Abstract
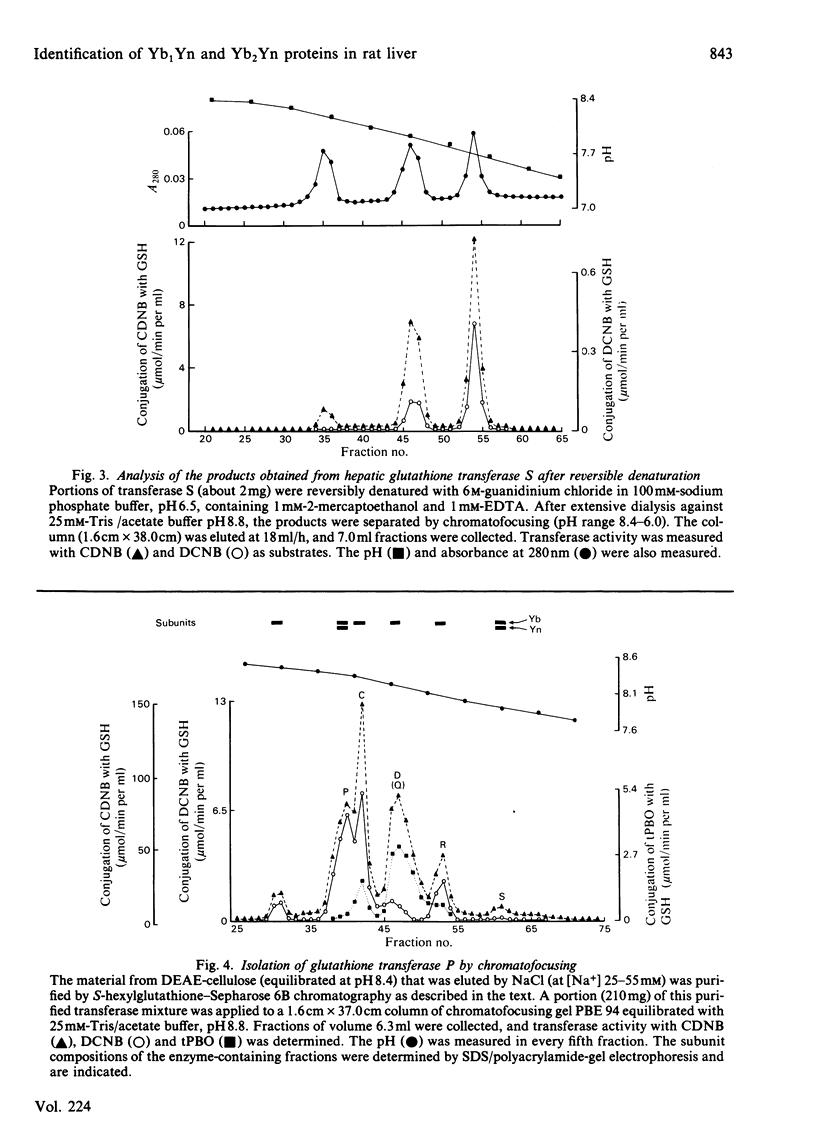
The glutathione S-transferases are dimeric proteins and comprise subunits of Mr 25 500 (Ya), 26 500 (Yn), 27 000 (Yb1 and Yb2) and 28 500 (Yc). Enzymes containing Ya and/or Yc subunits have been isolated as have forms containing binary combinations of Yn, Yb1 and Yb2 subunits. To date only one enzyme, transferase S, has been described that is a YbYn heterodimer [Hayes & Chalmers (1983) Biochem. J. 215, 581-588]; the identity of the Yb monomer found in transferase S has not been reported previously. The identification and isolation of a YnYn dimer (transferase N) from rat testis is now described. This has enabled structural and functional comparisons to be made between Yb1, Yb2 and Yn monomers. Reversible dissociation experiments between the YnYn and Yb1Yb1 homodimers and between the YnYn and Yb2Yb2 homodimers demonstrated that Yn monomers can hybridize with both Yb1 and Yb2 monomers. Reversible dissociation of transferases N and C (Yb1Yb2) showed that both Yb1 and Yb2 monomers can hybridize with Yn monomers under competitive conditions. The hydridization data suggest that transferase S represents the Yb2Yn subunit combination. A knowledge of the elution position from chromatofocusing columns of the Yb1Yn hybrid that was formed in vitro enabled a purification scheme to be devised for an enzyme from rat liver (transferase P) believed to consist of Yb1Yn subunits. A comparison of the chromatographic behaviour of the YnYn, Yb1Yb1 and Yb2Yb2 dimers on chromatofocusing and hydroxyapatite columns with the behaviour of transferases P and S on the same matrices suggests these two enzymes may be identified as the Yb1Yn and Yb2Yn dimers respectively. The catalytic activities and the inhibitory effects of non-substrate ligands on transferases P and S are significantly different and again suggest they comprise Yb1 and Yn subunits and Yb2 and Yn subunits respectively; transferase P exhibits a 6-fold higher specific activity for 1,2-dichloro-4-nitrobenzene than does transferase S, whereas, conversely, transferase S possesses a 9-fold higher specific activity for trans-4-phenylbut-3-en-2-one than does transferase P. The quaternary structure of transferases P and S was verified by using peptide mapping and 'Western blotting' techniques.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass N. M., Kirsch R. E., Tuff S. A., Marks I., Saunders S. J. Ligandin heterogeneity : evidence that the two non-identical subunits are the monomers of two distinct proteins. Biochim Biophys Acta. 1977 May 27;492(1):163–175. doi: 10.1016/0005-2795(77)90223-9. [DOI] [PubMed] [Google Scholar]
- Beale D., Ketterer B., Carne T., Meyer D., Taylor J. B. Evidence that the Ya and Yc subunits of glutathione transferase B (ligandin) are the products of separate genes. Eur J Biochem. 1982 Sep 1;126(3):459–463. doi: 10.1111/j.1432-1033.1982.tb06802.x. [DOI] [PubMed] [Google Scholar]
- Beale D., Meyer D. J., Taylor J. B., Ketterer B. Evidence that the Yb subunits of hepatic glutathione transferases represent two different but related families of polypeptides. Eur J Biochem. 1983 Dec 1;137(1-2):125–129. doi: 10.1111/j.1432-1033.1983.tb07805.x. [DOI] [PubMed] [Google Scholar]
- Bhargava M. M., Listowsky I., Arias I. M. Studies on subunit structure and evidence that ligandin is a heterodimer. J Biol Chem. 1978 Jun 25;253(12):4116–4119. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Eidne K. A., Kirsch R. E. Identity of ligandin in rat testis and liver. Biochem J. 1982 Apr 1;203(1):193–199. doi: 10.1042/bj2030193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey A. B., Friedberg T., Oesch F., Kreibich G. Studies on the subunit composition of rat liver glutathione S-transferases. J Biol Chem. 1983 Sep 25;258(18):11321–11325. [PubMed] [Google Scholar]
- Friedberg T., Milbert U., Bentley P., Guenther T. M., Oesch F. Purification and characterization of a new cytosolic glutathione S-transferase (glutathione S-transferase X) from rat liver. Biochem J. 1983 Dec 1;215(3):617–625. doi: 10.1042/bj2150617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthenberg C., Astrand I. M., Alin P., Mannervik B. Glutathione transferases in rat testis. Acta Chem Scand B. 1983;37(3):261–262. [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hayes J. D., Chalmers J. Bile acid inhibition of basic and neutral glutathione S-transferases in rat liver. Biochem J. 1983 Dec 1;215(3):581–588. doi: 10.1042/bj2150581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Clarkson G. H. Purification and characterization of three forms of glutathione S-transferase A. A comparative study of the major YaYa-, YbYb- and YcYc-containing glutathione S-transferases. Biochem J. 1982 Dec 1;207(3):459–470. doi: 10.1042/bj2070459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D. Rat liver glutathione S-transferases. A study of the structure of the basic YbYb-containing enzymes. Biochem J. 1983 Sep 1;213(3):625–633. doi: 10.1042/bj2130625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermodson M., Mahoney W. C. Separation of peptides by reversed-phase high-performance liquid chromatography. Methods Enzymol. 1983;91:352–359. doi: 10.1016/s0076-6879(83)91032-7. [DOI] [PubMed] [Google Scholar]
- Jakobson I., Askelöf P., Warholm M., Mannervik B. A steady-state-kinetic random mechanism for glutathione S-transferase A from rat liver. A model involving kinetically significant enzyme-product complexes in the forward reaction. Eur J Biochem. 1977 Jul 15;77(2):253–262. doi: 10.1111/j.1432-1033.1977.tb11664.x. [DOI] [PubMed] [Google Scholar]
- Jakoby W. B. The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv Enzymol Relat Areas Mol Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- Lai H. C., Li N., Weiss M. J., Reddy C. C., Tu C. P. The nucleotide sequence of a rat liver glutathione S-transferase subunit cDNA clone. J Biol Chem. 1984 May 10;259(9):5536–5542. [PubMed] [Google Scholar]
- Mannervik B., Guthenberg C. Glutathione transferase (human placenta). Methods Enzymol. 1981;77:231–235. doi: 10.1016/s0076-6879(81)77030-7. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Jensson H. Binary combinations of four protein subunits with different catalytic specificities explain the relationship between six basic glutathione S-transferases in rat liver cytosol. J Biol Chem. 1982 Sep 10;257(17):9909–9912. [PubMed] [Google Scholar]
- Pickett C. B., Telakowski-Hopkins C. A., Ding G. J., Argenbright L., Lu A. Y. Rat liver glutathione S-transferases. Complete nucleotide sequence of a glutathione S-transferase mRNA and the regulation of the Ya, Yb, and Yc mRNAs by 3-methylcholanthrene and phenobarbital. J Biol Chem. 1984 Apr 25;259(8):5182–5188. [PubMed] [Google Scholar]
- Redick J. A., Jakoby W. B., Baron J. Immunohistochemical localization of glutathione S-transferases in livers of untreated rats. J Biol Chem. 1982 Dec 25;257(24):15200–15203. [PubMed] [Google Scholar]
- Sheehan D., Mantle T. J. Evidence for two forms of ligandin (YaYa dimers of glutathione S-transferase) in rat liver and kidney. Biochem J. 1984 Mar 15;218(3):893–897. doi: 10.1042/bj2180893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D., Ryle C. M., Mantle T. J. Selective induction of glutathione S-transferase D in rat testis by phenobarbital. Biochem J. 1984 Apr 15;219(2):687–688. doi: 10.1042/bj2190687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons P. C., Vander Jagt D. L. Purification of glutathione S-transferases from human liver by glutathione-affinity chromatography. Anal Biochem. 1977 Oct;82(2):334–341. doi: 10.1016/0003-2697(77)90169-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]




