Abstract
Steady-state chemostat cultures of Azotobacter vinelandii were established in a simple defined medium that had been chemically purified to minimize Mo and that contained no utilizable combined N source. Growth was dependent on N2 fixation, the limiting nutrient being the Mo contaminating the system. The Mo content of the organisms was at least 100-fold lower than that of Mo-sufficient cultures, and they lacked the characteristic g = 3.7 e.p.r. feature of the MoFe-protein of nitrogenase. A characteristic of nitrogenase activity in vivo in Mo-limited populations was a disproportionately low activity for acetylene reduction, which was 0.3 to 0.1 of that expected from the rate of N2 reduction. Acetylene was also a poor substrate in comparison with protons as a substrate for nitrogenase, and did not markedly inhibit H2 evolution, in contrast with Mo-sufficient populations. In batch cultures in similar medium or 'spent' chemostat medium inoculated with Mo-limited organisms, the addition of Mo elicited a biphasic increased growth response at concentrations as low as 2.5 nM, provided that sufficient Fe was supplied. In this system V did not substitute for Mo, and Mo-deficient cultures ceased growth at a 25-fold lower population density compared with cultures supplemented with Mo. Nitrogenase component proteins could not be unequivocally detected by visual inspection of fractionated crude extracts of Mo-limited organisms. 35SO42-pulse-labelling studies also showed that the rate of synthesis of the MoFe-protein component of nitrogenase was too low to be quantified. However, the Fe-protein of nitrogenase was apparently synthesized at high rates. The discussion includes an evaluation of the possibility that A. vinelandii possesses an Mo-independent N2-fixation system.
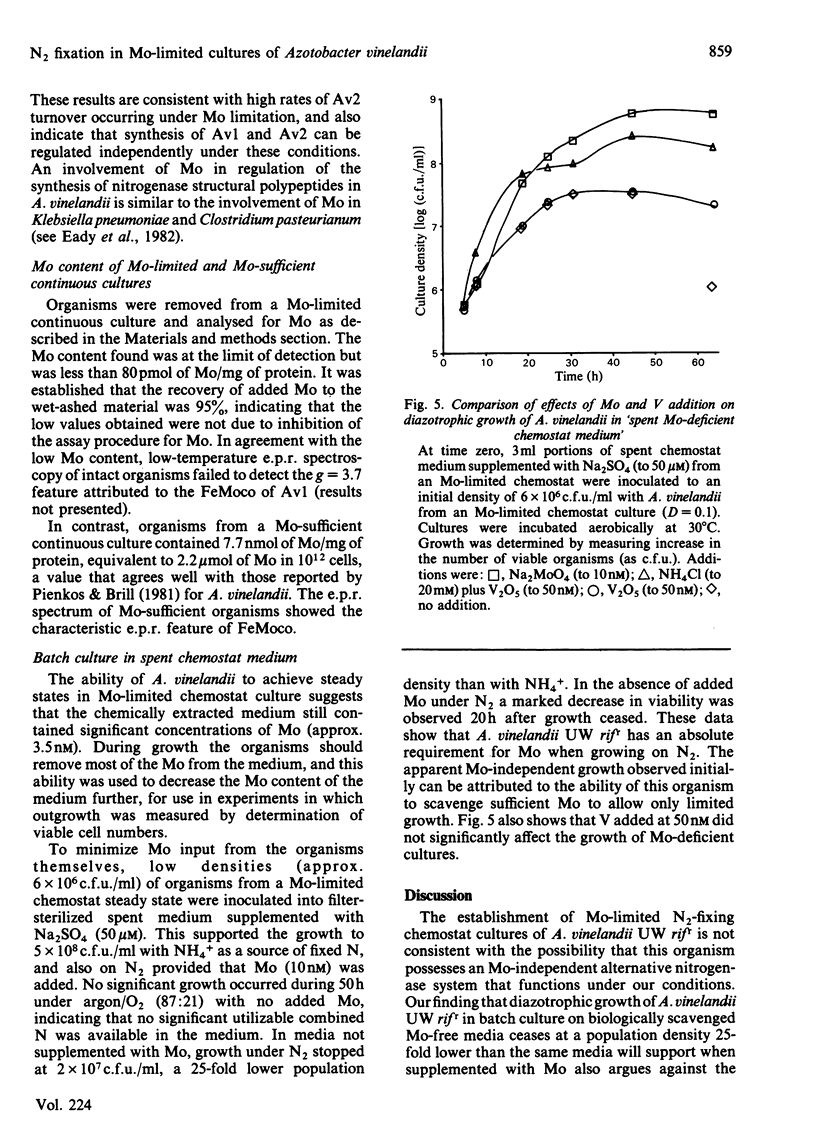
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benemann J. R., Smith G. M., Kostel P. J., McKenna C. E. Tungsten incorporation into Azotobacter vinelandii nitrogenase. FEBS Lett. 1973 Feb 1;29(3):219–221. doi: 10.1016/0014-5793(73)80023-7. [DOI] [PubMed] [Google Scholar]
- Bishop P. E., Jarlenski D. M., Hetherington D. R. Evidence for an alternative nitrogen fixation system in Azotobacter vinelandii. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7342–7346. doi: 10.1073/pnas.77.12.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Jarlenski D. M., Hetherington D. R. Expression of an alternative nitrogen fixation system in Azotobacter vinelandii. J Bacteriol. 1982 Jun;150(3):1244–1251. doi: 10.1128/jb.150.3.1244-1251.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop W. H., Henke L., Christopher J. P., Millar D. B. Photodestruction of acetylcholinesterase. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1980–1982. doi: 10.1073/pnas.77.4.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R. C., Fuchsman W. H., Hardy R. W. Nitrogenase from vanadium-grown Azotobacter: isolation, characteristics, and mechanistic implications. Biochem Biophys Res Commun. 1971 Feb 5;42(3):353–358. doi: 10.1016/0006-291x(71)90377-9. [DOI] [PubMed] [Google Scholar]
- Davis L. C., Shah V. K., Brill W. J., Orme-Johnson W. H. Nitrogenase. II. Changes in the EPR signal of component I (iron-molybdenum protein) of Azotobacter vinelandii nitrogenase during repression and derepression. Biochim Biophys Acta. 1972 Feb 28;256(2):512–523. doi: 10.1016/0005-2728(72)90079-5. [DOI] [PubMed] [Google Scholar]
- Davis L. C., Wang Y. L. In vivo and in vitro kinetics of nitrogenase. J Bacteriol. 1980 Mar;141(3):1230–1238. doi: 10.1128/jb.141.3.1230-1238.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESPOSITO R. G., WILSON P. W. Trace metal requirements of Azotobacter. Proc Soc Exp Biol Med. 1956 Dec;93(3):564–567. doi: 10.3181/00379727-93-22820. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Issack R., Kennedy C., Postgate J. R., Ratcliffe H. D. Nitrogenase synthesis in Klebsiella pneumoniae: comparison of ammonium and oxygen regulation. J Gen Microbiol. 1978 Feb;104(2):277–285. doi: 10.1099/00221287-104-2-277. [DOI] [PubMed] [Google Scholar]
- Hawkes T. R., McLean P. A., Smith B. E. Nitrogenase from nifV mutants of Klebsiella pneumoniae contains an altered form of the iron-molybdenum cofactor. Biochem J. 1984 Jan 1;217(1):317–321. doi: 10.1042/bj2170317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEELER R. F., VARNER J. E. Tungstate as an antagonist of molybdate in Azotobacter vinelandii. Arch Biochem Biophys. 1957 Aug;70(2):585–590. doi: 10.1016/0003-9861(57)90146-7. [DOI] [PubMed] [Google Scholar]
- Kallas T., Rebière M. C., Rippka R., Tandeau de Marsac N. The structural nif genes of the cyanobacteria Gloeothece sp. and Calothrix sp. share homology with those of Anabaena sp., but the Gloeothece genes have a different arrangement. J Bacteriol. 1983 Jul;155(1):427–431. doi: 10.1128/jb.155.1.427-431.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani H. H., Brill W. J. Nitrogenase V. The effect of Mo, W and V on the synthesis of nitrogenase components in Azotobacter vinelandii. Biochim Biophys Acta. 1974 Aug 7;362(1):160–166. doi: 10.1016/0304-4165(74)90037-3. [DOI] [PubMed] [Google Scholar]
- Partridge C. D., Yates M. G. Effect of chelating agents on hydrogenase in Azotobacter chroococcum. Evidence that nickel is required for hydrogenase synthesis. Biochem J. 1982 Apr 15;204(1):339–344. doi: 10.1042/bj2040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienkos P. T., Brill W. J. Molybdenum accumulation and storage in Klebsiella pneumoniae and Azotobacter vinelandii. J Bacteriol. 1981 Feb;145(2):743–751. doi: 10.1128/jb.145.2.743-751.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D., Mazur B. J., Haselkorn R. Isolation and physical mapping of nitrogen fixation genes from the cyanobacterium Anabaena 7120. J Biol Chem. 1982 Nov 10;257(21):13157–13163. [PubMed] [Google Scholar]
- Riddle G. D., Simonson J. G., Hales B. J., Braymer H. D. Nitrogen fixation system of tungsten-resistant mutants of Azotobacter vinelandii. J Bacteriol. 1982 Oct;152(1):72–80. doi: 10.1128/jb.152.1.72-80.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Chesshyre J. A., Wheeler C., Jones R., Woodley P. R., Postgate J. R. Genome size and complexity in Azotobacter chroococcum. J Gen Microbiol. 1984 Jul;130(7):1603–1612. doi: 10.1099/00221287-130-7-1603. [DOI] [PubMed] [Google Scholar]
- Scolnik P. A., Haselkorn R. Activation of extra copies of genes coding for nitrogenase in Rhodopseudomonas capsulata. Nature. 1984 Jan 19;307(5948):289–292. doi: 10.1038/307289a0. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Isolation of an iron-molybdenum cofactor from nitrogenase. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3249–3253. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]


