Abstract
Envenoming resulting from Apis honeybee stings pose a neglected public health concern, with clinical complications ranging from mild local reactions to severe systemic manifestations. This review explores the mechanisms underlying envenoming by honeybee sting, discusses diagnostic approaches, and reviews current pharmacological interventions. This section explores the diverse clinical presentations of honeybee envenoming, including allergic and non-allergic reactions, emphasizing the need for accurate diagnosis to guide appropriate medical management. Mechanistic insights into the honeybee venom’s impact on physiological systems, including the immune and cardiovascular systems, are provided to enhance understanding of the complexities of honeybee sting envenoming. Additionally, the article evaluates emerging diagnostic technologies and therapeutic strategies, providing a critical analysis of their potential contributions to improved patient outcomes. This article aims to provide current knowledge for healthcare professionals to effectively manage honeybee sting envenoming, thereby improving patient care and treatment outcomes.
Keywords: bee venom, bee sting, clinical envenoming, clinical management, venomous animals, Africanized bee, honeybee
1. Introduction
Africanized bees have displayed remarkable adaptability in the Americas representing a great public health concern for humans due to their propensity to attack even in mildly provoked situations. They tend to exhibit a high number of bees that attack from unusually far distances from the hive, persistently pursue their targets for extended periods, and release larger volumes of venom compared to other bee species (1–3). Accidents involving Africanized bees can lead to various clinical manifestations, determined by an individual’s sensitivity to the venom and the number of stings. The most common scenario occurs when an individual not sensitized to the venom receives a few stings. In such cases, the symptoms are typically limited to a localized inflammatory reaction, characterized by redness, pain, and local warmth. Often, these symptoms resolve without the need for medical intervention. Another presentation arises when an individual previously sensitized to one or more components of the venom experiences an immediate Gell and Coombs type I hypersensitivity reaction. This is a severe occurrence that can be triggered by just one sting, necessitating urgent medical attention. Manifestations include swelling of the glottis, bronchospasm, and anaphylactic shock. The third form of presentation results from multiple stings, leading to envenoming with a substantial amount of venom in the body. Alongside local signs such as pain, bleeding, bruising, redness, jaundice, and increased blood flow (hyperemia), these instances are frequently reported (4–8). In addition, systemic manifestations like difficulty breathing (dyspnea), neuroparalytic symptoms, intense burning headaches, nausea, vomiting, weakness (asthenia), muscle and joint pain, severe tremors, kidney failure, rhabdomyolysis, and shock (4–8) can occur due to the diverse fractions of the venom. Incidents leading to these symptoms are not uncommon (2, 9).
2. Epidemiology, bee venom, and clinical manifestations
Africanized bees emerged in Brazil during the 1950s when beekeepers introduced African bees (Apis mellifera scutellata) to the country. Renowned for their high productivity and disease resistance (10), these bees accidentally escaped and hybridized with European honey bees (Apis mellifera mellifera), established in Brazil since the early 19th century. The resulting hybrids demonstrated exceptional adaptability to the tropical climate, rapidly spreading throughout the Americas, excluding Canada (9, 11).
The success of Africanized bees in the Americas is attributed to ecological and genetic advantages over native pollinators, including higher reproductive rates, shorter development cycles, increased drone production, swarming frequency, enhanced disease resistance, and less selective nesting site choice (2, 10). A concerning trend is the escalating incidence of honeybee encounters in urban environments. This increase is primarily linked to pesticide use, deforestation, and declining floral resources, exacerbated by the proximity of bee habitats to human settlements (12). Africanized bee stings occur four to ten times more frequently than those of European bees, often involving group attacks (3, 11). Their extended pursuit of threats and increased venom delivery compared to other bee species (3) pose significant public health risks, leading Brazilian health authorities to classify bee-related incidents as a public health surveillance priority (13).
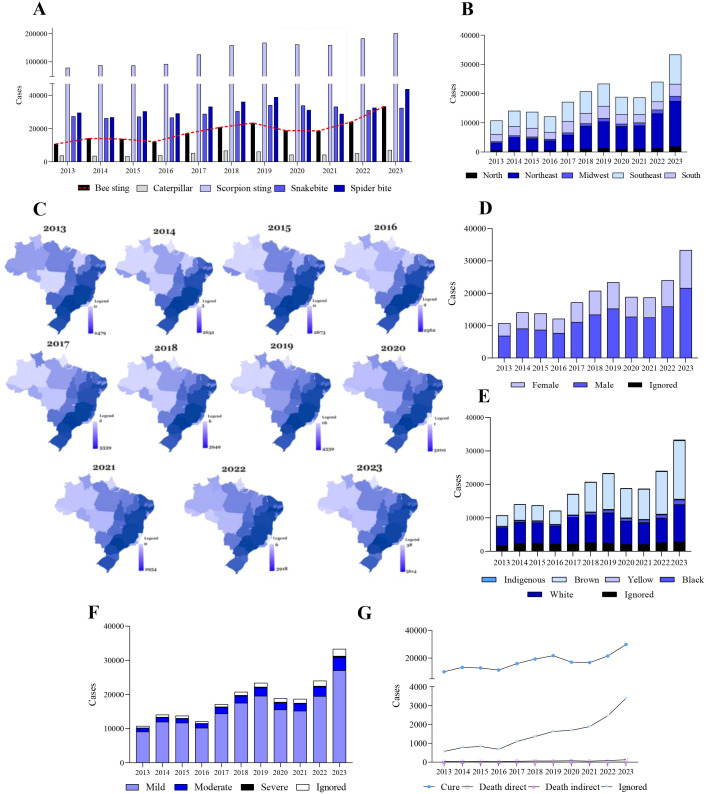
In Brazil, between 2013 and 2023, there were 206.746 reported cases of bee stings, with a notable increase in the year 2023, where 33,317 cases were reported, exceeding the number of snake cases (32,420 cases) leading to 649 direct fatalities and an additional 50 deaths indirectly attributed to bee stings ( Figure 1A ). As with envenoming by other venomous animals, the number of cases of envenoming by bees varies between Brazilian states ( Figures 1B, C ), although clinical and epidemiological studies are scarce (7, 14). It is estimated that the lethality rate is about 0.29%, with an annual rate average incidence of 6.89 per 100,000 inhabitants (15), although a temporal increase in reported cases is observed (6, 13, 14, 16, 17). In Amazonas State, the geographical landscape is cited as a contributing factor to the worsening of patients’ conditions (18). This assertion holds merit, considering the geographic obstacles and challenges encountered in route to medical assistance, often leading to fatal outcomes before reaching proper medical care (19). Most reported cases are concentrated in the Northeast, Southeast, and South regions ( Figures 1B, C ), affecting mainly men ( Figure 1D ) people of color ( Figure 1E ). However, the majority of cases are mild ( Figure 1F ) and progress towards cure ( Figure 1G ). However, the impact on patient health in moderate and severe cases is unknown. This is substantiated by the high population density and the diminishing presence of natural vegetation on mountains, in landfills, slums, and urban conglomerates, which can serve as habitats for bees. Consequently, these conditions may provoke bee swarms to launch extensive attacks (20, 21).
Figure 1.
Reported bee accidents and related deaths in Brazil (2013–2023) by state. (A) Bee accidents in Brazil per year. Except for 2020 and 2021, the others showed an increasing trend in cases. This drop refers to the years of the pandemic caused by the Sars-Cov-2 virus. (B) Annual reported bee accidents related to bee stings per region. (C) Distribution of cases of bee accidents according to state. (D) Distribution of cases of accidents caused by bees according to sex. (E) Distribution of cases of accidents caused by bees according to race. (F) Distribution of cases of accidents caused by bees according to clinical classification. (G) Distribution of cases of accidents caused by bees according to outcome. Graphs were produced using GraphPad Prism 9.0 software.
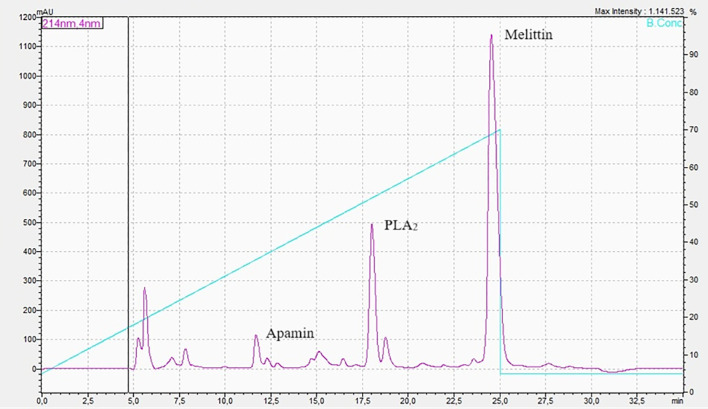
The Apis genus is responsible for most accidents, often resulting in severe outcomes and fatalities (22). Their venom is a complex mixture of proteins, low-molecular-weight peptides, amines, water, and mineral salts. Notably, melittin, phospholipase A2 (PLA2), hyaluronidase, apamin, and mast cell degranulating peptide are highly toxic components (23, 24). Various analytical methods, including electrophoresis, HPLC, HPLC-MS, GC-MS, liquid scintillation counting, ICP-MS, and stripping voltammetry, have been employed to characterize bee venom (25). Size-exclusion chromatography (SEC-HPLC) under isocratic conditions can identify melittin, apamin, and MCDP. However, due to the melittin/apamine ratio (30:1) and the venom’s chemical complexity, reverse-phase chromatography with C18 columns (RP-18) is necessary for comprehensive analysis, enabling the identification of apamin, hyaluronidase, MCDP, melittin, PLA2, procamine, tertiapin, and secapin (26). The abundance of A. mellifera venom components is reflected in the intensity of chromatographic peaks, with melittin being the most prominent, followed by PLA2, apamin, and other constituents (27) ( Figure 2 ).
Figure 2.
High-performance liquid chromatography of Apis mellifera venom.
Immediately following a bee sting, the venom delivery system remains embedded in the skin for approximately 30 seconds, allowing for venom release, with at least 90% injected within the first 20 seconds (28). While removing the stinger is a common initial response, it is unlikely to significantly reduce venom toxicity or quantity absorbed. On average, a bee sting injects 140-150 μg of venom, and the median lethal dose (LD50) is 2.8-3.5 mg/kg of body weight (26, 28). Thus, a non-allergic person weighing 60-70 kg could theoretically experience a 50% risk of fatality from 1,000-1,500 stings. However, fatalities have been reported with as few as 200-500 stings. Various factors influence envenoming severity, including time to medical care, age, weight, sting count, and individual characteristics (e.g., immune status, comorbidities, previous sensitization) (29). Individuals with asthma, allergic rhinitis, or a history of bee sting allergies are at increased risk for severe complications (30).
Typically, the clinical manifestations of bee envenomation can be categorized into local inflammatory reactions, allergic reactions, anaphylactic shock, and systemic toxic reactions (3, 9, 29). Initial symptoms are confined to the sting site, often presenting as pain, swelling, redness, and itching. Allergic reactions, classified as type I hypersensitivity reactions, usually occur within 10 minutes of the sting. These reactions can manifest as systemic urticaria, itching, angioedema, vomiting, or diarrhea. In severe cases, allergic reactions may progress to anaphylactic shock, causing bronchoconstriction (26). Importantly, even individuals without allergies can experience anaphylaxis due to systemic mastocytosis.
3. Bee venom: molecular basis of physiopathology
A typical bee sting elicits a local inflammatory response characterized by pain, edema, and erythema. Melittin, a primary bee venom allergen, is a potent inducer of acute pain (31). It has been demonstrated that intradermal melittin (5 μg in 50 μL saline) caused severe, 3-minute pain accompanied by local heat and swelling (32–35). Pain intensity and duration correlate with melittin dose, inducing mechanical hyperalgesia at the sting site and heat-thermal hyperalgesia in the surrounding area (34, 35). Animal studies extend these findings to include mechanical and thermal hyperalgesia, edema, and plasma extravasation lasting 72-96 hours (36–39).
Melittin activates the transient receptor potential vanilloid 1 (TRPV1) channel, a nonselective cation channel in peripheral sensory neurons, contributing to pain (40). TRPV1 also mediates pruritus (itching) (41, 42). Histamine, IL-31, IL-4, and cyclooxygenase (COX), lipoxygenase (LOX), and PLA2 pathway products stimulate TRPV1, promoting itching (31, 43). Another mechanism by which melittin induces itching is via serotonin release due to pore formation and mast cell degranulation (31), as well as by increased transcriptional regulation of voltage-gated sodium channels in neurons associated with itch (44, 45). Additionally, melittin enhances nociceptor activity by modulating G protein-coupled receptors (GPCRs), leading to hyperalgesia and allodynia (31).
Another critical effect of venom components, particularly allergens, is hemorrhage. While primarily associated with IgE-mediated anaphylaxis, allergens can also trigger non-IgE-dependent inflammatory responses (46). Bradykinin (BK), whose production increases during envenomation due to melittin’s action, contributes to anaphylactic symptoms. Melittin directly activates PLA2, mimicking BK’s effects on tracheal tone and inducing angioedema (47), leading to airway obstruction, asphyxia, and severe gastrointestinal symptoms resembling acute abdomen (46).
A complex interplay between inflammation and coagulation arises, as plasma kinin formation cascade activation results in factor XII binding, autoactivation, and conversion of prekallikrein to kallikrein. Kallikrein cleaves high-molecular-weight kininogen, releasing vasoactive BK (48). BK subsequently activates and modulates coagulation, particularly factor XII (49). Both BK and BK 1-5 inhibit thrombin-induced platelet aggregation, while thrombin plays a crucial role in coagulation and platelet activation (50, 51). Additionally, BK enhances nitric oxide (NO) production in endothelial cells, inhibiting thrombocyte adhesion through angiotensin II blockade (52)
Interestingly, BK induces prolonged thrombolysis via the bradykinin B2 receptor (B2) and prostacyclin (PGI2) (53, 54). This reveals BK’s potential to activate plasminogen, which can be antagonized by angiotensin-converting enzyme (ACE) inhibitors through amplified tissue plasminogen activator effects (53, 55). ACE inhibition modulates fibrinolytic balance, with angiotensin II-mediated increases in plasminogen activation inhibitor-1 playing a crucial role (56). Consequently, coagulation disorders, including decreased fibrinogen activity and prolonged prothrombin and partial thromboplastin times, have been reported in experimental and clinical bee venom envenomation (57).
In summary, melittin induces BK release and ACE dysfunction, leading to coagulation and fibrinolysis imbalances. Additionally, impaired vascular smooth muscle contractility following envenomation exacerbates hemorrhagic episodes (57, 58). Elevated BK levels contribute to reduced vascular tone through epithelial effects (48, 52), while melittin and PLA2 may further decrease muscle contractility, potentially inducing hemorrhage.
Bee venom PLA2 hydrolyzes phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, and phosphatidylserine 2-acyl bonds, disrupting plasma membrane integrity. This process releases lysophospholipids and fatty acids, inducing inflammation and further membrane damage. Notably, purified PLA2 lacks hydrolytic activity against erythrocyte membrane phospholipids, but its combination with melittin synergistically enhances hemolysis beyond that achievable by either component alone (59).
Skeletal and cardiac muscle cell membranes exhibit greater resistance to PLA2, although both crude venom and melittin exert toxic effects on the cardiovascular system. The exact mechanisms underlying these effects and potential contributions from other venom components remain unclear (60). Melittin initially increases, then decreases the spontaneous beating rate of cultured cardiac myocytes, ultimately causing their degeneration (61).
Wistar rats exhibited electrocardiographic (ECG) changes, enzyme alterations, and morphological lesions resembling acute myocardial infarction (AMI) type 8, suggesting a direct toxic effect of the venom on cardiac muscle, primarily attributed to melittin (62, 63). Bee venom induces extensive endothelial damage, collagen degradation, and smooth muscle cell migration within the aorta (64). Melittin and apamin provoke coronary artery vasospasm, facilitating platelet aggregation and thrombosis. At lower concentrations, melittin induces transient relaxation through an endothelium-dependent mechanism involving NO production and activation of smooth muscle charybdotoxin-sensitive K+ channels, but at higher concentrations, it causes contraction. Apamin, while not directly affecting coronary artery contraction or relaxation, inhibits NO and prostanoid production, modulating the coronary artery’s relaxation response to melittin via smooth muscle apamin-sensitive K+ channels (65). Although bee venom triggers endogenous vasodilatory amine release, paradoxical coronary vasoconstriction remains possible, especially in the context of endothelial damage, potentially leading to acute coronary syndrome (66).
Africanized bee venom induces myonecrosis both in vivo and in vitro (67). Specifically, melittin triggers phospholipid breakdown, generating free fatty acids and diacylglycerol in equine and human skeletal muscle primary cultures. At higher concentrations, it also breaks down triglycerides. Additionally, melittin alters calcium (Ca2+) release thresholds in skeletal muscle terminal cisternae fractions (68). Membrane rupture initiates a cascade of intracellular changes, with increased intracellular calcium levels being a critical factor in subsequent cellular dysfunction and death. Melittin and PLA2 synergistically contribute to skeletal muscle cell myonecrosis (69).
Apis mellifera venom contains a protein with a conserved C1q domain (70). C1q activates the classical complement pathway by binding to antibody Fc regions, leading to C1r and C1s activation. Subsequently, C1s cleaves C4, generating pro-inflammatory anaphylatoxins C4a and C4b (71). While C1q is present in A. mellifera venom, recombinant C1q failed to recognize IgE from bee venom allergic patients (70), suggesting a need for further investigation into its role in complement activation during bee stings.
Interestingly, melittin’s hydrophilic head shares similarities with the C1q sequence. Envenomation induces melittin-mediated, antigen-independent IgG and C1q aggregation, triggering the classical pathway and producing anaphylatoxins C3a and C5a (72). C5a rapidly induces physiological responses, including mast cell degranulation, potentially leading to fatal anaphylaxis (73). Bee sting envenomation elicits acute allergic and inflammatory responses. A. mellifera venom and melittin activate the NLRP3 inflammasome, leading to procaspase-1 cleavage and neutrophil recruitment to the sting site. Mast cells play a protective role by degrading and neutralizing A. mellifera toxins post-degranulation (74). Melittin activates the 5-lipoxygenase pathway in neutrophils, releasing arachidonate and inducing neutrophilia (75, 76). Additionally, PLA2 from Apis mellifera lamarckii (Egyptian honeybee) venom hydrolyzes phosphatidylcholine, inhibits platelet aggregation, and impairs blood coagulation by inhibiting the extrinsic pathway (77).
4. Role of oxidative stress, inflammation, and coagulation
Redox homeostasis alterations in venomous animal envenomation victims contribute to secondary or long-term complications, with oxidative stress being a key factor. Bee sting envenomation disrupts hepatic metabolism, elevating plasma alanine transaminase (ALT) and aspartate aminotransferase (AST)levels, indicating hepatotoxicity. Additionally, it induces caspase-1 activation and pro-inflammatory molecule secretion through H2O2 overproduction (78).
One of the most reported clinical complications following bee sting envenomation is ischemic stroke with hemorrhagic transformation. Although the pathophysiology remains unclear, proposed mechanisms involve systemic immune-mediated vasoconstriction and a prothrombotic state, leading to ischemia and subsequent stroke (79), similar to other venomous animal envenomations (80). These events occur through platelet aggregation, coagulation cascade activation via tissue thromboplastin release from damaged tissue phospholipids (81), and rapid declines in platelet count with coagulopathy (82). Combined with hemolysis and endothelial damage, widespread fibrin thrombi formation can lead to vessel occlusion, progressing to hemorrhagic or occlusive transformations.
Apis mellifera venom targets various cells, with hemolysis and rhabdomyolysis being particularly damaging due to oxidative stress, primarily mediated by melittin and PLA2. Hemolysis can occur directly through venom action or indirectly, while rhabdomyolysis is primarily venom-induced but may also involve inflammation, vascular congestion, and edema (83). PLA2-mediated hemolysis results from red blood cell membrane lipid disruption, releasing free hemoglobin (Hb) (83, 84). Free Hb induces macrophage programmed death, shifting macrophage polarization towards a cytotoxic phenotype, exacerbating inflammation and tissue damage (85–87). Additionally, Hb spontaneously oxidizes and reacts with NO, converting hemoglobin to methemoglobin (MtHb).
MtHb is highly pro-oxidant, readily releasing ferric heme, which easily crosses cell membranes and increases oxidative damage. Rhabdomyolysis, another significant contributor to oxidative stress, releases myoglobin, reactive oxygen species (ROS), and uric acid into the bloodstream. Myoglobin undergoes oxidation similar to Hb, releasing free iron and inducing free radical formation. These events can collectively damage the liver and kidneys (83).
Ferroptosis, a ROS-dependent cell death characterized by iron accumulation and lipid peroxidation, may contribute to liver and kidney damage (88). Ferroptosis inducers like erastin or RSL3 increase intracellular iron, generate excessive ROS, and enhance lipid peroxidation through lipoxygenase (ALOX) or EGLN prolyl hydroxylases (PHDs) (88, 89). Hepatocellular death, occurring through apoptosis, necrosis, or pyroptosis, is a common response to various liver diseases (90). Animal studies reveal A. mellifera venom-induced sinusoidal and centrilobular congestion, eosinophilia, cytoplasmic vacuolation, intraparenchymal hemorrhage, centrilobular necrosis, and apoptosis (83). Clinically, bee sting envenomation patients exhibit elevated ALT, AST, and bilirubin levels, consistent with hemolysis, thrombotic microangiopathy, and acute liver injury (91). Hepatic vessel occlusion due to microthrombi formation can also lead to ischemia and necrosis (92). Additionally, venom-induced oxidative stress, evidenced by increased malondialdehyde (MDA) and glutathione (GSH) levels, contributes to liver damage (93).
Elevated MDA and GSH levels indicate lipid peroxidation in liver tissue, a process mediated by ROS generated through various mechanisms, including neutrophil activity (94). This lipid peroxidation serves as a pivot for inflammation, as evidenced by increased TNF-α levels in liver tissue (93). The potential involvement of neutrophil extracellular traps (NETs) in this process warrants further investigation. Nox enzyme-dependent NET formation is well-characterized, involving increased ROS production, neutrophil granule and nuclear membrane disintegration, and the release of neutrophil elastase (NE) and myeloperoxidase (MPO) (95). NE and MPO interact with the neutrophil nucleus, cleaving histones and facilitating chromatin decondensation, ultimately leading to the release of DNA decorated with granular content into the extracellular environment for antigen capture (96, 97). While NET formation has been observed in snakebite envenomation (98), its role in multiple bee sting envenomation remains unexplored.
Oxidative stress induced by bee venom contributes to acute kidney injury. Bee venom and melittin disrupt renal cell redox homeostasis by inhibiting α-MG uptake through a PLA2-oxidative stress-Ca2+ pathway (99). This process involves increased arachidonic acid and lipid peroxide production, along with elevated Ca2+ uptake, suggesting a link between PLA2 activation, Ca2+, and oxidative stress in renal cells (99). Acute kidney injury, primarily affecting proximal tubules, is a common complication of bee sting envenomation (100, 101). Redox imbalance, characterized by lipid peroxidation and membrane protein denaturation, leading to altered membrane fluidity, enzyme function, and ion transport, plays a crucial role in renal failure pathogenesis. The kidney’s high sensitivity to oxidative stress due to its rich polyunsaturated fatty acid content renders it susceptible to tubular necrosis caused by bee venom-induced redox imbalance (102).
Disseminated intravascular coagulation (DIC) with associated thrombocytopenia presents another potential mechanism for kidney injury. Despite its prevalence, effective treatment and management strategies remain limited. Given platelets’ sensitivity to external stimuli, including ROS, their interplay with oxidative stress is plausible, especially considering platelet apoptosis exacerbates oxidative stress induced by the hemorrhagic, hemolytic, and necrotic effects of bee venom components. Numerous case studies have documented initial thrombocytopenia and alterations in hemostatic and renal systems. Platelets play a crucial role in thromboinflammation (80), explaining the pathogenesis of renal changes in bee sting envenomation.
Roodt et al. (83) reported pulmonary congestion, septal enlargement, atelectasis, emphysema, intra-alveolar hemorrhagic foci, and arterial lesions with acute edema in mice following experimental envenomation with Apis mellifera mellifera venom from different regions of Buenos Aires, Argentina. While pulmonary alterations in bee envenomation remain unclear clinically, proposed mechanisms include pro-thrombotic state-induced congestion due to platelet aggregates, fibrin deposition, and erythrocyte accumulation, similar to snake envenomation. Pulmonary edema may result from catecholamine-induced myocarditis, myocardial ischemia due to coronary vasoconstriction, and direct cardiotoxin effects on the myocardium (103–106). Additionally, blood leukocyte mobilization, as observed in other venomous animal envenomations, could contribute to acute lung injury.
5. Clinical complications in honeybee stings envenoming
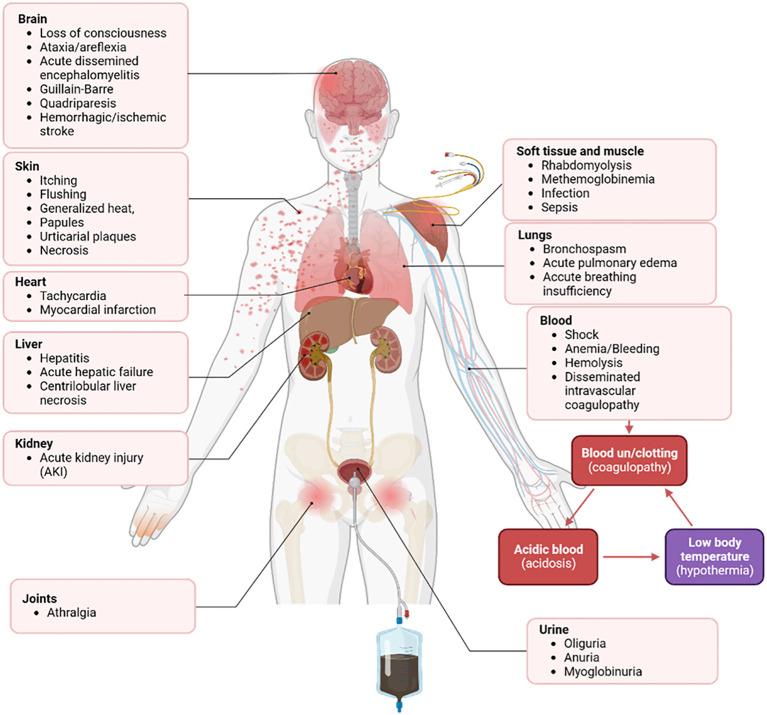
A wide array of clinical complications can arise from multiple bee stings ( Figure 3 ). These complications manifest locally or systemically, with variable onset, organ involvement, and overall impact. While frequently reported, the underlying pathogenesis of rarer reactions remains largely undefined ( Table 1 ).
Figure 3.
Spectrum of severe systemic effects from bee stings. Multiple bee stings trigger highly severe systemic reactions, exhibiting a broad spectrum of clinical manifestations.
Table 1.
Complications associated with honeybee stings envenoming.
| System/Organ | Clinical complications | Reference |
|---|---|---|
| Cardiovascular | Acute myocardial ischemia | (107–109) |
| Acute myocardial injury | (110) | |
| Atrial fibrillation | (111) | |
| Hypertension | (112, 113) | |
| Kounis syndrome (KS) | (111, 114–120) | |
| Left ventricular hypertrophy | (121) | |
| Left ventricular systolic dysfunction | (108, 122) | |
| Mobitz type 2 heart block | (123) | |
| Myocardial damage | (112, 124) | |
| Pericardial effusion | (125) | |
| Pericarditis epistenocardica | (126) | |
| Subendocardial hemorrhage | (121) | |
| Takotsubo cardiomyopathy | (127) | |
| Digestory | Boerhaave’s syndrome | (107) |
| Gastrointestinal hemorrhage | (113, 128) | |
| Hematologic | Acute limb ischemia | (129) |
| Acute femoral thrombosis | (129) | |
| Anemia | (130) | |
| Bleeding | (4) | |
| Brachial artery thrombosis | (131) | |
| Deep vein thrombosis (DVT) | (132) | |
| Disseminated intravascular coagulation | (112) | |
| Hematochezia | (133) | |
| Hemolysis | (29, 91, 112, 134) | |
| Shock | (18, 112, 125, 135, 136) | |
| Thrombotic microangiopathy | (91) | |
| Thrombotic thrombocytopenic purpura (TTP) | (137) | |
| Hepatic | Acute liver injury | (91) |
| Adrenal hemorrhage | (138) | |
| Hepatic damage | (124) | |
| Hepatic dysfunction | (29, 112) | |
| Ischemic hepatitis | (133) | |
| Immune | Anaphylactic shock | (4, 73, 107, 108, 114, 121, 122, 133, 139–143) |
| Mast cell activation syndrome | (116) | |
| Multisystem | Cardiopulmonary arrest | (143) |
| Multiorgan failure | (79, 92, 134, 136, 144–146) | |
| Multiorgan injury | (147) | |
| Muscular | Hemiparesis | (148, 149) |
| Rhabdomyolysis | (18, 29, 112, 135, 148, 150–155) | |
| Nervous | Acute bilateral cerebellar infarction | (156) |
| Axonal motor polyneuropathy | (157, 158) | |
| Cavernous sinus thrombosis | (159) | |
| Coma | (112, 136) | |
| Convulsion | (159) | |
| Encephalitis | (128) | |
| Guillain-Barre syndrome | (160) | |
| Hemorrhagic/Ischemic stroke | (134, 136, 148, 149, 161–165) | |
| Intracerebral hemorrhage | (136) | |
| Multiple acute cerebral infarcts | (148, 149, 159) | |
| Subarachnoid hemorrhage | (4, 121, 149) | |
| Subdural hemorrhage | (149) | |
| Tonsillar herniation | (133) | |
| Transcortical motor aphasia | (166) | |
| Renal | Acute kidney injury | (29, 91, 108, 113, 135, 150, 153, 155, 159, 167) |
| Acute kidney failure | (18, 112, 134, 136, 147, 148, 151, 154) | |
| Respiratory | Acute pulmonary emphysema | (141) |
| Acute respiratory distress syndrome (ARDS) | (18, 112, 115, 125, 134, 135, 168) | |
| Bronchial obstruction | (141) | |
| Laryngeal congestion | (138) | |
| Laryngeal edema | (138, 139) | |
| Pulmonary congestion | (139, 141, 169) | |
| Pulmonary edema | (113, 125, 127, 139, 141) | |
| Pulmonary hemorrhage | (141) | |
| Traquea congestion | (138) | |
| Traquea edema | (138) | |
| Skin | Angioedema | (169, 170) |
| Grover’s Disease (GD) | (171) | |
| Urinary | Anuria | (115, 125) |
| Hematuria | (130, 133) | |
| Hemoglobinuria | (29) | |
| Visual | Cataract | (172, 173) |
| Central retinal artery occlusion (CRAO) | (124, 140) | |
| Conjunctival chemosis | (136, 174–178) | |
| Conjunctival congestion | (172) | |
| Conjunctival hyperemia | (177–179) | |
| Conjunctival injection | (175) | |
| Conjunctival ischemia | (175) | |
| Corneal abrasions | (179–182) | |
| Corneal decompensation | (172) | |
| Corneal edema | (172, 175, 181, 183–186) | |
| Cornea epithelial defect | (172) | |
| Corneal infiltration | (172, 175, 180) | |
| Corneal scarring | (172, 186) | |
| Descemet membrane fold | (174) | |
| Endophthalmitis | (184, 187) | |
| Episcleral hyperemia | (178) | |
| Eyelid edema | (175, 179) | |
| Glaucoma | (172) | |
| Keratoconjunctivitis | (188) | |
| Keratopathy | (180, 186) | |
| Optic disc hyperemia | (189) | |
| Optic neuritis | (190) | |
| Optic neuropathy | (185) | |
| Retinal striae | (189) | |
| Scleritis | (184) | |
| Striate keratopathy | (177) | |
| Subconjunctival hemorrhage | (176, 185, 187) | |
| Uveitis | (189, 191) | |
| Vitritis | (189) |
Melittin, phospholipase A2, apamin, and hyaluronidase are the primary toxic components of Apis mellifera bee venom. These substances significantly contribute to the development of clinical complications following multiple bee stings (192). The cardiovascular system is particularly vulnerable, with ischemic events, including acute myocardial infarction and Kounis syndrome (allergy-induced acute coronary syndrome), being common sequelae. Other potential cardiac complications include takotsubo cardiomyopathy, atrial fibrillation, and cardiac damage (193, 194).
Hemorrhage can occur in various locations following a bee sting, including the digestive, nervous, and respiratory systems, potentially leading to gastrointestinal, subarachnoid, or pulmonary hemorrhage, respectively. Additionally, hematological complications such as ischemia, anemia, thrombosis, hemolysis, disseminated intravascular coagulation (DIC), and shock, often culminating in hypovolemia, may arise. While hemolysis occurs in 17-22% of wasp sting cases, DIC is less common but can trigger thromboplastin release and microthrombi formation (192, 194).
Bee stings can induce hepatitis due to liver damage, manifested by elevated transaminases, alkaline phosphatases, and bilirubin levels. However, rhabdomyolysis and cardiac damage can also increase transaminases, complicating liver injury diagnosis. Severe liver damage may progress to liver failure. In rats, melittin has been implicated in liver injury through vasoconstriction and glycogenolysis (194).
Anaphylactic shock, an IgE-mediated immune response causing hypoperfusion and vasodilation, poses a severe risk to individuals with previous bee sting exposure or allergies. This life-threatening condition can lead to organ injury and death. Additionally, mast cell activation syndrome, characterized by excessive mast cell production and inflammatory effects, can trigger multisystem complications, organ failure, and mortality rates exceeding those of anaphylaxis (194).
Rhabdomyolysis, characterized by skeletal muscle breakdown, is frequently associated with bee envenoming, evidenced by elevated creatine phosphokinase (CPK) and bilirubin levels, and can contribute to acute kidney injury (AKI) (18, 192). Hemiparesis, or muscle weakness following ischemic stroke, is another potential muscular complication leading to immobilization or reduced physical activity (195).
Bee sting envenomation can lead to various neurological complications, including stroke (both ischemic and hemorrhagic), behavioral changes, ataxia, areflexia, encephalomyelitis, and Guillain-Barré syndrome. Ischemic and hemorrhagic strokes pose significant risks due to their potentially fatal outcomes. Subarachnoid intracranial hemorrhage often complicates ischemic stroke, undergoing hemorrhagic transformation. While the exact mechanisms remain unclear, two primary theories have been proposed: 1) an immune-mediated systemic reaction causing vasoconstriction and a prothrombotic state leading to ischemia and subsequent stroke; and 2) disseminated intravascular coagulation (DIC) triggered by tissue thromboplastin release, coupled with hemolysis, resulting in vessel occlusion, ischemic stroke, and eventual hemorrhagic transformation (9, 34).
Furthermore, behavioral changes, ataxia, and areflexia typically accompany degeneration or obstruction in specific regions of the brain and cerebellum. In this context, some cases displaying these complications have also experienced strokes, indicating a potential connection (196). It is well-established that the execution of any movement entails the coordinated action of agonist and synergist muscles, which contract to facilitate the movement, while antagonist muscles relax to allow it, and fixator muscles stabilize posture and prevent unintended shifts (196). Hence, considering that bee venom, particularly melittin, induces rhabdomyolysis, it is plausible that the partial loss of motor function in these cases may also be linked to rhabdomyolysis (18, 192).
Rhabdomyolysis, hemolysis, and hypotension can adversely affect the renal system, elevating creatine kinase and bilirubin levels, and ultimately leading to acute kidney injury (AKI) and renal failure. Multiple bee sting envenomation often causes glomerular and peritubular vasoconstriction, potentially resulting in ischemic injury and acute tubular necrosis. The accumulation of myoglobin, Tamm-Horsfall proteins, and uric acid within the tubules contributes to cast formation, further obstructing tubules and inducing ischemia. Anuria and hematuria are additional associated renal complications (192).
Clinical respiratory complications include acute pulmonary edema, pulmonary hemorrhage, and acute respiratory distress syndrome (ARDS). Bee venom’s ability to increase vascular permeability contributes to edema formation in various tissues, including the lungs, trachea, conjunctiva, and subcutaneous areas (194). Alveolar capillary damage can precipitate diffuse alveolar hemorrhage (DAH), potentially progressing to ARDS (197, 198). The pathogenesis of ARDS in bee sting envenomation remains unclear, although melittin and phospholipase A2 are suspected contributors to acute lung injury. Venom-induced inflammation can cause extensive tissue damage, culminating in severe cases of ARDS and multisystemic cardiorespiratory arrest (199, 200).
Ocular complications, though uncommon, encompass a wide range of issues. These include eyelid edema, conjunctival congestion, corneal abrasions, optic neuropathy, ptosis, purulent ocular secretions, conjunctival and episcleral hyperemia, symblepharon, macular retinal striae, ciliary congestion, corneal edema with Descemet’s membrane folding, and even vision loss. Additionally, lens abscess, partial iris atrophy, and cataract formation have been reported. The retained stinger’s direct venom action, coupled with the rapid immune response, primarily causes corneal injury (201, 202).
Clinical complications from bee sting envenomation can be severe, life-threatening, and multi-systemic. These complications, potentially triggered by a single sting, allergies, or anaphylaxis, can impact various bodily systems. Moreover, a high number of bee stings can exacerbate these complications, affecting the cardiovascular, nervous, hematological, or respiratory systems ( Table 2 ).
Table 2.
Clinical complications associated with honeybee stings envenoming in the entire patient cohort and in patients who died.
| Case | Age | Sex | Country | Number of Stings | Time to Treatment | Time to Death | Comorbidity | Clinical complications |
System | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | Male | Turkey | 5989 | 3 h | 12 days | – | Acute kidney failure Acute respiratory distress syndrome (ARDS) Convulsion Hemolysis Hypertension Ischemic stroke Multiorgan failure |
Nervous Hematologic Respiratory Urinary Multiorgan |
(134) |
| 2 | 8 | Male | Nigeria | – | 50 min | 36 h | Sickle cell anemia | Hematuria Hemolysis |
Hematologic | (130) |
| 3 | 25 | Male | Australia | 1 | – | 40 h | – | Acute respiratory distress syndrome (ARDS) Anaphylactic shock Bleeding Cerebral tonsillar herniation Disseminated intravascular coagulation (DIC) Hematuria Ischemic hepatitis Myocardial damage |
Cardiovascular Hepatic Hematologic Immune Nervous Urinary Respiratory |
(133) |
| 4 | 38 | Male | South Africa | 32 | – | – | – | Subarachnoid hemorrhage Subendocardial hemorrhage |
Cardiovascular Nervous |
(121) |
| 5 | 58 | Male | South Africa | 1 | – | – | – | Laryngeal edema Left ventricular hypertrophy Pulmonary congestion Pulmonary edema |
Cardiovascular Respiratory |
(121) |
| 6 | 58 | Male | South Africa | 10 | No medical care | – | – | Anaphylactic shock | Immune | (121) |
| 7 | 38 | Female | Hungary | 1 | No medical care | 4 min | – | Acute respiratory distress syndrome (ARDS) Anaphylactic shock |
Immune Respiratory |
(73) |
| 8 | 25 | Male | Australia | 1 | – | Asthma | Cardiorespiratory arrest Laryngeal edema Pulmonary congestion |
Cardiovascular Respiratory |
(139) | |
| 9 | 36 | Female | Australia | 40-50 | 15 min | >29 min | Asthma Psoriasis |
Cardiorespiratory arrest Laryngeal edema |
Cardiovascular Respiratory |
(139) |
| 10 | 54 | Male | Australia | 1 | 15 min | >15 min | Osteoporosis | Cardiorespiratory arrest Laryngeal edema Pulmonary congestion Pulmonary edema |
Cardiovascular Respiratory |
(139) |
| 11 | 70 | Male | Brazil | – | 20 h | – | Diabetes Hypertension |
Acute respiratory distress syndrome (ARDS) Anuria Cardiorespiratory arrest |
Cardiovascular Respiratory Urinary |
(18) |
| 12 | 39 | Male | Honduras | – | 3 h | 37 h | – | Anaphylactic shock Anuria Rhabdomyolysis |
Immune Multiorgan Muscular Urinary |
(145) |
| 13 | 19 | Male | Brazil | 2000 | 0 h | 23 days | – | Acute respiratory distress syndrome (ARDS) Anuria Cardiorespiratory arrest Pericardial effusion Pulmonary edema Shock |
Cardiovascular Hematologic Urinary Respiratory |
(125) |
| 14 | 46 | Female | Brazil | 1 | 2 h | 2 h | Obesity | Anaphylactic shock Bleeding Subarachnoid hemorrhage Pulmonary edema Pulmonary hemorrhage |
Hematologic Immune |
(4) |
| 15 | 48 | Male | Turkey | – | – | 3 days | – | Encephalitis Hepatitis Gastrointestinal haemorrhage |
Digestory Hepatic Nervous |
(128) |
| 16 | 59 | Male | Italy | – | – | – | – | Bronchial obstruction Cardiorespiratory arrest Laryngeal edema Pulmonary congestion Pulmonary edema Pulmonary emphysema Pulmonary hemorrhage |
Cardiovascular Respiratory |
(141) |
| 17 | 67 | Male | Iran | 20 | 3 days | 5 days | Type-2 diabetes Hypertension Myocardial infarction Ventricular aneurysm |
Cardiorespiratory arrest Thrombotic thrombocytopenic purpura (TTP) |
Cardiovascular Hematologic Respiratory |
(137) |
| 18 | 59 | Male | India | – | – | 5 days | Parkinson disease | Adrenal hemorrhage Laryngeal congestion Laryngeal edema Tracheal congestion Tracheal edema Pulmonary congestion Pulmonary edema Rhabdomyolysis |
Muscular Urinary Respiratory |
(138) |
| 19 | 42 | Male | Iran | – | – | 30 min – 1 h | – | Anaphylactic shock Cardiorespiratory arrest |
Cardiovascular Immune Respiratory |
(143) |
| 20 | 41 | Female | India | 1 | 3 h | 7 days | – | Disseminated intravascular coagulation (DIC) Hemiparesis Hemorrhagic/Ischemic stroke |
Hematologic Nervous |
(149) |
| 21 | 66 | Male | Argentina | >500 | – | 36 h | Hypertension | Anaphylactic shock Multiorgan failure |
Immune Multiorgan |
(203) |
Defining envenomation by sting number remains challenging, as the lethal venom dose is 2.8-3.5 mg/kg body weight (26, 28). Severe complications like disseminated intravascular coagulation, hematuria, ischemic hepatitis, myocardial damage, and death have been reported in adults with fewer than 30, even as few as one sting, particularly in sensitized individuals ( Table 2 ). This highlights the unpredictable nature of bee sting envenomation.
Differentiating the causes and assessing the risks of death linked to either the direct toxic effects of venom or the allergic syndrome triggered by honeybee stings poses a formidable challenge, demanding thorough investigation and vigilant patient monitoring. Consequently, all incidents involving bee stings should be regarded as envenoming, and their significance, even in instances involving only a few stings, should not be underestimated. This recognition was underscored during the Apilic antivenom’s phase I/II clinical trial, wherein a recommendation was made to administer two vials of antivenom to patients with more than five stings. For those with fewer than five stings, the use of apilic antivenom is not advised, except when dictated by medical judgment (5).
6. Prevention of bee stings
Preventing severe systemic reactions to bee stings requires a multi-faceted approach. Clothing choices, such as wearing light-colored, smooth-textured garments, can deter bees. Avoiding scented products and maintaining personal hygiene by wearing clean clothes and showering regularly can also reduce the risk of stings. Minimizing exposure to bee-attracting factors, including flowering plants and food remnants, is crucial (204).
When confronted by a solitary stinging insect, remaining calm and still is crucial to avoid provocation. However, if multiple insects attack, swiftly retreating indoors or to a sheltered area is essential. Submerging in water to escape is ill-advised, as some bee species, like Africanized honeybees, may continue to sting upon resurfacing (205).
Individuals with a history of severe allergic reactions to insect stings should carry an epinephrine auto-injector and wear medical identification. Prompt medical attention is crucial in case of an allergic reaction (206). Integrating these preventive measures into daily routines significantly reduces the risk of severe systemic reactions from bee stings, enhancing safety in various environments.
7. Clinical complications diagnostic
Preventive exams identify diseases in asymptomatic individuals. Bee sting envenomations often present with multiple, late-diagnosed complications. Early diagnosis is crucial for effective management and improved outcomes ( Supplementary Table 1 ). Although warning signs exist, monitoring challenges persist for general clinicians (207).
8. Pharmacological interventions
Current management and treatment protocols for honeybee sting envenomation primarily address analgesia and allergic reactions, with limited guidance on other complications. Consequently, treatment options and associated risks for these complications remain scarce. Given the diverse clinical manifestations of honeybee sting envenomation, adapting treatment protocols from other conditions may be beneficial. It is essential to consider both potential benefits and risks when implementing treatment plans, as some interventions may exacerbate complications or cause adverse effects. A comprehensive understanding of treatment options and their associated risks for various clinical complications is crucial for optimal patient care ( Supplementary Table 2 ).
Symptomatic treatments for severe bee sting envenomations often prove ineffective. To address this, the Center for the Study of Venoms and Venomous Animals, Brazil initiated the development of a bee sting antivenom. Initial efforts focused on venom biochemical characterization to understand its composition. Traditional horse-based antivenom production faced challenges due to high allergic reaction rates and anaphylactic shock in horses. To mitigate these risks, researchers explored various strategies, including cobalt-60 irradiation (208). Irradiation significantly altered the chromatographic profiles of native Apis venom. While irradiated venom combined with IFA or SBA-15 produced similar antibody titers compared to native venom and IFA, these titers were notably lower. Importantly, irradiation reduced venom toxicity while preserving its immunogenicity, and IFA enhanced antibody production (208). However, successful immunization required removing all allergenic components from the venom, leaving only melittin and PLA2 for immunization.
Apilic antivenom demonstrated partial neutralization of venom effects, including hematocrit, vascular permeability, myeloperoxidase activity, edema, plasma CK activity, venom phospholipase and hyaluronidase activity, and cytotoxicity in kidney cell cultures (209, 210). These findings indicate the antivenom’s potential efficacy against Africanized bee (A. mellifera) venom and melittin in vivo and in vitro. Subsequent phase I/II clinical trials were initiated to evaluate the antivenom’s safety and efficacy. Given the absence of established clinical protocols for antivenom evaluation, a new protocol was developed and approved by ethics regulatory (The Brazilian Committee of Ethics in Research – CONEP) and sanitation agencies (Brazilian Health Regulatory Agency – ANVISA). This protocol outlined specific, adjuvant, symptomatic, and complementary treatments, in addition to standard clinical trial guidelines for heterologous antivenoms. It represented the first clinical trial specifically designed to assess the efficacy and safety of an Africanized bee venom antivenom (5).
The Apilic antivenom proved to be safe and demonstrated efficacy in neutralizing various venom effects, including hematocrit, vascular permeability, and enzyme activity, both in vivo and in vitro. These findings highlight the antivenom’s potential for treating Africanized bee (A. mellifera) envenomation. At the time, the pharmacokinetics of Africanized bee venom in humans was reported for the first time. The concentrations of melittin and PLA2 varied between 0.03 ng/mL and 587.35 ng/mL during hospitalization and follow-up, and interestingly, it was possible to observe that the blood concentration of PLA2 and mainly melittin increases again, especially after 10 days hospitalization, but without any clinical symptoms (8)). Mass spectrometry assays, allowed to determine the presence and relative levels of melittin in the participants. It should be noted that these issues were expected, as accidents involving AHB are peculiar and different from all other accidents involving venomous animals described. A phase III clinical trial is essential to confirm these observations, optimize dosing, and fully evaluate the antivenom’s efficacy (8).
The Apilic antivenom production process aligns with established antivenom manufacturing standards (211). However, producing pilot batches compliant with Good Manufacturing Practices (GMP) has presented challenges. Small-scale production for clinical trials is particularly difficult for pharmaceutical industries due to the associated risks and investments. Collaborating with Contract Development and Manufacturing Organizations (CDMOs) could facilitate technology transfer and small-batch production (212).
9. Final remarks
The increasing incidence of bee sting envenomation necessitates a comprehensive understanding of its underlying mechanisms, clinical manifestations, and potential complications to inform effective public health interventions. This review elucidates the intricate effects of bee venom and its associated health consequences. While existing research provides valuable insights into venom composition and its impact, clinical management guidelines for bee sting envenomation remain limited. This review fills a critical knowledge gap by providing a comprehensive overview of the clinical implications of bee venom exposure. Our analysis underscores the complexity of venom-human interactions and the severity of potential outcomes. The development of novel treatment strategies, including an antivenom, is imperative to reduce mortality and long-term complications. However, until such interventions become available, alternative pharmacological approaches must be explored, considering their associated risks.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The APC was funded by FAPESP Proc 2021/11936-3 (RF). We are thankful to the Coordination of Superior Level Staff Improvement (CAPES) for the scholarship n° 88887.674376/2022-00 (JC) and 88887.826358/2023-00 (DA), and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo Research Foundation), scholarships to JC n° 2022/16060-1, to AP n° 2023/09921-3, FAPESP Process 2021/11936-3 and 2023/01554-1 (RF). We also thank the The National Council for Scientific and Technological Development (CNPq) scholarship to MP n° 305778/2023-4 and to AP n° 151190/2023-2, and the Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM), scholarship to FC n° 01.02.016301.01070/2023-13. RF is a CNPq PQ1C fellow researcher 303224/2018-5 and CNPq PQ1D research fellow n° 301608/2022-9. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Author contributions
JC: Conceptualization, Data curation, Methodology, Project administration, Writing – original draft. PR: Data curation, Investigation, Writing – review & editing. AP: Data curation, Investigation, Writing – original draft. BJ: Investigation, Visualization, Writing – review & editing. DA: Investigation, Visualization, Writing – review & editing. FP: Writing – review & editing. AA: Writing – review & editing. FC: Visualization, Writing – original draft, Writing – review & editing. MP: Writing – original draft, Writing – review & editing. RF: Formal analysis, Funding acquisition, Project administration, Visualization, Writing – original draft, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1437413/full#supplementary-material
References
- 1. Scott Schneider S, DeGrandi-Hoffman G, Smith DR. T HE A FRICAN H ONEY B EE : factors contributing to a successful biological invasion. Annu Rev Entomol. (2004) 49:351–76. doi: 10.1146/annurev.ento.49.061802.123359 [DOI] [PubMed] [Google Scholar]
- 2. Ferreira RS, Almeida RAMB, Barraviera SRCS, Barraviera B. Historical perspective and human consequences of africanized bee stings in the americas. J Toxicol Environ Health Part B. (2012) 15:97–108. doi: 10.1080/10937404.2012.645141 [DOI] [PubMed] [Google Scholar]
- 3. Pucca MB, Cerni FA, Oliveira IS, Jenkins TP, Argemí L, Sørensen CV, et al. Bee updated: current knowledge on bee venom and bee envenoming therapy. Front Immunol. (2019) 10:2090. doi: 10.3389/fimmu.2019.02090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Oliveira FA, Guimarães JV, dos Reis MA, Teixeira VdePA. Acidente humano por picadas de abelhas africanizadas. Rev Soc Bras Med Trop. (2000) 33:403–5. doi: 10.1590/S0037-86822000000400012 [DOI] [PubMed] [Google Scholar]
- 5. Barbosa AN, Boyer L, Chippaux J-P, Medolago NB, Caramori CA, Paixão AG, et al. A clinical trial protocol to treat massive Africanized honeybee (Apis mellifera) attack with a new apilic antivenom. J Venomous Anim Toxins including Trop Dis. (2017) 23:14. doi: 10.1186/s40409-017-0106-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diniz AG, Belmino JF, Araújo KA, Vieira AT, Leite Rde S. Epidemiology of honeybee sting cases in the state of ceará, northeastern Brazil. Rev Inst Med Trop Sao Paulo. (2016) 58:40–5. doi: 10.1590/S1678-9946201658040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Linard AT, Barros R, Sousa J, Leite R. Epidemiology of bee stings in Campina Grande, Paraíba state, Northeastern Brazil. J Venomous Anim Toxins including Trop Dis. (2014) 20:13. doi: 10.1186/1678-9199-20-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barbosa AN, Ferreira RS, de Carvalho FCT, Schuelter-Trevisol F, Mendes MB, Mendonça BC, et al. Single-arm, multicenter phase I/II clinical trial for the treatment of envenomings by massive africanized honey bee stings using the unique apilic antivenom. Front Immunol. (2021) 12:653151. doi: 10.3389/fimmu.2021.653151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Almeida RAM de B, Olivo TET, Mendes RP, Barraviera SRCS, Souza L do R, Martins JG, et al. Africanized honeybee stings: how to treat them. Rev Soc Bras Med Trop. (2011) 44:755–61. doi: 10.1590/S0037-86822011000600020 [DOI] [PubMed] [Google Scholar]
- 10. Scott Schneider S, DeGrandi-Hoffman G, Smith DR. The African honey bee: factors contributing to a successful biological invasion. Annu Rev Entomol. (2004) 49:351–76. doi: 10.1146/annurev.ento.49.061802.123359 [DOI] [PubMed] [Google Scholar]
- 11. Harpur BA, Kadri SM, Orsi RO, Whitfield CW, Zayed A. Defense Response in Brazilian Honey Bees (Apis mellifera scutellata × spp.) Is Underpinned by Complex Patterns of Admixture. Genome Biol Evol. (2020) 12:1367–77. doi: 10.1093/gbe/evaa128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zaluski R, Kadri SM, de Souza EA, da Silva VMC, da Silva JRC, Rodrigues-Orsi P, et al. Africanized honeybees in urban areas: a public health concern. Rev Soc Bras Med Trop. (2014) 47:659–62. doi: 10.1590/0037-8682-0254-2013 [DOI] [PubMed] [Google Scholar]
- 13. de Oliveira SK, Trevisol DJ, Parma GC, Ferreira Júnior RS, Barbosa AN, Barraviera B, et al. Honey bee envenoming in Santa Catarina, Brazil, 2007 through 2017: an observational, retrospective cohort study. Rev Soc Bras Med Trop. (2019) 52. doi: 10.1590/0037-8682-0418-2018 [DOI] [PubMed] [Google Scholar]
- 14. Marques MRV, Araújo KAM, Tavares AV, Vieira AA, Leite RS. Epidemiology of envenomation by Africanized honeybees in the state of Rio Grande do Norte, Northeastern Brazil. Rev Bras Epidemiologia. (2020) 23. doi: 10.1590/1980-549720200005 [DOI] [PubMed] [Google Scholar]
- 15. Kono IS, Freire RL, Caldart ET, Rodrigues F de S, Santos JA, Freire LGD, et al. Bee stings in Brazil: Epidemiological aspects in humans. Toxicon. (2021) 201:59–65. doi: 10.1016/j.toxicon.2021.08.014 [DOI] [PubMed] [Google Scholar]
- 16. Chippaux J-P. Epidemiology of envenomations by terrestrial venomous animals in Brazil based on case reporting: from obvious facts to contingencies. J Venomous Anim Toxins including Trop Dis. (2015) 21:13. doi: 10.1186/s40409-015-0011-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Costa AG, Chaves BA, Murta FLG, Sachett JAG, Sampaio VS, Silva VC, et al. Hymenoptera stings in Brazil: a neglected health threat in Amazonas State. Rev Soc Bras Med Trop. (2018) 51:80–4. doi: 10.1590/0037-8682-0109-2017 [DOI] [PubMed] [Google Scholar]
- 18. Mendonça-da-Silva I, Monteiro WM, Sachett JAG, Barbosa ES, Cordeiro-dos-Santos M, Lacerda MVG, et al. Bee sting envenomation severe cases in Manaus, Brazilian Amazon: clinical characteristics and immune markers of case reports. Rev Soc Bras Med Trop. (2021) 54:e20200319. doi: 10.1590/0037-8682-0319-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cristino JS, Salazar GM, MaChado VA, Honorato E, Farias AS, Vissoci JRN, et al. A painful journey to antivenom: The therapeutic itinerary of snakebite patients in the Brazilian Amazon (The QUALISnake Study). PloS Negl Trop Dis. (2021) 15:e0009245. doi: 10.1371/journal.pntd.0009245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baum KA, Tchakerian MD, Thoenes SC, Coulson RN. Africanized honey bees in urban environments: A spatio-temporal analysis. Landsc Urban Plan. (2008) 85:123–32. doi: 10.1016/j.landurbplan.2007.10.005 [DOI] [Google Scholar]
- 21. de Toledo VDAA, Toral FLB, de Miranda SB, Shiraishi A, Hashimoto JH, da Silva WR. Ocorrência e coleta de colônias e de enxames de abelhas africanizadas na zona urbana de Maringá, Estado do Paraná, Brasil. Acta Sci. (2006) 28:353–9. doi: 10.4025/actascianimsci.v28i3.53 [DOI] [Google Scholar]
- 22. Schmidt JO. Clinical consequences of toxic envenomations by Hymenoptera. Toxicon. (2018) 150:96–104. doi: 10.1016/j.toxicon.2018.05.013 [DOI] [PubMed] [Google Scholar]
- 23. Grunwald T, Bockisch B, Spillner E, Ring J, Bredehorst R, Ollert M. Molecular cloning and expression in insect cells of honeybee venom allergen acid phosphatase (Api m 3). J Allergy Clin Immunol. (2006) 117:848–54. doi: 10.1016/j.jaci.2005.12.1331 [DOI] [PubMed] [Google Scholar]
- 24. Pereira AFM, Cavalcante JS, Angstmam DG, Almeida C, Soares GS, Pucca MB, et al. Unveiling the pain relief potential: harnessing analgesic peptides from animal venoms. Pharmaceutics. (2023) 15:2766. doi: 10.3390/pharmaceutics15122766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. El Mehdi I, Falcão SI, Boujraf S, Mustapha H, Campos MG, Vilas-Boas M. Analytical methods for honeybee venom characterization. J Adv Pharm Technol Res. (2022) 13:154–60. doi: 10.4103/japtr.japtr_166_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fitzgerald KT, Flood AA. Hymenoptera stings. Clin Tech Small Anim Pract. (2006) 21:194–204. doi: 10.1053/j.ctsap.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 27. Elena Ionete R, Romina Dinca O, Tamaian R, Irina Geana E. Exploring apis mellifera venom compounds using highly efficient methods. Smart Energy and Sustainable Environment (2013) 16:97–108. [Google Scholar]
- 28. Schumacher M, Tveten M, Egen N. Rate and quantity of delivery of venom from honeybee stings. J Allergy Clin Immunol. (1994) 93:831–5. doi: 10.1016/0091-6749(94)90373-5 [DOI] [PubMed] [Google Scholar]
- 29. de Toledo LFM, Moore DCBC, Caixeta DMDL, Salú MDS, Farias CVB, Azevedo ZMA. Multiple bee stings, multiple organs involved: a case report. Rev Soc Bras Med Trop. (2018) 51:560–2. doi: 10.1590/0037-8682-0341-2017 [DOI] [PubMed] [Google Scholar]
- 30. Ediger D, Terzioglu K, Ozturk RT. Venom allergy, risk factors for systemic reactions and the knowledge levels among Turkish beekeepers. Asia Pac Allergy. (2018) 8:e15. doi: 10.5415/apallergy.2018.8.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen J, Guan S-M, Sun W, Fu H. Melittin, the major pain-producing substance of bee venom. Neurosci Bull. (2016) 32:265–72. doi: 10.1007/s12264-016-0024-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koyama N, Hirata K, Hori K, Dan K, Yokota T. Computer-assisted infrared thermographic study of axon reflex induced by intradermal melittin. Pain. (2000) 84:133–9. doi: 10.1016/S0304-3959(99)00192-X [DOI] [PubMed] [Google Scholar]
- 33. Koyama N, Hirata K, Hori K, Dan K, Yokota T. Biphasic vasomotor reflex responses of the hand skin following intradermal injection of melittin into the forearm skin. Eur J Pain. (2002) 6:447–53. doi: 10.1016/S1090-3801(02)00029-0 [DOI] [PubMed] [Google Scholar]
- 34. Sumikura H, Andersen OK, Drewes AM, Arendt-Nielsen L. A comparison of hyperalgesia and neurogenic inflammation induced by melittin and capsaicin in humans. Neurosci Lett. (2003) 337:147–50. doi: 10.1016/S0304-3940(02)01325-3 [DOI] [PubMed] [Google Scholar]
- 35. Sumikura H, Andersen OK, Drewes AM, Arendt-Nielsen L. Secondary heat hyperalgesia induced by melittin in humans. Eur J Pain. (2006) 10:121–1. doi: 10.1016/j.ejpain.2005.01.013 [DOI] [PubMed] [Google Scholar]
- 36. Lariviere WR, Melzack R. The bee venom test: a new tonic-pain test. Pain. (1996) 66:271–7. doi: 10.1016/0304-3959(96)03075-8 [DOI] [PubMed] [Google Scholar]
- 37. Chen J, Luo C, Li H-L, Chen H-S. Primary hyperalgesia to mechanical and heat stimuli following subcutaneous bee venom injection into the plantar surface of hindpaw in the conscious rat: a comparative study with the formalin test. Pain. (1999) 83:67–76. doi: 10.1016/S0304-3959(99)00075-5 [DOI] [PubMed] [Google Scholar]
- 38. Chen H-S, Chen J. Secondary heat, but not mechanical, hyperalgesia induced by subcutaneous injection of bee venom in the conscious rat: effect of systemic MK-801, a non-competitive NMDA receptor antagonist. Eur J Pain. (2000) 4:389–401. doi: 10.1053/eujp.2000.0197 [DOI] [PubMed] [Google Scholar]
- 39. Chen J, Chen H-S. Pivotal role of capsaicin-sensitive primary afferents in development of both heat and mechanical hyperalgesia induced by intraplantar bee venom injection. Pain. (2001) 91:367–76. doi: 10.1016/S0304-3959(00)00458-9 [DOI] [PubMed] [Google Scholar]
- 40. Chen Y-N, Li K-C, Li Z, Shang G-W, Liu DN, Lu ZM, et al. Effects of bee venom peptidergic components on rat pain-related behaviors and inflammation. Neuroscience. (2006) 138:631–40. doi: 10.1016/j.neuroscience.2005.11.022 [DOI] [PubMed] [Google Scholar]
- 41. Tsagareli MG, Nozadze I, Tsiklauri N, Carstens MI, Gurtskaia G, Carstens E. Thermal hyperalgesia and mechanical allodynia elicited by histamine and non-histaminergic itch mediators: respective involvement of TRPV1 and TRPA1. Neuroscience. (2020) 449:35–45. doi: 10.1016/j.neuroscience.2020.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cavalcante J dos S, Nogueira Júnior FA, Bezerra Jorge RJ, Almeida C. Pain modulated by Bothrops snake venoms: Mechanisms of nociceptive signaling and therapeutic perspectives. Toxicon. (2021) 201:105–14. doi: 10.1016/j.toxicon.2021.08.016 [DOI] [PubMed] [Google Scholar]
- 43. Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. (2017) 171:217–228.e13. doi: 10.1016/j.cell.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cevikbas F, Lerner EA. Physiology and pathophysiology of itch. Physiol Rev. (2020) 100:945–82. doi: 10.1152/physrev.00017.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yu Y-Q, Zhao Z-Y, Chen X-F, Xie F, Yang Y, Chen J. Activation of tetrodotoxin-resistant sodium channel naV1.9 in rat primary sensory neurons contributes to melittin-induced pain behavior. Neuromolecular Med. (2013) 15:209–17. doi: 10.1007/s12017-012-8211-0 [DOI] [PubMed] [Google Scholar]
- 46. Kaplan AP. Kinins, airway obstruction, and anaphylaxis. Anaphylaxis (2010) 95:67–84. doi: 10.1159/000315938 [DOI] [PubMed] [Google Scholar]
- 47. Silva A, Amrani Y, Trifilieff A, Landry Y. Involvement of B2 receptors in the bradykinin-induced relaxation of Guinea-pig isolated trachea. Br J Pharmacol. (1995) 114:103–8. doi: 10.1111/j.1476-5381.1995.tb14912.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Joseph K, Kaplan AP. Formation of bradykinin: A major contributor to the innate inflammatory response. Advances in Immunology (2005) 86:159–208. doi: 10.1016/S0065-2776(04)86005-X [DOI] [PubMed] [Google Scholar]
- 49. Schmaier AH. Assembly, activation, and physiologic influence of the plasma kallikrein/kinin system. Int Immunopharmacol. (2008) 8:161–5. doi: 10.1016/j.intimp.2007.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Murphey LJ, Malave HA, Petro J, Biaggioni I, Byrne DW, Vaughan DE, et al. Bradykinin and its metabolite bradykinin 1-5 inhibit thrombin-induced platelet aggregation in humans. J Pharmacol Exp Ther. (2006) 318:1287–92. doi: 10.1124/jpet.106.104026 [DOI] [PubMed] [Google Scholar]
- 51. Cleary DB, Ehringer WD, Maurer MC. Establishing the inhibitory effects of bradykinin on thrombin. Arch Biochem Biophys. (2003) 410:96–106. doi: 10.1016/S0003-9861(02)00677-X [DOI] [PubMed] [Google Scholar]
- 52. Landmesser U, Drexler H. Effect of angiotensin II type 1 receptor antagonism on endothelial function: role of bradykinin and nitric oxide. J Hypertens. (2006) 24:S39–43. doi: 10.1097/01.hjh.0000220405.38622.23 [DOI] [PubMed] [Google Scholar]
- 53. Gryglewski RJ, Uracz W, Chłopicki S, Marcinkiewicz E. Bradykinin as A major endogenous regulator of endothelial function. Pediatr Pathol Mol Med. (2002) 21:279–90. doi: 10.1080/02770930290056514 [DOI] [PubMed] [Google Scholar]
- 54. Gryglewski RJ, Święs J, Uracz W, Chłopicki S, Marcinkiewicz E. Mechanisms of angiotensin-converting enzyme inhibitor induced thrombolysis in Wistar rats. Thromb Res. (2003) 110:323–9. doi: 10.1016/j.thromres.2003.08.005 [DOI] [PubMed] [Google Scholar]
- 55. Pretorius M, Rosenbaum D, Vaughan DE, Brown NJ. Angiotensin-converting enzyme inhibition increases human vascular tissue-type plasminogen activator release through endogenous bradykinin. Circulation. (2003) 107:579–85. doi: 10.1161/01.CIR.0000046268.59922.A4 [DOI] [PubMed] [Google Scholar]
- 56. Felmeden DC, Lip GY. The renin-angiotensin-aldosterone system and fibrinolysis. J Renin-Angiotensin-Aldosterone System. (2000) 1:240–4. doi: 10.3317/jraas.2000.036 [DOI] [PubMed] [Google Scholar]
- 57. Prado M, Solano-Trejos G, Lomonte B. Acute physiopathological effects of honeybee (Apis mellifera) envenoming by subcutaneous route in a mouse model. Toxicon. (2010) 56:1007–17. doi: 10.1016/j.toxicon.2010.07.005 [DOI] [PubMed] [Google Scholar]
- 58. Sciani JM, Marques-Porto R, Lourenço A, Orsi R de O, Junior RSF, Barraviera B, et al. Identification of a novel melittin isoform from Africanized Apis mellifera venom. Peptides (NY). (2010) 31:1473–9. doi: 10.1016/j.peptides.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 59. Vogt W, Patzer P, Lege L, Oldigs H-D, Wille G. Synergism between phospholipase A and various peptides and SH-reagents in causing haemolysis. Naunyn-Schmiedebergs Archiv fur Pharmakologie. (1970) 265:442–54. doi: 10.1007/BF00997079 [DOI] [PubMed] [Google Scholar]
- 60. Habermann E. Bee and wasp venoms. Sci (1979). (1972) 177:314–22. doi: 10.1126/science.177.4046.314 [DOI] [PubMed] [Google Scholar]
- 61. Okamoto T, Isoda H, Kubota N, Takahata K, Takahashi T, Kishi T, et al. Melittin cardiotoxicity in cultured mouse cardiac myocytes and its correlation with calcium overload. Toxicol Appl Pharmacol. (1995) 133:150–63. doi: 10.1006/taap.1995.1136 [DOI] [PubMed] [Google Scholar]
- 62. Ferreira DB, Costa RS, Oliveira JSM, Muccillo G. Cardiac noradrenaline in experimental rat envenomation with africanized bee venom. Exp Toxicologic Pathol. (1994) 45:507–11. doi: 10.1016/S0940-2993(11)80516-6 [DOI] [PubMed] [Google Scholar]
- 63. Ferreira DB, Costa RS, De Oliveira JAM, Muccillo G. An infarct-like myocardial lesion experimentally induced in wistar rats with Africanized bee venom. J Pathol. (1995) 177:95–102. doi: 10.1002/path.1711770114 [DOI] [PubMed] [Google Scholar]
- 64. Florea A, Crăciun C. Bee (Apis mellifera) Venom Produced Toxic Effects of Higher Amplitude in Rat Thoracic Aorta than in Skeletal Muscle—An Ultrastructural Study. Microscopy Microanalysis. (2012) 18:304–16. doi: 10.1017/S1431927611012876 [DOI] [PubMed] [Google Scholar]
- 65. Černe K, Kristan KČ, Budihna MV, Stanovnik L. Mechanisms of changes in coronary arterial tone induced by bee venom toxins. Toxicon. (2010) 56:305–12. doi: 10.1016/j.toxicon.2010.03.010 [DOI] [PubMed] [Google Scholar]
- 66. Senthilkumaran S, David SS, Menezes RG, Thirumalaikolundusubramanian P. Acute myocardial infarction triggered by bee sting: An alternative view. Emergency Med Australasia. (2013) 25:615–5. doi: 10.1111/1742-6723.12140 [DOI] [PubMed] [Google Scholar]
- 67. Azevedo-Marques MM, Ferreira DB, Costa RS. Rhabdomyonecrosis experimentally induced in Wistar rats by Africanized bee venom. Toxicon. (1992) 30:344–8. doi: 10.1016/0041-0101(92)90875-6 [DOI] [PubMed] [Google Scholar]
- 68. Fletcher JE, Tripolitis L, Beech J. Bee venom melittin is a potent toxin for reducing the threshold for calcium-induced calcium release in human and equine skeletal muscle. Life Sci. (1992) 51:1731–8. doi: 10.1016/0024-3205(92)90302-6 [DOI] [PubMed] [Google Scholar]
- 69. Ownby CL, Powell JR, Jiang M, Fletcher JE. Melittin and phospholipase A2 from bee (Apis mellifera) venom cause necrosis of murine skeletal muscle in vivo . Toxicon. (1997) 35:67–80. doi: 10.1016/S0041-0101(96)00078-5 [DOI] [PubMed] [Google Scholar]
- 70. de Graaf DC, Brunain M, Scharlaken B, Peiren N, Devreese B, Ebo DG, et al. Two novel proteins expressed by the venom glands of Apis mellifera and Nasonia vitripennis share an ancient C1q-like domain. Insect Mol Biol. (2010) 19:1–10. doi: 10.1111/j.1365-2583.2009.00913.x [DOI] [PubMed] [Google Scholar]
- 71. Schartz ND, Tenner AJ. The good, the bad, and the opportunities of the complement system in neurodegenerative disease. J Neuroinflamm. (2020) 17:354. doi: 10.1186/s12974-020-02024-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Efetov KA, Shiriaev NV. Intensification of the interaction of IgG with C1q in the presence of melittin as a possible reason for anaphylaxis under the effect of bee venom. Ukr Biokhim Zh. (1999) 73:49–54. [PubMed] [Google Scholar]
- 73. Törő K, Borka K, Kardos M, Kristóf I, Sótonyi P. Expression and function of C5a receptor in a fatal anaphylaxis after honey bee sting*. J Forensic Sci. (2011) 56:526–8. doi: 10.1111/j.1556-4029.2010.01681.x [DOI] [PubMed] [Google Scholar]
- 74. Palm NW, Medzhitov R. Role of the inflammasome in defense against venoms. Proc Natl Acad Sci. (2013) 110:1809–14. doi: 10.1073/pnas.1221476110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nielsen OH, Bouchelouche PN, Berild D. Arachidonic acid and calcium metabolism in rnelittin stimulated neutrophils. Mediators Inflammation. (1992) 1:313–7. doi: 10.1155/S0962935192000462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. França FO, Benvenuti LA, Fan HW, Dos Santos DR, Hain SH, Picchi-Martins FR, et al. Severe and fatal mass attacks by “killer” bees (Africanized honey bees–Apis mellifera scutellata) in Brazil: clinicopathological studies with measurement of serum venom concentrations. Q J Med. (1994) 87:269–282. [PubMed] [Google Scholar]
- 77. Darwish DA, Masoud HMM, Abdel-Monsef MM, Helmy MS, Zidan HA, Ibrahim MA. Phospholipase A2 enzyme from the venom of Egyptian honey bee Apis mellifera lamarckii with anti-platelet aggregation and anti-coagulation activities. J Genet Eng Biotechnol. (2021) 19:10. doi: 10.1186/s43141-020-00112-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lee K-S, Kim B-Y, Park M-J, Deng Y, Kim J-M, Kim Y-H, et al. Bee venom induces acute inflammation through a H2O2-mediated system that utilizes superoxide dismutase. Toxins (Basel). (2022) 14:558. doi: 10.3390/toxins14080558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Guzel M, Akar H, Erenler AK, Baydin A, Kayabas A. Acute ischemic stroke and severe multiorgan dysfunction due to multiple bee stings. Turk J Emerg Med. (2016) 16:126–8. doi: 10.1016/j.tjem.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cavalcante JS, de Almeida DEG, Santos-Filho NA, Sartim MA, de Almeida Baldo A, Brasileiro L, et al. Crosstalk of inflammation and coagulation in bothrops snakebite envenoming: endogenous signaling pathways and pathophysiology. Int J Mol Sci. (2023) 24:11508. doi: 10.3390/ijms241411508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Popescu NI, Lupu C, Lupu F. Disseminated intravascular coagulation and its immune mechanisms. Blood. (2022) 139:1973–86. doi: 10.1182/blood.2020007208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zolfagharian H, Mohajeri M, Babaie M. Honey bee venom (Apis mellifera) contains anticoagulation factors and increases the blood-clotting time. J Pharmacopuncture. (2015) 18:7–11. doi: 10.3831/KPI.2015.18.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. de Roodt AR, Lanari LC, Lago NR, Bustillo S, Litwin S, Morón-Goñi F, et al. Toxicological study of bee venom (Apis mellifera mellifera) from different regions of the province of Buenos Aires, Argentina. Toxicon. (2020) 188:27–38. doi: 10.1016/j.toxicon.2020.09.014 [DOI] [PubMed] [Google Scholar]
- 84. Elieh Ali Komi D, Shafaghat F, Zwiener RD. Immunology of bee venom. Clin Rev Allergy Immunol. (2018) 54:386–96. doi: 10.1007/s12016-017-8597-4 [DOI] [PubMed] [Google Scholar]
- 85. Cavalcante JS, Brito IM da C, De Oliveira LA, De Barros LC, Almeida C, Rossini BC, et al. Experimental Bothrops atrox Envenomation: Blood Plasma Proteome Effects after Local Tissue Damage and Perspectives on Thromboinflammation. Toxins (Basel). (2022) 14:613. doi: 10.3390/toxins14090613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cavalcante JS, Borges da Silva WRG, de Oliveira LA, Brito IMC, Muller KS, J. Vidal IS, et al. Blood plasma proteome alteration after local tissue damage induced by Bothrops erythromelas snake venom in mice. J Proteomics. (2022) 269:104742. doi: 10.1016/j.jprot.2022.104742 [DOI] [PubMed] [Google Scholar]
- 87. Cavalcante J dos S, de Almeida CAS, Clasen MA, da Silva EL, de Barros LC, Marinho AD, et al. A fingerprint of plasma proteome alteration after local tissue damage induced by Bothrops leucurus snake venom in mice. J Proteomics. (2022) 253:104464. doi: 10.1016/j.jprot.2021.104464 [DOI] [PubMed] [Google Scholar]
- 88. Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. (2021) 22:266–82. doi: 10.1038/s41580-020-00324-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. (2021) 31:107–25. doi: 10.1038/s41422-020-00441-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Conde de la Rosa L, Goicoechea L, Torres S, Garcia-Ruiz C, Fernandez-Checa JC. Role of oxidative stress in liver disorders. Livers. (2022) 2:283–314. doi: 10.3390/livers2040023 [DOI] [Google Scholar]
- 91. Witharana RA, Dissanayake A, Karunaratne I, Wijesinghe S. A rare case of micro-angiopathic hemolytic anemia due to envenoming by giant asian honey bee (Apis dorsata). Wilderness Environ Med. (2021) 32:340–3. doi: 10.1016/j.wem.2021.01.008 [DOI] [PubMed] [Google Scholar]
- 92. Navaradnam P, Suganthan N, Kumanan T, Sujanitha V, Mayorathan U. Kounis syndrome and multiorgan failure following multiple wasp stings. Cureus. (2021) 13. doi: 10.7759/cureus.14606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Senturk A, Dalkiran B, Acikgoz B, Aksu I, Acikgoz O, Kiray M. The effects of bee venom on liver and skeletal muscle in exhaustive swimming rats. Biol Futur. (2022) 73:237–44. doi: 10.1007/s42977-022-00115-6 [DOI] [PubMed] [Google Scholar]
- 94. Cichoż-Lach H. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. (2014) 20:8082. doi: 10.3748/wjg.v20.i25.8082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ravindran M, Khan MA, Palaniyar N. Neutrophil extracellular trap formation: physiology, pathology, and pharmacology. Biomolecules. (2019) 9:365. doi: 10.3390/biom9080365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. de Bont CM, Boelens WC, Pruijn GJM. NETosis, complement, and coagulation: a triangular relationship. Cell Mol Immunol. (2019) 16:19–27. doi: 10.1038/s41423-018-0024-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Vorobjeva NV, Chernyak BV. NETosis: molecular mechanisms, role in physiology and pathology. Biochem (Moscow). (2020) 85:1178–90. doi: 10.1134/S0006297920100065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Katkar GD, Sundaram MS, NaveenKumar SK, Swethakumar B, Sharma RD, Paul M, et al. NETosis and lack of DNase activity are key factors in Echis carinatus venom-induced tissue destruction. Nat Commun. (2016) 7:11361. doi: 10.1038/ncomms11361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Han HJ, Park SH, Lee JH, Yoon BC, Park KM, Mar WC, et al. Involvement of oxidative stress in bee venom-induced inhibition of na+/glucose cotransporter in renal proximal tubule cells. Clin Exp Pharmacol Physiol. (2002) 29:564–8. doi: 10.1046/j.1440-1681.2002.03685.x [DOI] [PubMed] [Google Scholar]
- 100. dos Reis MA, Costa RS, Coimbra TM, Dantas M, Gomes UA. Renal changes induced by envenomation with africanized bee venom in female wistar rats. Kidney Blood Press Res. (1997) 20:271–7. doi: 10.1159/000174157 [DOI] [PubMed] [Google Scholar]
- 101. dos Reis MA, Costa RS, Coimbra TM, Teixeira VPA. Acute renal failure in experimental envenomation with africanized bee venom. Ren Fail. (1998) 20:39–51. doi: 10.3109/08860229809045088 [DOI] [PubMed] [Google Scholar]
- 102. Gyurászová M, Gurecká R, Bábíčková J, Tóthová Ľ. Oxidative stress in the pathophysiology of kidney disease: implications for noninvasive monitoring and identification of biomarkers. Oxid Med Cell Longev. (2020) 2020:1–11. doi: 10.1155/2020/5478708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. De Matos IM, Rocha OA, Leite R, Freire-Maia L. Lung oedema induced by Tityus serrulatus scorpion venom in the rat. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. (1997) 118:143–8. doi: 10.1016/S0742-8413(97)00086-8 [DOI] [PubMed] [Google Scholar]
- 104. Freire-Maia L, Almeida HO, Cunha-Melo JR, Azevedo AD, Barroso J. Mechanism of the pulmonary edema induced by intravenous injection of scorpion toxin in the rat. Agents Actions. (1978) 8:113–8. doi: 10.1007/BF01972412 [DOI] [PubMed] [Google Scholar]
- 105. Freire-Maia L, de Matos IM. Heparin or a PAF antagonist (BN-52021) prevents the acute pulmonary edema induced by Tityus serrulatus scorpion venom in the rat. Toxicon. (1993) 31:1207–10. doi: 10.1016/0041-0101(93)90137-8 [DOI] [PubMed] [Google Scholar]
- 106. Coelho FM, Pessini AC, Coelho AM, Pinho VS, Souza DG, Arantes EC, et al. Platelet activating factor receptors drive CXC chemokine production, neutrophil influx and edema formation in the lungs of mice injected with Tityus serrulatus venom. Toxicon. (2007) 50:420–7. doi: 10.1016/j.toxicon.2007.04.009 [DOI] [PubMed] [Google Scholar]
- 107. Sheshala K, Misra KC, Hemanth C, Appasani S. Bee sting to boerhaave's syndrome. Indian J Crit Care Med. (2021) 25:346–8. doi: 10.5005/jp-journals-10071-23770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pirasath S, Senthan V, Seneviratne MH. Kounis syndrome: Acute myocardial infarction following multiple bee stings. SAGE Open Med Case Rep. (2021) 9:2050313X2199920. doi: 10.1177/2050313X21999206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mehdi Z, Gupta M, Garg A, Kaur N. Beyond anaphylaxis : acute myocardial infarction with is- chemic stroke after bee sting a case report. Iranian Journal of Emergency Medicine (2023) 10:3–6. doi: 10.22037/ijem.v10i1.40469 [DOI] [Google Scholar]
- 110. Bindu C, Manuprakash S, Srinivasa B. Acute myocardial injury in multiple bee stings - Case report. Int J Med Sci Public Health. (2013) 2:1107. doi: 10.5455/ijmsph.2013.200620132 [DOI] [Google Scholar]
- 111. Gopinath B, Kumar G, Nayaka R, Ekka M. Kounis syndrome and atrial fibrillation after bee sting: A case report. J Family Med Prim Care. (2022) 11:7460. doi: 10.4103/jfmpc.jfmpc_901_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. França FOS, Benvenuti LA, Fan HW. Severe and fatal mass attacks by ‘killer’ bees (Africanized honey bees—Apis mellifera scutellata) in Brazil: clinicopathological studies with measurement of serum venom concentrations. QJM: Int J Med. (1994) 87:269–282. doi: 10.1093/oxfordjournals.qjmed.a068927 [DOI] [PubMed] [Google Scholar]
- 113. Mellyana O, Adelina D, Kusuma J. Acute kidney injury due to multiple bee stings in a 3 years old girl. Med Hospitalia : J Clin Med. (2019) 6:64–70. doi: 10.36408/mhjcm.v6i1.382 [DOI] [Google Scholar]
- 114. Tsuruta K, Yokoi K, Yoshioka G, Chen W, Jojima K, Hongo H, et al. Different types of Kounis syndrome caused by different episodes of bee sting anaphylaxis: Misfortunes never come singly. J Cardiol cases. (2022) 26:81–4. doi: 10.1016/j.jccase.2022.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. E Khoda MM, Islam RN, Rahim MA, Mansur MA. Wcn23-0329 kounis syndrome with fatal outcome: A case report. Kidney Int Rep. (2023) 8:S5. doi: 10.1016/j.ekir.2023.02.011 [DOI] [Google Scholar]
- 116. Aytekin G, Çölkesen F, Yıldız E, Arslan Ş, Oltulu P. Kounis syndrome in a patient with secondary mast cell activation syndrome after a bee sting. Ann Med Res. (2020) 1. doi: 10.5455/annalsmedres.2019.10.671 [DOI] [Google Scholar]
- 117. Kamalesh TN, Nishanth S, Akanksh MD, Naveen K. Kounis syndrome: is it a heralder or just a mimicker of acute coronary syndrome? J OF Clin AND Diagn Res. (2023) 17. doi: 10.7860/JCDR/2023/60999.18040 [DOI] [Google Scholar]
- 118. Thwe EE, Sudnik P, Dobrovolschi C, Krishnamurthy M. Kounis syndrome: an allergic acute coronary syndrome due to a bee sting. Cureus. (2022) 14. doi: 10.7759/cureus.26395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Katsanou K, Tsiafoutis I, Kounis NG. Timeo apis mellifera and dona ferens: bee sting-induced Kounis syndrome. Clin Chem Lab Med (CCLM). (2018) 56:e197–200. doi: 10.1515/cclm-2018-0002 [DOI] [PubMed] [Google Scholar]
- 120. Acehan S, Satar S, Gulen M, Yucel C, Segmen MS. Angina and arrhythmia symptoms following multiple bee stings: kounis syndrome. Wilderness Environ Med. (2022) 33:417–21. doi: 10.1016/j.wem.2022.06.003 [DOI] [PubMed] [Google Scholar]
- 121. du Toit-Prinsloo L, Morris NK, Meyer P, Saayman G. Deaths from bee stings: a report of three cases from Pretoria, South Africa. Forensic Sci Med Pathol. (2016) 12:81–5. doi: 10.1007/s12024-015-9737-x [DOI] [PubMed] [Google Scholar]
- 122. Le H-Y, Tien ND, Son PN, Viet Hoa LT, Phuong LL, Hai PD. Extracorporeal membrane oxygenation support in refractory anaphylactic shock after bee stings: A case report. Perfusion. (2023) 38:1308–10. doi: 10.1177/02676591221103540 [DOI] [PubMed] [Google Scholar]
- 123. Chaudry A. Mobitz type-2 heart block after a bee-sting. Cureus. (2020) 12. doi: 10.7759/cureus.11856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Su Z, Hu Z, Wang L, Wang Y, Fang X, Ye P. Visual loss caused by central retinal artery occlusion after bee sting: A case report. Front Med (Lausanne). (2021) 8:707978. doi: 10.3389/fmed.2021.707978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. de Azevedo RV, de Paiva RB, Ades F, David CM. Síndrome de envenenamento por 2000 picadas de abelhas africanizadas. Relato caso. Rev Bras Ter Intensiva. (2006) 18:99–103. doi: 10.1590/S0103-507X2006000100016 [DOI] [PubMed] [Google Scholar]
- 126. Dural İE. Kounis Syndrome with Pericarditis Epistenocardica : a case report. (2022), 1–12. doi: 10.21203/rs.3.rs-1342895/v1 [DOI] [Google Scholar]
- 127. Seecheran RV, Ramdin R, Singh S, Seecheran V, Persad S, Peram L, et al. Africanized honey bee sting-induced stress-related cardiomyopathy: A bee or octopus trap. Cureus. (2021) 13. doi: 10.7759/cureus.16681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Önder Ö, Aktan AH. Encephalitis and toxic hepatitis caused by bee sting: an unusual case report. Turkish J Intensive Care. (2021) 19:196–9. doi: 10.4274/tybd.galenos.2021.86548 [DOI] [Google Scholar]
- 129. Ratnayake GM, Weerathunga PN, Dilrukshi MSA, Amara Witharana EWR, Jayasinghe S. Giant honey bee (Apis dorsata) sting and acute limb ischemia: a case report and review of the literature. BMC Res Notes. (2018) 11:327. doi: 10.1186/s13104-018-3422-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Odinaka K, Achigbu K, Ike I, Iregbu F. Bee sting envenomation resulting in gross haematuria in an eight-year-old Nigerian male with sickle cell anaemia: A case report. Nigerian Med J. (2015) 56:69. doi: 10.4103/0300-1652.149175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Akgul MH, Bagırov E. Brachial artery thrombosis following bee sting, case report. Int J Surg Case Rep. (2021) 78:184–6. doi: 10.1016/j.ijscr.2020.11.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Cil M, Leblebisatan G, Leblebisatan S, Barutcu A, Cil MK, Kilinc Y. Deep vein thrombosis after a wild bee sting. J Pediatr Hematol Oncol. (2022) 44:e241–2. doi: 10.1097/MPH.0000000000002072 [DOI] [PubMed] [Google Scholar]
- 133. Farhat E, Tegg E, Mohammed S, Grzechnik E, Favaloro EJ. Not as sweet as honey: A rare case of an apparent factor V “inhibitor” in association with bee sting anaphylaxis. Am J Hematol. (2018) 93:965–70. doi: 10.1002/ajh.25121 [DOI] [PubMed] [Google Scholar]
- 134. Akyıldız B, Özsoylu S, Öztürk MA, İnci A, Düzlü Ö, Yıldırım A. A fatal case caused by massive honey bee stings. Turkish J Pediatr. (2016) 57:611–4. [PubMed] [Google Scholar]
- 135. Rauf A, Vijayan A, Hashitha V, John S, Peringat J. Rhabdomyolysis and acute kidney injury following multiple bee stings in a child: A case report. J Pediatr Crit Care. (2021) 8:252. doi: 10.4103/jpcc.jpcc_38_21 [DOI] [Google Scholar]
- 136. Babikir H, Ibrahim N, Altayeb Z, Ahmed A. A rare acute haemorrhagic stroke and severe multiorgan dysfunction following massive honeybee stings. Sudan J Paediatr. (2021) 121:209–14. doi: 10.24911/SJP.106-1598962628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Khalighi Z, Azami G, Shafiei E, Sahebi A, Mozafari A. Thrombotic thrombocytopenic purpura following honeybee envenomation: A case report. Int J Med Toxicol Forensic Med. (2020) 10:28794–28794. doi: 10.32598/ijmtfm.v10i2.28794 [DOI] [Google Scholar]
- 138. Mukesh R, Rekha JS, Shaha KK, Mylapalli J. Adrenal hemorrhage and rhabdomyolysis following mass bee sting envenomation: An autopsy based case report. J Indian Soc Toxicol. (2020) 16:47–50. doi: 10.31736/2020v16i2/47-50 [DOI] [Google Scholar]
- 139. Riches KJ, Gillis D, James RA. An autopsy approach to bee sting-related deaths. Pathology. (2002) 34:257–62. doi: 10.1080/00313020220131327 [DOI] [PubMed] [Google Scholar]
- 140. Pujari S, Ranjan R, Verghese S, Manayath G, Narendran V. Case report of central retinal artery occlusion following bee sting injury: A possible link? Oman J Ophthalmol. (2021) 14:112. doi: 10.4103/ojo.ojo_27_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Mondello C, Baldino G, Cianci V, Forzese E, Asmundo A, Ieni A, et al. Postmortem biochemistry and immunohistochemistry in anaphylactic death due to hymenoptera sting: A forensic case report. Int J Environ Res Public Health. (2023) 20:5640. doi: 10.3390/ijerph20095640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Bui PV, Haas NL, Herrman NWC, Macias M, Hoch V, Schaeffer W, et al. Epinephrine administered for anaphylaxis unmasking a type 1 brugada pattern on electrocardiogram. J Emerg Med. (2019) 56:444–7. doi: 10.1016/j.jemermed.2018.12.045 [DOI] [PubMed] [Google Scholar]
- 143. Fakhar M, Zakariaei Z, Sharifpour A, Soleymani M, Zakariaei A. Fatal outcome following multiple bee stings: A rare case. Clin Case Rep. (2022) 10:e05303. doi: 10.1002/ccr3.5303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Contreras Zuniga E, Zuluaga SX, Casas Quiroga IC. Envenenamiento por múltiples picaduras de abejas y choque anafiláctico secundario: descripción de un caso clínico y revisión de la literatura. Acta toxicológica Argent. (2008) 16:27–32. [Google Scholar]
- 145. Izaguirre González AI, Martínez-Zepeda ER, Rivas-Godoy AF, Sánchez-Sierra LE, Díaz-Robbio I, Izaguirre González AI, et al. Falla multiorgánica secundaria a múltiples picaduras de abeja (Apis mellifera). Rev la Facultad Medicina (México). (2018) 61:31–7. [Google Scholar]
- 146. Lubis M, Puspitasari R, Saragih RA. Multiple bee stings induced multiorgan dysfunction in a 4 year old female. Pediatr Oncall. (2019) 16:19–20. doi: 10.7199/ped.oncall.2019.30 [DOI] [Google Scholar]
- 147. Sunny JM, Abrencillo R. Massive bee envenomation treated by therapeutic plasma exchange. J Clin Apher. (2021) 36:654–7. doi: 10.1002/jca.21898 [DOI] [PubMed] [Google Scholar]
- 148. Jain J, Banait S, Srivastava A, Lodhe R. Stroke intracerebral multiple infarcts: Rare neurological presentation of honey bee bite. Ann Indian Acad Neurol. (2012) 15:163. doi: 10.4103/0972-2327.95008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Gupta A. Acute fatal stroke associated with honeybee sting. J Neurol Neuromedicine. (2019) 5:18–21. doi: 10.29245/2572.942X/2019/1.1262 [DOI] [Google Scholar]
- 150. Betten DP, Richardson WH, Tong TC, Clark RF. Massive honey bee envenomation-induced rhabdomyolysis in an adolescent. Pediatrics. (2006) 117:231–5. doi: 10.1542/peds.2005-1075 [DOI] [PubMed] [Google Scholar]
- 151. Hiran S, Pande TK, Pani S, Gupta R, Vishwanathan KA. Rhabdomyolysis due to multiple honey bee stings. Postgrad Med J. (1994) 70:937–7. doi: 10.1136/pgmj.70.830.937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Seelarathna RMM, Navakumaran M, Kumanan T. Bee stings an unusual cause of severe rhabdomyolysis: A case report and literature review. Jaffna Med J. (2020) 32:42–3. doi: 10.4038/jmj.v32i2.107 [DOI] [Google Scholar]
- 153. Constantino K, Pawlukiewicz AJ, Spear L. A case report on rhabdomyolysis after multiple bee stings. Cureus. (2020) 12. doi: 10.7759/cureus.9501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Geoffroy S, Fremery A, Lambert Y, Marty C, Elenga N. Case report: acute kidney failure due to massive envenomation of a two-year-old child caused by killer bee stings. Am J Trop Med Hyg. (2021) 105:222–4. doi: 10.4269/ajtmh.20-1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Sathi S, Garg AK, Chand J, Singh MK, Saini VS, Trivedi A. Severe acute kidney injury after multiple honey bee stings. Int J Nephrol Kidney Fail. (2020) 6. doi: 10.16966/2380-5498.195 [DOI] [Google Scholar]
- 156. Mahale R, Mehta A, Shankar A, Buddaraju K, John A, Javali M, et al. Isolated posterior circulation stroke following honey-bee sting. Neurol India. (2016) 64:116. doi: 10.4103/0028-3886.178053 [DOI] [PubMed] [Google Scholar]
- 157. Saini AG, Sankhyan N, Suthar R, Singhi P. Acute axonal polyneuropathy following honey-bee sting. J Child Neurol. (2014) 29:674–6. doi: 10.1177/0883073813517262 [DOI] [PubMed] [Google Scholar]
- 158. Poddar K, Poddar S, Singh A. Acute polyradiculoneuropathy following honey bee sting. Ann Indian Acad Neurol. (2012) 15:137. doi: 10.4103/0972-2327.95000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Walter O, Macrine O, George Michael OO. Case Report: An unusual case of bee envenomation presenting with acute kidney injury, cavernous venous thrombosis and multiple episodes of convulsions. Global J Med Clin Case Rep. (2020) 7:103–6. doi: 10.17352/2455-5282.000109 [DOI] [Google Scholar]
- 160. Zuluaga C, Lara J, Berrouet MC. Síndrome de Guillain-Barré asociado a accidente apídico : reporte de caso. Medicina Upb. (2021) 40:77–81. doi: 10.18566/medupb.v40n1.a11 [DOI] [Google Scholar]
- 161. Ramlackhansingh AF, Seecheran N. Africanised honey bee sting-induced ischaemic stroke. BMJ Case Rep. (2020) 13:e234877. doi: 10.1136/bcr-2020-234877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Varuni K, Sivansuthan S, Joseph Piratheepan G, Gajanthan R. A case report on unusual cause of young ischemic Cerebrovascular Accident: a rare complication of honey bee stings. Jaffna Med J. (2018) 30:32–4. doi: 10.4038/jmj.v30i2.25 [DOI] [Google Scholar]
- 163. Kabra R, Andhale A, Acharya S, Kumar S, Sawant R. Acute ischemic stroke post honeybee sting: A rare case report. Cureus. (2022) 14. doi: 10.7759/cureus.31851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Abhishek BR, Velayutham SS, Jeyaraj KM, Sowmini PR, Kumar MS, Sarvanan SV, et al. Thrombolysis in ischemic stroke after bee sting: A rare scenario. Ann Indian Acad Neurol. (2021) 24:985. doi: 10.4103/aian.AIAN_770_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Rathnayaka N, Ranathunga AN, Rathnayaka B, Kularatne SAM. Massive cerebral infarct following multiple honeybee stings. Ceylon Med J. (2021) 66:151. doi: 10.4038/cmj.v66i3.9494 [DOI] [PubMed] [Google Scholar]
- 166. Sancar E, Ararat E, Avci S. Acute ischemic stroke associated with allergic reaction case report. Ann Med Case Rep. (2020) 2:1015. [Google Scholar]
- 167. Ryakitimbo A, Kennedy M, Shao E, Itana ME, Mbwasi R, Kinabo G, et al. Acute kidney injury in a Tanzanian boy following multiple bee stings in resource-limited setting: a case report. Oxf Med Case Rep. (2018) 2018:omy070. doi: 10.1093/omcr/omy070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Singer E, Lande L. Critical upper airway edema after a bee sting to the uvula. Wilderness Environ Med. (2022) 33:236–8. doi: 10.1016/j.wem.2022.02.001 [DOI] [PubMed] [Google Scholar]
- 169. da Silva GAR, Pires KL, Soares DC de S, Ferreira MR, Ferry FR de A, Motta RN, et al. RRH: envenoming syndrome due to 200 stings from Africanized honeybees. Rev Inst Med Trop Sao Paulo. (2013) 55:61–4. doi: 10.1590/S0036-46652013000100011 [DOI] [PubMed] [Google Scholar]
- 170. Mathew A, Chrispal A, David T. Acute myocardial injury and rhabdomyolysis caused by multiple bee stings. J Assoc Physicians India. (2011) 59:518–20. [PubMed] [Google Scholar]
- 171. Haniff S, Butler ME, Abou-Jaoude EA, Lenahan ML. An unusual trigger of grover’s disease (GD). Cureus. (2023) 15. doi: 10.7759/cureus.40648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Gudiseva H, Uddaraju M, Pradhan S, Das M, Mascarenhas J, Srinivasan M, et al. Ocular manifestations of isolated corneal bee sting injury, management strategies, and clinical outcomes. Indian J Ophthalmol. (2018) 66:262. doi: 10.4103/ijo.IJO_600_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Dau M, Ngu B, Norina T, Jaffar T, Hussein A. Case series of cornea bee sting : different approaches of management. Journal of Biomedical and Clinical Sciences (JBCS) (2019) 4:7–10. [Google Scholar]
- 174. Dai XX, Hao JL, Zhou DD, Teng SY, Hui P, Lu CW. A successfully managed case of corneal bee sting: A case report and literature review. Iran Red Crescent Med J. (2019) 21:0–4. doi: 10.5812/ircmj.99335.Case [DOI] [Google Scholar]
- 175. Chilibeck C, Wang N, Murphy C. Making a bee-line for the eye: a limbal sting and retained honey bee. Clin Exp Optom. (2021) 104:538–40. doi: 10.1080/08164622.2021.1878837 [DOI] [PubMed] [Google Scholar]
- 176. Duff- Lynes SM, Martin P, Horn EP. Management of bulbar conjunctival injury by honeybee sting: A case report of a retained honeybee stinger. Am J Ophthalmol Case Rep. (2022) 25:101365. doi: 10.1016/j.ajoc.2022.101365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Grüb M, Mielke J, Schlote T. Bienenstichverletzung der Hornhaut - eine Kasuistik1. Klin Monbl Augenheilkd. (2001) 218:747–50. doi: 10.1055/s-2001-18669 [DOI] [PubMed] [Google Scholar]
- 178. Isawumi MA, Hassan M. Honeybee sting of the sclera: occular features, treatment, outcome and presumed pathogenesis. Pan Afr Med J. (2014) 17. doi: 10.11604/pamj.2014.17.30.3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Bhalerao S, Singh P, Rani P, Rathi V. The sting of a honey bee: An unusual subconjunctival foreign body. Indian J Ophthalmol. (2017) 65:1226. doi: 10.4103/ijo.IJO_533_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Newman AR, Beckman TJ, Meiklejohn BD, Green MD. Migration of retained tarsal bee stinger onto the ocular surface causing superficial keratopathy. Digital J Ophthalmol. (2022) 28:31–3. doi: 10.5693/djo.02.2021.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Chen MC, Melnychuk V. Corneal bee sting: improvement in the acute stage in the absence of treatment. Pan Afr Med J. (2020) 37. doi: 10.11604/pamj.2020.37.54.20267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Davidorf OA, Ng AE, Davidorf JM. Retained eyelid bee stinger: A case of secondary corneal abrasion. Am J Ophthalmol Case Rep. (2020) 18:100670. doi: 10.1016/j.ajoc.2020.100670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Chauhan D. Corneal honey bee sting: endoilluminator-assisted removal of retained stinger. Int Ophthalmol. (2012) 32:285–8. doi: 10.1007/s10792-012-9553-1 [DOI] [PubMed] [Google Scholar]
- 184. Dogra M, Narang S, Sood S, Gupta P. Successful management of bee sting induced Aspergillus fumigatus endophthalmitis and scleritis. Indian J Ophthalmol. (2018) 66:461. doi: 10.4103/ijo.IJO_889_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Ahmed M, Lee CS, McMillan B, Jain P, Wiley L, Odom JV, et al. Predicting visual function after an ocular bee sting. Int Ophthalmol. (2019) 39:1621–6. doi: 10.1007/s10792-018-0978-z [DOI] [PubMed] [Google Scholar]
- 186. Tyagi M, Reddy S, Basu S, Pappuru R, Dave V. Endoscopic visualization-assisted corneal bee sting removal. Indian J Ophthalmol. (2021) 69:423. doi: 10.4103/ijo.IJO_1161_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Al Amry M, Al Ghadeer H, Al Gethami AR. Bee sting presumed endophthalmitis: a devastating ocular outcome. Int J Retina Vitreous. (2021) 7:52. doi: 10.1186/s40942-021-00320-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Limaiem R, Chaabouni A, El Maazi A, Mnasri H, Mghaieth F, El Matri L. Lésions oculaires par piqûre d’abeille. À propos d’un cas. J Fr Ophtalmol. (2009) 32:277–9. doi: 10.1016/j.jfo.2009.01.007 [DOI] [PubMed] [Google Scholar]
- 189. Rishi E, Rishi P. Intraocular inflammation in a case of bee sting injury. GMS Ophthalmol cases. (2018) 8:Doc02. doi: 10.3205/oc000084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. del Carmen Zambrano-Infantino R, Piñerúa-Gonsálvez JF, Montaño C, Rodríguez C. Neuritis óptica por picadura de abeja. Investigacion Clinica (Venezuela). (2013) 54:180–5. [PubMed] [Google Scholar]
- 191. da Cunha CEX, Ribeiro MVMR, Soares ARG, Castro LR, Jucá JV de O. Uveíte anterior hipertensiva após picada de abelha: um relato de caso. Rev Med (Rio J). (2023) 102. doi: 10.11606/issn.1679-9836.v102i1e-194512 [DOI] [Google Scholar]
- 192. Silva GBDJ, Vasconcelos AGJ, Rocha AMT, Vasconcelos VR, Barros JN, Fujishima JS, et al. Acute kidney injury complicating bee stings – a review. Rev Inst Med Trop Sao Paulo. (2017) 59:e25. doi: 10.1590/s1678-9946201759025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. RodrÍguez-Ruiz C, Puig-Carrión G, Delgado-Nieves A, López-Candales A. Kounis syndrome: A more commonly encountered cause of acute coronary syndrome. Heart Views. (2019) 20:122. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_43_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Ruwanpathirana P, Priyankara D. Clinical manifestations of wasp stings: a case report and a review of literature. Trop Med Health. (2022) 50:82. doi: 10.1186/s41182-022-00475-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Wist S, Clivaz J, Sattelmayer M. Muscle strengthening for hemiparesis after stroke: A meta-analysis. Ann Phys Rehabil Med. (2016) 59:114–24. doi: 10.1016/j.rehab.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 196. Pedroso JL, Vale TC, Braga-Neto P, Dutra LA, França MC, Jr., Teive HAG, et al. Acute cerebellar ataxia: differential diagnosis and clinical approach. Arq Neuropsiquiatr. (2019) 77:184–93. doi: 10.1590/0004-282x20190020 [DOI] [PubMed] [Google Scholar]
- 197. Uzel IF, Arslan M, Demirhan O. Anaphylaxis and diffuse alveolar hemorrhage following bee sting. Respir Case Rep. (2013) 2:10–3. doi: 10.5505/respircase.2013.00710 [DOI] [Google Scholar]
- 198. Lara AR, Schwarz MI. Diffuse alveolar hemorrhage. Chest. (2010) 137:1164–71. doi: 10.1378/chest.08-2084 [DOI] [PubMed] [Google Scholar]
- 199. Thumtecho S, Suteparuk S, Sitprija V. Pulmonary involvement from animal toxins: the cellular mechanisms. J Venomous Anim Toxins including Trop Dis. (2023) 29:e20230026. doi: 10.1590/1678-9199-jvatitd-2023-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Cai Z-Y, Xu B-P, Zhang W-H, Peng H-W, Xu Q, Yu H-B, et al. Acute respiratory distress syndrome following multiple wasp stings treated with extracorporeal membrane oxygenation: A case report. World J Clin cases. (2022) 10:11122–7. doi: 10.12998/wjcc.v10.i30.11122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Semler-Collery A, Hayek G, Ramadier S, Perone J-M. A case of conjunctival bee sting injury with review of the literature on ocular bee stings. Am J Case Rep. (2019) 20:1284–9. doi: 10.12659/AJCR.917592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Çaça I, Ari S, Ülü K, Ayata A. Bee sting of the cornea: A case study and review of the literature. Ann Ophthalmol. (2006) 38:77–80. doi: 10.1385/AO:38:1:77 [DOI] [PubMed] [Google Scholar]
- 203. De D, Caso Clínico UN, De R, Contreras Zuniga LLE, Zuluaga SX. Resumen: Envenenamiento por múltiples picaduras de abejas y choque anafiláctico secundario. acta toxicológica argentina (2008) 16:27–32. [Google Scholar]
- 204. CDC C for DC and P . Bees, wasps, and hornets. (2018). [Google Scholar]
- 205. Greene A, Breisch NL. Avoidance of bee and wasp stings: an entomological perspective. Curr Opin Allergy Clin Immunol. (2005) 5:337–41. doi: 10.1097/01.all.0000173781.58154.53 [DOI] [PubMed] [Google Scholar]
- 206. Sicherer SH, Simons FER, Mahr TA, Abramson SL, Dinakar C, Fleisher TA, et al. Epinephrine for first-aid management of anaphylaxis. Pediatrics. (2017) 139. doi: 10.1542/peds.2016-4006 [DOI] [PubMed] [Google Scholar]
- 207. Cavalcante J dos S, de Almeida DEG, Moraes MS, Santos SR, Pincinato PM, Riciopo PM, et al. Challenges and opportunities in clinical diagnostic routine of envenomation using blood plasma proteomics. Toxins (Basel). (2023) 15:180. doi: 10.3390/toxins15030180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Ferreira Junior RS, Anderlini RP, Pimenta DC, De Oliveira Orsi R, Barraviera B, Sant’Anna OA. New Nanostructured Silica Adjuvant (SBA-15) Employed to Produce Antivenom in Young Sheep Using Crotalus durissus terrificus and Apis mellifera Venoms Detoxified by Cobalt-60. J Toxicol Environ Health A. (2010) 73:926–33. doi: 10.1080/15287391003745069 [DOI] [PubMed] [Google Scholar]
- 209. Teixeira-Cruz JM, Monteiro-MaChado M, Cons BL, Martins-Ferreira J, da Cunha LER, Barraviera B, et al. Antagonism of in vitro and in vivo activities of Apis mellifera venom by antiapilic serum. Toxicon. (2016) 117:102–3. doi: 10.1016/j.toxicon.2016.04.003 [DOI] [Google Scholar]
- 210. Teixeira-Cruz JM, Strauch MA, Monteiro-MaChado M, Tavares-Henriques MS, de Moraes JA, Ribeiro da Cunha LE, et al. A novel apilic antivenom to treat massive, africanized honeybee attacks: A preclinical study from the lethality to some biochemical and pharmacological activities neutralization. Toxins (Basel). (2021) 13:30. doi: 10.3390/toxins13010030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Oliveira Orsi R, Zaluski R, de Barros LC, Barraviera B, Pimenta DC, Ferreira Junior RS. Standardized guidelines for Africanized honeybee venom production needed for development of new apilic antivenom. J Toxicol Environ Health Part B. (2024) 27:73–90. doi: 10.1080/10937404.2023.2300786 [DOI] [PubMed] [Google Scholar]
- 212. Ferreira Junior RS, Morales MM, Barretti P, Barraviera B. Launching a CDMO in Brazil aiming to develop biopharmaceuticals for clinical trials. J Venomous Anim Toxins including Trop Dis. (2022) 28:e20220017. doi: 10.1590/1678-9199-jvatitd-2022-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.