ubiquitin protein ligase that specifically targets p53 for degradation (16, 17, 28). The HPV-16 E6-8S/9A/10T mutant is defective in p53 degradation yet retains the ability to activate telomerase, demonstrating that these two functions of E6 are separate and distinct(20). Expression of HPV-16 E6 does not induce telomerase in human foreskin fibroblasts (20) or in IMR90 cells (32). It has been suggested that a cell-type-specific ability of HPV-16 E6 to induce c-myc expression is responsible for this differential telomerase activation (35). In this study, we show that upregulation of c-myc does not directly correlate with telomerase activation, indicating that other regulators of hTERT expression are also involved. We also demonstrate that activation of telomerase by HPV-16 E6 does not require upregulation of c-myc, yet intact E boxes in the hTERT promoter are required for HPV-16 E6-mediated transactivation. In addition, the ability of E6 to bind its cellular partner E6AP appears to be important for the induction of hTERT.
Expression levels of c-myc do not correlate with telomerase activity.
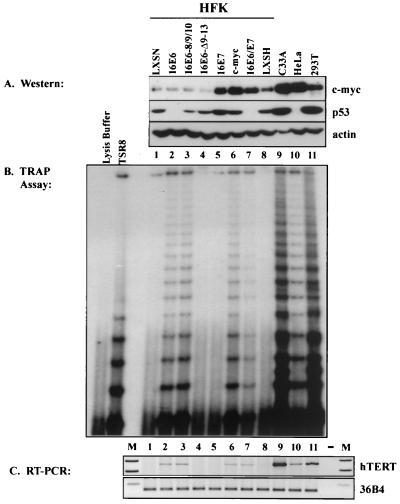
To address the mechanism by which HPV-16 E6 activates telomerase in HFKs, we transduced HFKs with retroviruses encoding HPV-16 E6, E6-8S/9A/10T, and E6-Δ9-13 (5, 8, 25). In addition, the HPV-16 oncogene E7, E6-E7 (13), c-myc (provided by R. Eisenman), and vector controls (LXSN and LXSH) were transduced into HFKs. After selection in 50 μg of G418 (GIBCO BRL)/ml or 8 μg of hygromycin B (Roche)/ml, cells were harvested in parallel for telomeric repeat amplification protocol (TRAP) assay as previously described (18), reverse transcription (RT)-PCR, and Western blotting of nuclear extracts. Nuclear extracts were made as previously described (27), except that the homogenization step was omitted. The transformed cell lines C33A, HeLa, and 293T were also harvested in parallel to serve as positive controls for telomerase activity.
Expression of HPV-16 E6, E6-8S/9A/10T, c-myc, or E6 and E7 activated telomerase, as seen in Fig. 1B. As recently published, E6 activates telomerase by inducing transcription of the telomerase hTERT gene (31) (Fig. 1C). In each case, induction of telomerase activity was directly correlated with expression of hTERT RNA (Fig. 1C). Overexpression of c-myc was sufficient to activate telomerase in HFKs and may be necessary for hTERT induction, as c-Myc protein was detected in all cells with active telomerase. Interestingly, the steady-state levels of c-Myc protein in each of these cell lines did not correlate with expression of hTERT (Fig. 1). Telomerase was activated both with high (lanes 6, 7, 9, 10, and 11) and with low (lanes 2 and 3) levels of c-Myc nuclear protein (Fig. 1A and B). Conversely, no telomerase activation was detected in the HPV-16 E7-expressing cells, which contain high levels of c-Myc (lane 5). Therefore, activation of telomerase by HPV-16 E6 in HFKs is independent of c-Myc induction. It should be noted that c-myc expression is elevated in cells actively proliferating and decreased in cells with lower proliferation rates (14). We have observed slightly elevated levels of c-Myc in HFKs expressing HPV-16 E6 and E6 mutants in some experiments (data not shown). However, in those experiments, LXSN-HFKs had undetectable c-Myc expression. Therefore, we attribute the differences in expression of c-Myc to variation in the proliferation rate rather than to specific induction of c-myc by HPV-16 E6.
FIG. 1.
c-Myc expression levels do not correlate with telomerase activity in HFKs. Cells were transduced with retroviruses to express the indicated genes. (A) Western blots. Nuclear extracts were made and 20 μg of total protein was loaded per lane on an SDS–8% polyacrylamide gel and subsequently transferred to a polyvinylidene difluoride membrane. Blots were probed with c-Myc mouse monoclonal antibody (C-33; Santa Cruz), p53 mouse monoclonal antibody (Ab-6; Calbiochem), and actin goat polyclonal antibody (Santa Cruz). (B) Telomerase activity. TRAP assays were performed using 5 μg of whole-cell extract per lane (17). Lysis buffer was used as a negative control. TSR8 is a synthetic oligonucleotide of eight telomeric repeats (Intergen). (C) hTERT RNA expression. RT-PCR was carried out with RNA isolated from the transduced HFKs. The RT reaction was done using random hexamers (GIBCO) and the Superscript II System (GIBCO) and followed by RNase H treatment. Specific amplification of hTERT and the positive control 36B4 was done using forward primer 5′CGAGCTGCTCAGGTCTTTCTTTTATG3′ and reverse primer 5′CCACGACGTAGTCCATGTTCACAATC3′ for hTERT and forward primer 5′TGCCAGTGTCTGTCTGCAGA3′ and reverse primer 5′ACAAAGGCAGATGGATCAGC3′ for 36B4. The negative control (−) contained no RNA. M, marker lanes.
The induction of c-myc by HPV-16 E7 was not sufficient to activate telomerase. Presumably, E7 induces c-myc by binding retinoblastoma protein, thereby releasing E2F to activate c-myc in a manner similar to that demonstrated for simian virus 40 large T antigen (1, 6). Given the comparable c-Myc expression levels in lanes 5 and 6 of Fig. 1A, it is perplexing that HPV-16 E7 does not induce hTERT. Perhaps E7 inhibits c-myc-mediated telomerase activation. However, as shown with the cells expressing both E6 and E7, E7 did not inhibit E6-mediated telomerase activation. Interestingly, in a previous study, Garbe et al. found that E7 expression promoted telomerase activation (11). Benzo[a]pyrene treatment of HMECs generated mortal extended-life cultures. Subsequent E7 transduction did not immediately activate telomerase, but after 2 to 4 months of culture, these cells had detectable telomerase activity that gradually increased with further passaging (11). This finding suggests an epigenetic mechanism of telomerase activation facilitated by E7 (11). Activation of telomerase by HPV-16 E6, on the other hand, is detectable within the first passage after selection, arguing that E6 directly affects another regulator of hTERT transcription (11, 20).
Both HPV-16 E6 and c-myc require intact E boxes to activate the hTERT promoter.
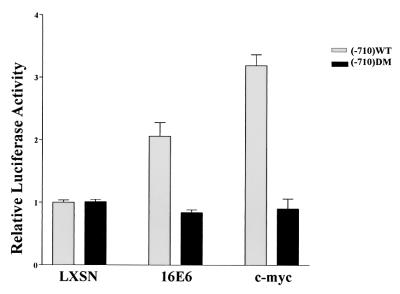
The in vivo expression data suggested that activation of hTERT by HPV-16 E6 was independent of c-Myc protein levels. To address which transcription factor binding sites are required for activation by E6 and c-myc, we performed hTERT reporter luciferase assays in HFKs. First, two pGL3 luciferase reporter constructs were generated. An approximately 800-bp region of the hTERT promoter (from −710 to +76, the translation start site) was cloned into pGL3-Basic (Promega) from pXP2 constructs provided by K.-J. Wu (35). An identical clone was made, with mutations at both proximal E boxes (CACGTG mutated to CACCTG). The constructs were cotransfected with retrovirus expression vectors LXSN, LXSN-E6, and LXSN-c-myc into HFKs using FuGENE6 (Roche). Cells were harvested 24 h after transfection, and lysates were assayed for luciferase activity (Promega) and total protein concentration (Bio-Rad). As previously published, c-myc activated the hTERT promoter approximately threefold and activation is dependent on intact E boxes (29, 35) (Fig. 2). Coexpression of HPV-16 E6 activated the hTERT promoter twofold. In comparison to the in vivo induction of hTERT by E6 seen in Fig. 1, the rather modest transactivation of this fragment of the hTERT promoter by E6 may indicate that the reporter assays do not accurately reflect the endogenous promoter. Nevertheless, E6-mediated activation of the hTERT promoter was completely abolished by point mutations within the E box, indicating that E6-mediated induction of hTERT is also E box dependent.
FIG. 2.
Intact E boxes are required for activation of hTERT by either c-myc or HPV-16 E6. Cells were cotransfected with pGL3-hTERT(−710) WT or DM and the indicated retrovirus construct. Luciferase values were normalized for protein concentration and are graphed relative to the vector control value for each promoter construct. The graph represents four experiments done either in duplicate or in triplicate. DM, double mutant E boxes.
Activation of telomerase by HPV-16 E6 is therefore independent of c-Myc expression levels but dependent on intact E boxes within the hTERT promoter. This finding suggests a few possible mechanisms of hTERT activation by HPV-16 E6. First, E6 may allow c-Myc to have access to the E boxes either by removing a repressor or by altering the local chromatin structure. Second, E6 may alter the expression of cofactor that preferentially targets c-Myc to the hTERT promoter. Both of these mechanisms may be unnecessary when high levels of c-myc are present, as suggested by the c-myc transductions. A third possible mechanism involves another unidentified transcription factor that, in the presence of E6, may transactivate the hTERT promoter independently of c-Myc.
Ability of E6 to activate telomerase correlates with ability to bind E6AP.
Though it has been shown that E6-mediated telomerase activation is clearly independent of p53 degradation (20) (Fig. 1), some studies have suggested that p53 is an inhibitor of telomerase activity (21, 22). We observed no correlation between p53 expression levels and hTERT expression (Fig. 1). Both the c-myc-transduced and the E6-8S/9A/10T-transduced HFKs induced hTERT despite the presence of p53. The E6-8S/9A/10T mutant does not target p53 for degradation yet retains its ability to activate telomerase. Interestingly, a deletion mutant in the same region, E6-Δ9-13, cannot activate telomerase or target p53 for degradation. While the ability to bind and target p53 for degradation strongly correlates with ability to bind E6AP (8, 23), other targets of E6- and E6AP-mediated ubiquitination appear to exist. Other researchers have proposed that the human homolog of the Drosophila discs large tumor suppressor protein (hDLG) (19) and a novel putative GAP protein, E6 (E6-targeted protein 1) (9, 10), are targets of E6 and E6AP.
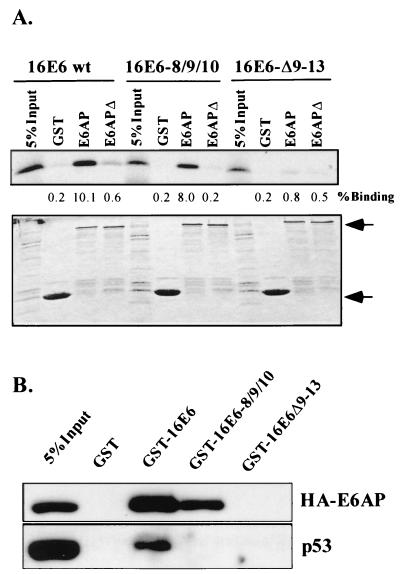
We hypothesize that the ability of E6 to bind E6AP may correlate with its ability to activate telomerase, irrespective of its ability to target p53 for degradation. To address this hypothesis, binding assays were performed to determine whether the E6-8S/9A/10T and E6-Δ9-13 mutants, both having lost the ability to target p53 for degradation, can bind to E6AP. Glutathione S-tranferase (GST)-tagged E6AP proteins were produced and purified as previously described (17). These proteins were incubated with 35S-radiolabeled E6 proteins (TNT kit; Promega) for 2 h in binding buffer (phosphate-buffered saline, 1% NP-40, 2mM dithiothreitol, protease inhibitors) at 4°C. Complexes were precipitated with glutathione-Sepharose (Amersham Pharmacia) and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography. Figure 3A shows that while both wild-type (wt) E6 and E6-8S/9A/10T bind to E6AP, E6-Δ9-13 does not. This interaction was further confirmed by incubating GST-E6 proteins with lysates from HFKs transiently transfected with an LXSN–hemagglutinin (HA)-E6AP construct. Again, both wt E6 and E6-8S/9A/10T interact strongly with HA-E6AP, while E6-Δ9-13 does not (Fig. 3B). This experiment also confirms that though both wt E6 and E6-8S/9A/10T bind E6AP, only wt E6 interacts with p53. Therefore, E6-mediated telomerase activation correlates well with the ability of E6 to bind E6AP. Other investigators have also found that E6 mutants with decreased binding to E6AP inefficiently immortalize epithelial cells (23). In addition, all E6 mutants that bind E6AP and target p53 for degradation can activate telomerase, further suggesting that E6AP binding is an important component of E6-mediated telomerase activation (20). We propose that E6 and E6AP may target a regulator of hTERT transcription.
FIG. 3.
Ability of E6 to activate telomerase correlates with ability to bind E6AP. (A) E6 binding assay. 35S-radiolabeled E6 proteins were incubated with purified GST proteins and precipitated with glutathione-Sepharose. E6APΔ is a mutant with the E6-binding region (amino acids 391 to 408) deleted. Protein complexes were analyzed by SDS-PAGE and autoradiography (upper panel). Binding was subsequently quantitated by phosphorimaging using ImageQuant. Percent binding indicates the average percent binding of three independent experiments. Lower panel is a Coomassie blue-stained gel to show loading of GST proteins. Arrows indicate GST-E6AP proteins and GST. (B) E6AP binding assay. HFKs were transiently transfected with HA-E6AP. Cells were lysed in binding buffer (phosphate-buffered saline, 1% NP-40, 2 mM dithiothreitol, 10% glycerol). Lysates were incubated with purified GST proteins and precipitated with glutathione-Sepharose. Protein complexes were separated by SDS-PAGE, transferred to polyvinylidene difluoride membrane, and Western blotted for HA-E6AP (mouse monoclonal antibody 16B12; Babco) and p53 (mouse monoclonal antibody Ab-6; Calbiochem). Results represent four independent experiments.
Given these data with HFKs, we suggest that while c-Myc is sufficient to activate telomerase, the mechanism by which HPV-16 E6 activates telomerase is not dependent on increasing c-Myc protein levels, either by protein stabilization or by upregulation. Induction of hTERT by E6 is, however, dependent on the presence of intact E boxes in the hTERT proximal promoter. Since the ability of E6 to bind E6AP correlates strongly with the ability to induce hTERT, we are currently examining possible targets of E6 and E6AP for roles in hTERT transcriptional regulation.
Acknowledgments
We thank R. N. Eisenman for the LXSN-c-myc construct, K.-J. Wu for the pXP2-TERT-Luc constructs, and Peter Howley for the GST-E6AP and GST-E6APΔ expression constructs.
This work was supported by a grant from NIH, CA64795, to D.A.G. L.G. is supported in part by a Viral Oncology Training Grant (PHS 5 T32 CA 09229-23).
REFERENCES
- 1.Batsche E, Lipp M, Cremisi C. Transcriptional repression and activation in the same cell type of the human c-MYC promoter by the retinoblastoma gene protein: antagonisation of both effects by SV40 T antigen. Oncogene. 1994;9:2235–2243. [PubMed] [Google Scholar]
- 2.Bodnar A G, Ouellette M, Frolkis M, Holt S E, Chiu C P, Morin G B, Harley C B, Shay J W, Lichtsteiner S, Wright W E. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 3.Cong Y S, Wen J, Bacchetti S. The human telomerase catalytic subunit hTERT: organization of the gene and characterization of the promoter. Hum Mol Genet. 1999;8:137–142. doi: 10.1093/hmg/8.1.137. [DOI] [PubMed] [Google Scholar]
- 4.Counter C M, Hahn W C, Wei W, Caddle S D, Beijersbergen R L, Lansdorp P M, Sedivy J M, Weinberg R A. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc Natl Acad Sci USA. 1998;95:14723–14728. doi: 10.1073/pnas.95.25.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crook T, Tidy J A, Vousden K H. Degradation of p53 can be targeted by HPV E6 sequences distinct from those required for p53 binding and trans-activation. Cell. 1991;67:547–556. doi: 10.1016/0092-8674(91)90529-8. [DOI] [PubMed] [Google Scholar]
- 6.Dyson N, Howley P M, Munger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 7.Elenbaas B, Spirio L, Koerner F, Fleming M D, Zimonjic D B, Donaher J L, Popescu N C, Hahn W C, Weinberg R A. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 2001;15:50–65. doi: 10.1101/gad.828901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster S A, Demers G W, Etscheid B G, Galloway D A. The ability of human papillomavirus E6 proteins to target p53 for degradation in vivo correlates with their ability to abrogate actinomycin D-induced growth arrest. J Virol. 1994;68:5698–5705. doi: 10.1128/jvi.68.9.5698-5705.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Q, Singh L, Kumar A, Srinivasan S, Wazer D E, Band V. Human papillomavirus type 16 E6-induced degradation of E6Tp1 correlates with its ability to immortalize human mammary epithelial cells. J Virol. 2001;75:4459–4466. doi: 10.1128/JVI.75.9.4459-4466.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao Q, Srinivasan S, Boyer S N, Wazer D E, Band V. The E6 oncoproteins of high-risk papillomaviruses bind to a novel putative GAP protein, E6TP1, and target it for degradation. Mol Cell Biol. 1999;19:733–744. doi: 10.1128/mcb.19.1.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garbe J, Wong M, Wigington D, Yaswen P, Stampfer M R. Viral oncogenes accelerate conversion to immortality of cultured conditionally immortal human mammary epithelial cells. Oncogene. 1999;18:2169–2180. doi: 10.1038/sj.onc.1202523. [DOI] [PubMed] [Google Scholar]
- 12.Hahn W C, Counter C M, Lundberg A S, Beijersbergen R L, Brooks M W, Weinberg R A. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 13.Halbert C L, Demers G W, Galloway D A. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J Virol. 1991;65:473–478. doi: 10.1128/jvi.65.1.473-478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henriksson M, Luscher B. Proteins of the Myc network: essential regulators of cell growth and differentiation. Adv Cancer Res. 1996;68:109–182. doi: 10.1016/s0065-230x(08)60353-x. [DOI] [PubMed] [Google Scholar]
- 15.Horikawa I, Cable P L, Afshari C, Barrett J C. Cloning and characterization of the promoter region of human telomerase reverse transcriptase gene. Cancer Res. 1999;59:826–830. [PubMed] [Google Scholar]
- 16.Huibregtse J M, Scheffner M, Howley P M. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991;10:4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huibregtse J M, Scheffner M, Howley P M. Localization of the E6-AP regions that direct human papillomavirus E6 binding, association with p53, and ubiquitination of associated proteins. Mol Cell Biol. 1993;13:4918–4927. doi: 10.1128/mcb.13.8.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiyono T, Foster S A, Koop J I, McDougall J K, Galloway D A, Klingelhutz A J. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- 19.Kiyono T, Hiraiwa A, Fujita M, Hayashi Y, Akiyama T, Ishibashi M. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc Natl Acad Sci USA. 1997;94:11612–11616. doi: 10.1073/pnas.94.21.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klingelhutz A J, Foster S A, McDougall J K. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380:79–82. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- 21.Kusumoto M, Ogawa T, Mizumoto K, Ueno H, Niiyama H, Sato N, Nakamura M, Tanaka M. Adenovirus-mediated p53 gene transduction inhibits telomerase activity independent of its effects on cell cycle arrest and apoptosis in human pancreatic cancer cells. Clin Cancer Res. 1999;5:2140–2147. [PubMed] [Google Scholar]
- 22.Li H, Cao Y, Berndt M C, Funder J W, Liu J P. Molecular interactions between telomerase and the tumor suppressor protein p53 in vitro. Oncogene. 1999;18:6785–6794. doi: 10.1038/sj.onc.1203061. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Chen J J, Gao Q, Dalal S, Hong Y, Mansur C P, Band V, Androphy E J. Multiple functions of human papillomavirus type 16 E6 contribute to the immortalization of mammary epithelial cells. J Virol. 1999;73:7297–7307. doi: 10.1128/jvi.73.9.7297-7307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyerson M, Counter C M, Eaton E N, Ellisen L W, Steiner P, Caddle S D, Ziaugra L, Beijersbergen R L, Davidoff M J, Liu Q, Bacchetti S, Haber D A, Weinberg R A. hEST2, the putative human telomerase catalytic subunit gene, is upregulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 25.Mietz J A, Unger T, Huibregtse J M, Howley P M. The transcriptional transactivation function of wild-type p53 is inhibited by SV40 large T-antigen and by HPV-16 E6 oncoprotein. EMBO J. 1992;11:5013–5020. doi: 10.1002/j.1460-2075.1992.tb05608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakayama J, Tahara H, Tahara E, Saito M, Ito K, Nakamura H, Nakanishi T, Tahara E, Ide T, Ishikawa F. Telomerase activation by hTRT in human normal fibroblasts and hepatocellular carcinomas. Nat Genet. 1998;18:65–68. doi: 10.1038/ng0198-65. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Russell D W. Molecular cloning: a laboratory manual. 3rd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 2001. pp. 17.4–17.11. [Google Scholar]
- 28.Scheffner M, Werness B A, Huibregtse J M, Levine A J, Howley P M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 29.Takakura M, Kyo S, Kanaya T, Hirano H, Takeda J, Yutsudo M, Inoue M. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999;59:551–557. [PubMed] [Google Scholar]
- 30.Vaziri H, Benchimol S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol. 1998;8:279–282. doi: 10.1016/s0960-9822(98)70109-5. [DOI] [PubMed] [Google Scholar]
- 31.Veldman T, Horikawa I, Barrett J C, Schlegel R. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J Virol. 2001;75:4467–4472. doi: 10.1128/JVI.75.9.4467-4472.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Xie L Y, Allan S, Beach D, Hannon G J. Myc activates telomerase. Genes Dev. 1998;12:1769–1774. doi: 10.1101/gad.12.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinrich S L, Pruzan R, Ma L, Ouellette M, Tesmer V M, Holt S E, Bodnar A G, Lichtsteiner S, Kim N W, Trager J B, Taylor R D, Carlos R, Andrews W H, Wright W E, Shay J W, Harley C B, Morin G B. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat Genet. 1997;17:498–502. doi: 10.1038/ng1297-498. [DOI] [PubMed] [Google Scholar]
- 34.Wick M, Zubov D, Hagen G. Genomic organization and promoter characterization of the gene encoding the human telomerase reverse transcriptase (hTERT) Gene. 1999;232:97–106. doi: 10.1016/s0378-1119(99)00108-0. [DOI] [PubMed] [Google Scholar]
- 35.Wu K J, Grandori C, Amacker M, Simon-Vermot N, Polack A, Lingner J, Dalla-Favera R. Direct activation of TERT transcription by c-MYC. Nat Genet. 1999;21:220–224. doi: 10.1038/6010. [DOI] [PubMed] [Google Scholar]