Abstract
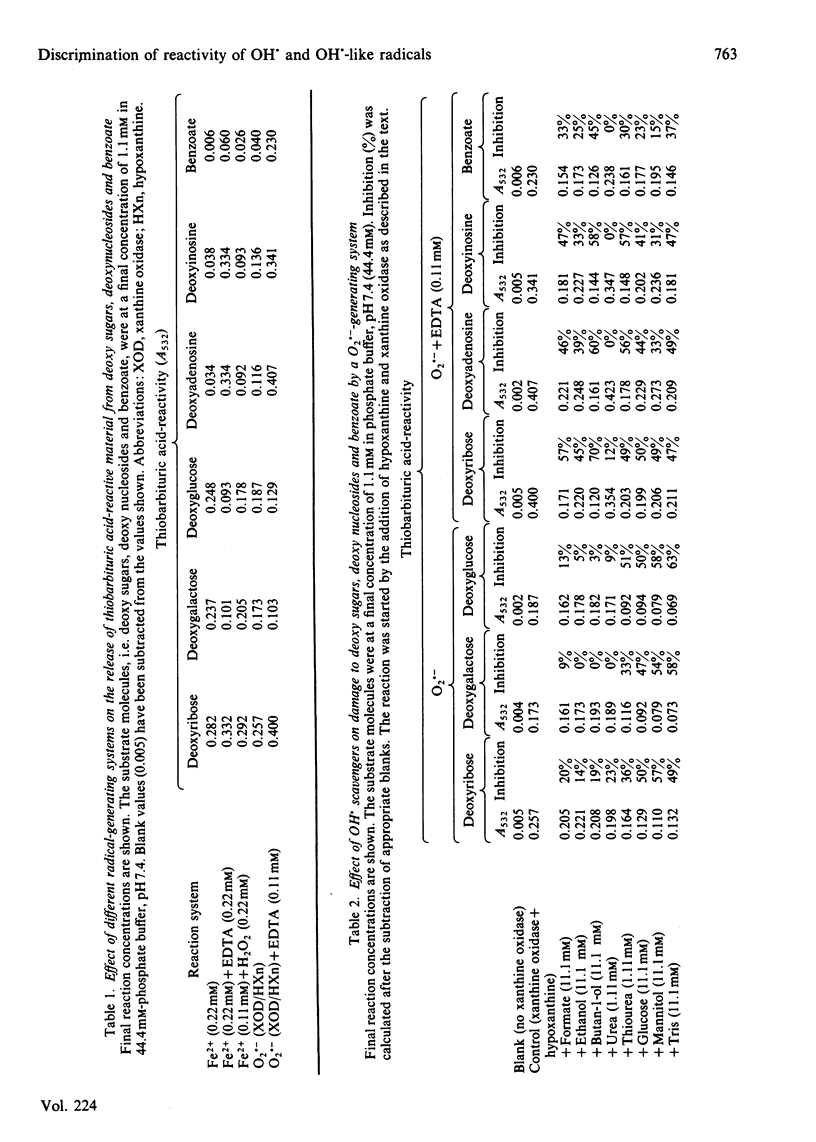
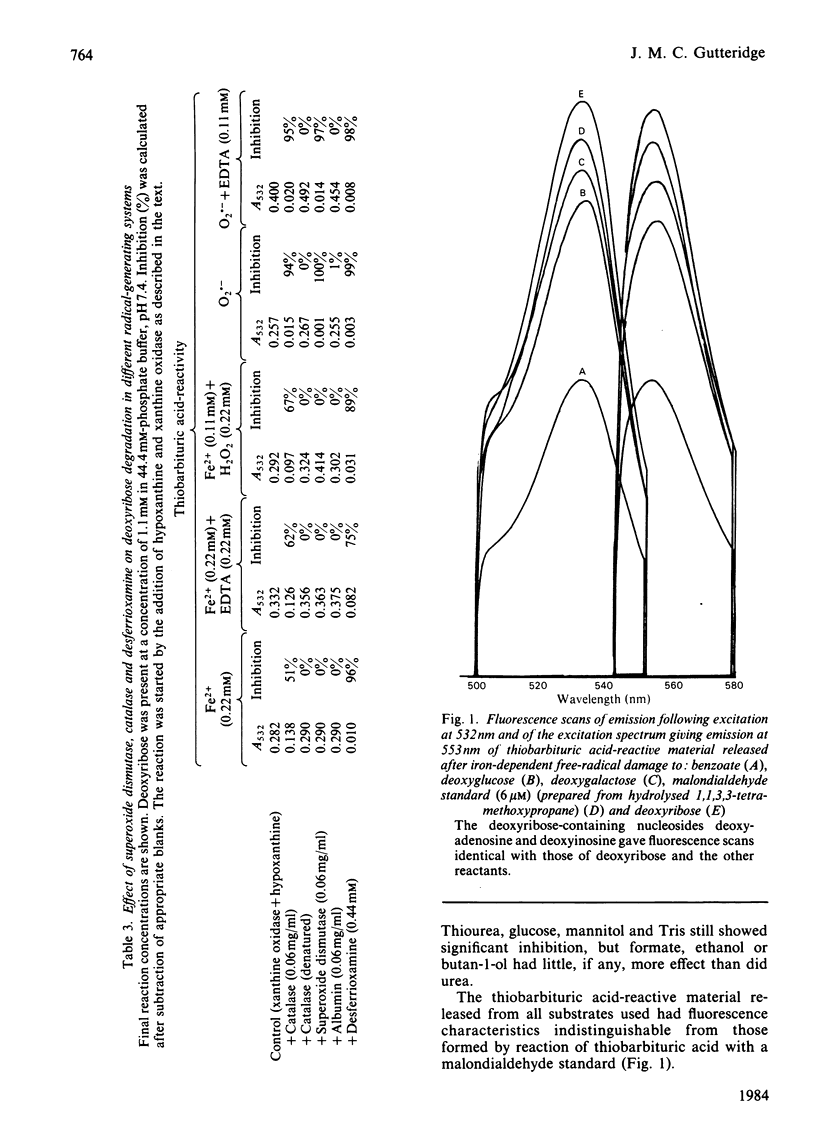
Hydroxyl radicals (OH.) can be formed in aqueous solution by a superoxide (O2.-)-generating system in the presence of a ferric salt or in a reaction independent of O2.- by the direct addition of a ferrous salt. OH. damage was detected in the present work by the release of thiobarbituric acid-reactive material from deoxy sugars, nucleosides and benzoate. The carbohydrates deoxyribose, deoxygalactose and deoxyglucose were substantially degraded by the iron(II) salt and the iron(III) salt in the presence of an O2.- -generating system, whereas deoxyinosine, deoxyadenosine and benzoate were not. Addition of EDTA to the reaction systems producing radicals greatly enhanced damage to deoxyribose, deoxyinosine, deoxyadenosine and benzoate, but decreased damage to deoxygalactose and deoxyglucose. Further, OH. scavengers were effective inhibitors only when EDTA was present. Inhibition by catalase and desferrioxamine confirmed that H2O2 and iron salts were essential for these reactions. The results suggest that, in the absence of EDTA, iron ions bind to the carbohydrate detector molecules and bring about a site-specific reaction on the molecule. This reaction is poorly inhibited by most OH. scavengers, but is strongly inhibited by scavengers such as mannitol, glucose and thiourea, which can themselves bind iron ions, albeit weakly. In the presence of EDTA, however, iron is removed from these binding sites to produce OH. in 'free' solution. These can be readily intercepted by the addition of OH. scavengers.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Floyd R. A. DNA-ferrous iron catalyzed hydroxyl free radical formation from hydrogen peroxide. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1209–1215. doi: 10.1016/0006-291x(81)90748-8. [DOI] [PubMed] [Google Scholar]
- Floyd R. A. Direct demonstration that ferrous ion complexes of di- and triphosphate nucleotides catalyze hydroxyl free radical formation from hydrogen peroxide. Arch Biochem Biophys. 1983 Aug;225(1):263–270. doi: 10.1016/0003-9861(83)90029-2. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Free-radical damage to lipids, amino acids, carbohydrates and nucleic acids determined by thiobarbituric acid reactivity. Int J Biochem. 1982;14(7):649–653. doi: 10.1016/0020-711x(82)90050-7. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Lipid peroxidation and possible hydroxyl radical formation stimulated by the self-reduction of a doxorubicin-iron (III) complex. Biochem Pharmacol. 1984 Jun 1;33(11):1725–1728. doi: 10.1016/0006-2952(84)90340-x. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Quinlan G. J., Wilkins S. Mitomycin C-induced deoxyribose degradation inhibited by superoxide dismutase. A reaction involving iron, hydroxyl and semiquinone radicals. FEBS Lett. 1984 Feb 13;167(1):37–41. doi: 10.1016/0014-5793(84)80828-5. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Richmond R., Halliwell B. Inhibition of the iron-catalysed formation of hydroxyl radicals from superoxide and of lipid peroxidation by desferrioxamine. Biochem J. 1979 Nov 15;184(2):469–472. doi: 10.1042/bj1840469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Rowley D. A., Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Detection of 'free' iron in biological systems by using bleomycin-dependent degradation of DNA. Biochem J. 1981 Oct 1;199(1):263–265. doi: 10.1042/bj1990263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Stocks J. Caeruloplasmin: physiological and pathological perspectives. Crit Rev Clin Lab Sci. 1981;14(4):257–329. doi: 10.3109/10408368109105866. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Thiobarbituric acid-reactivity following iron-dependent free-radical damage to amino acids and carbohydrates. FEBS Lett. 1981 Jun 15;128(2):343–346. doi: 10.1016/0014-5793(81)80113-5. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Toeg D. Iron-dependent free radical damage to DNA and deoxyribose. Separation of TBA-reactive intermediates. Int J Biochem. 1982;14(10):891–893. doi: 10.1016/0020-711x(82)90071-4. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Wilkins S. Copper salt-dependent hydroxyl radical formation. Damage to proteins acting as antioxidants. Biochim Biophys Acta. 1983 Aug 23;759(1-2):38–41. doi: 10.1016/0304-4165(83)90186-1. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Ahluwalia S. Hydroxylation of p-coumaric acid by horseradish peroxidase. The role of superoxide and hydroxyl radicals. Biochem J. 1976 Mar 1;153(3):513–518. doi: 10.1042/bj1530513a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett. 1981 Jun 15;128(2):347–352. doi: 10.1016/0014-5793(81)80114-7. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron chelates: is it a mechanism for hydroxyl radical production in biochemical systems? FEBS Lett. 1978 Aug 15;92(2):321–326. doi: 10.1016/0014-5793(78)80779-0. [DOI] [PubMed] [Google Scholar]
- Hoe S., Rowley D. A., Halliwell B. Reactions of ferrioxamine and desferrioxamine with the hydroxyl radical. Chem Biol Interact. 1982 Jul 15;41(1):75–81. doi: 10.1016/0009-2797(82)90018-7. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- Miller G. G., Raleigh J. A. Action of some hydroxyl radical scavengers on radiation-induced haemolysis. Int J Radiat Biol Relat Stud Phys Chem Med. 1983 Apr;43(4):411–419. doi: 10.1080/09553008314550471. [DOI] [PubMed] [Google Scholar]
- Paschen W., Weser U. Problems concerning the biochemical action of superoxide dismutase (erythrocuprein). Hoppe Seylers Z Physiol Chem. 1975 Jun;356(6):727–737. doi: 10.1515/bchm2.1975.356.s1.727. [DOI] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Role of iron and ethylenediaminetetraacetic acid in the bactericidal activity of a superoxide anion-generating system. Arch Biochem Biophys. 1981 May;208(2):512–519. doi: 10.1016/0003-9861(81)90539-7. [DOI] [PubMed] [Google Scholar]
- Rowley D. A., Halliwell B. Superoxide-dependent and ascorbate-dependent formation of hydroxyl radicals in the presence of copper salts: a physiologically significant reaction? Arch Biochem Biophys. 1983 Aug;225(1):279–284. doi: 10.1016/0003-9861(83)90031-0. [DOI] [PubMed] [Google Scholar]
- Samuni A., Aronovitch J., Godinger D., Chevion M., Czapski G. On the cytotoxicity of vitamin C and metal ions. A site-specific Fenton mechanism. Eur J Biochem. 1983 Dec 1;137(1-2):119–124. doi: 10.1111/j.1432-1033.1983.tb07804.x. [DOI] [PubMed] [Google Scholar]
- Samuni A., Chevion M., Czapski G. Unusual copper-induced sensitization of the biological damage due to superoxide radicals. J Biol Chem. 1981 Dec 25;256(24):12632–12635. [PubMed] [Google Scholar]
- Schuessler H., Freundl K. Reactions of formate and ethanol radicals with bovine serum albumin studied by electrophoresis. Int J Radiat Biol Relat Stud Phys Chem Med. 1983 Jul;44(1):17–29. doi: 10.1080/09553008314550831. [DOI] [PubMed] [Google Scholar]
- Willson R. L. Free radical protection: why vitamin E, not vitamin C, beta-carotene or glutathione? Ciba Found Symp. 1983;101:19–44. [PubMed] [Google Scholar]
- Wong S. F., Halliwell B., Richmond R., Skowroneck W. R. The role of superoxide and hydroxyl radicals in the degradation of hyaluronic acid induced by metal ions and by ascorbic acid. J Inorg Biochem. 1981 Apr;14(2):127–134. doi: 10.1016/s0162-0134(00)80033-1. [DOI] [PubMed] [Google Scholar]
- Youngman R. J., Elstner E. F. Oxygen species in paraquat toxicity: the crypto-OH radical. FEBS Lett. 1981 Jul 6;129(2):265–268. doi: 10.1016/0014-5793(81)80180-9. [DOI] [PubMed] [Google Scholar]
- Youngman R. J., Osswald W. F., Elstner E. F. Crypto - OH. radical production by nitrofurantoin. Biochem Pharmacol. 1982 Feb 15;31(4):603–606. doi: 10.1016/0006-2952(82)90169-1. [DOI] [PubMed] [Google Scholar]


