Abstract
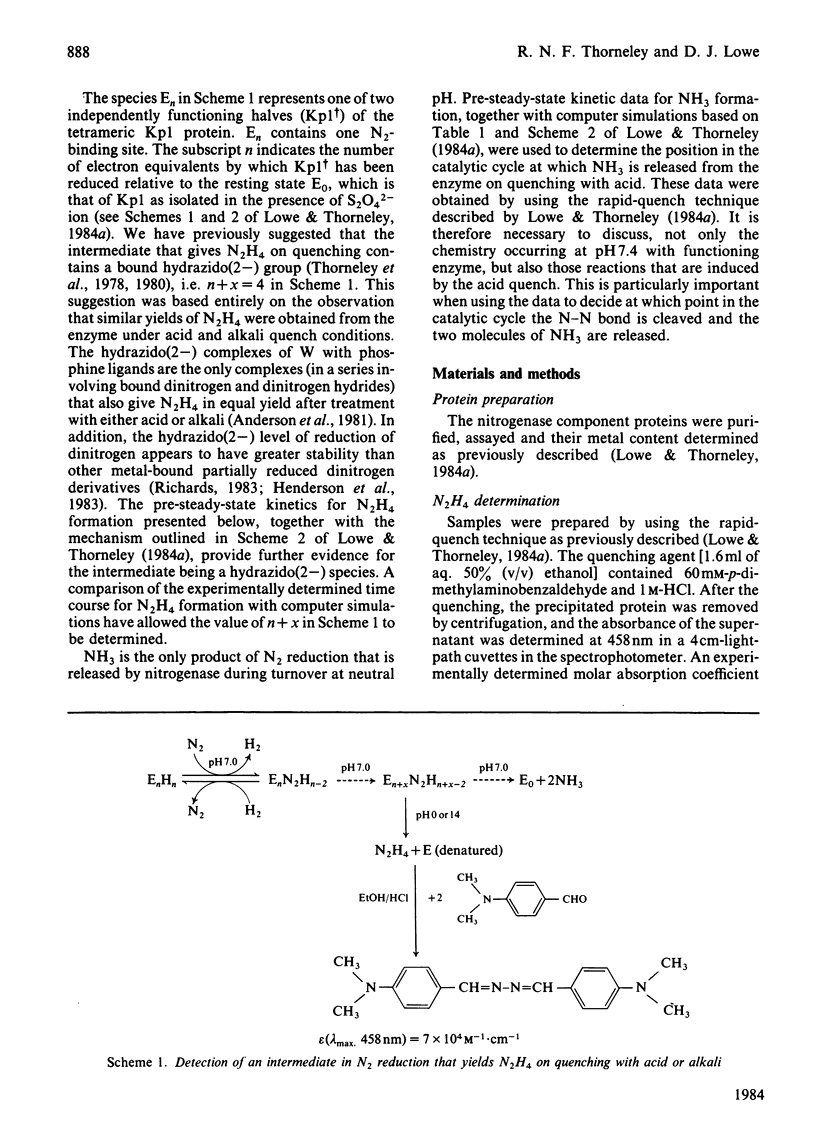
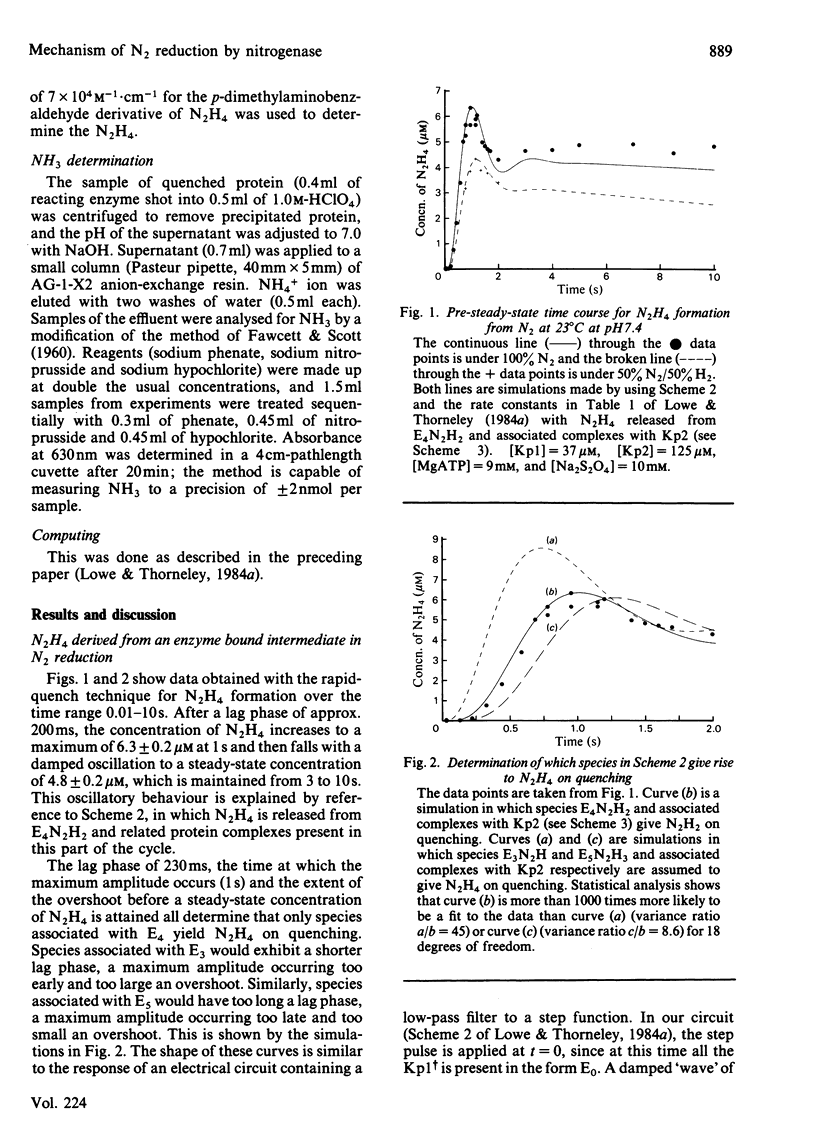
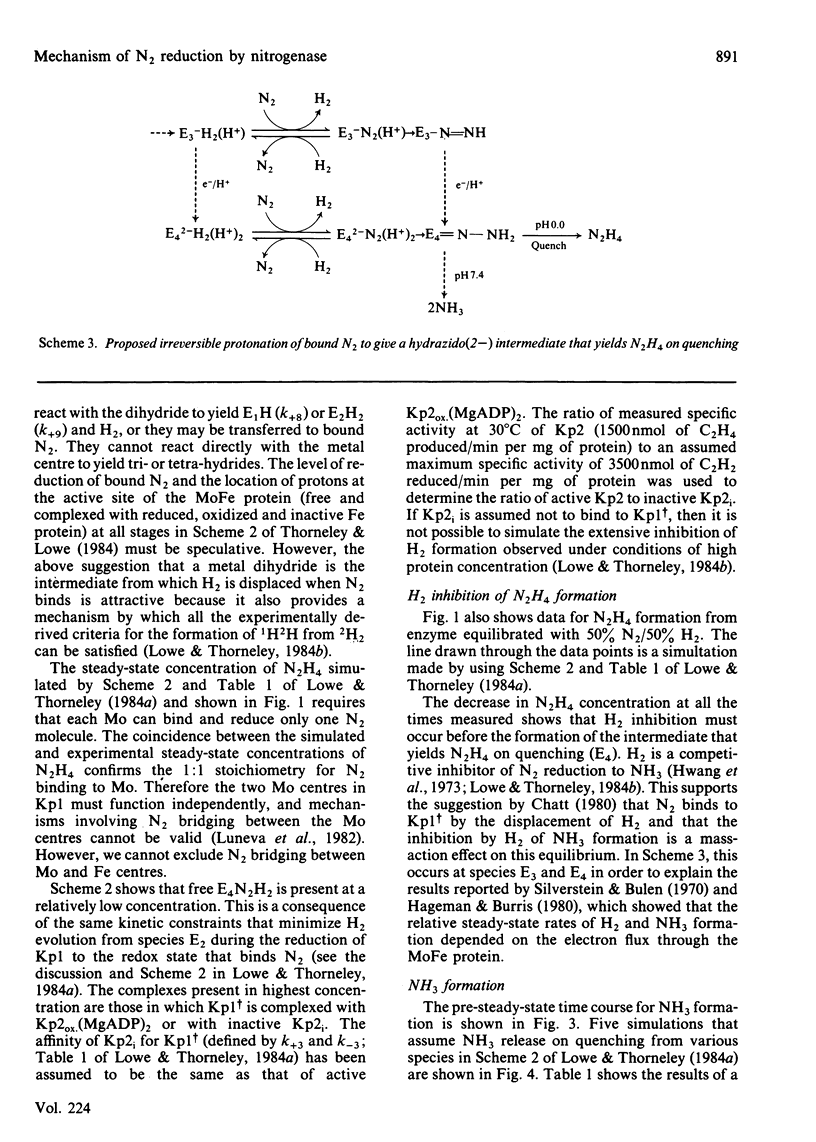
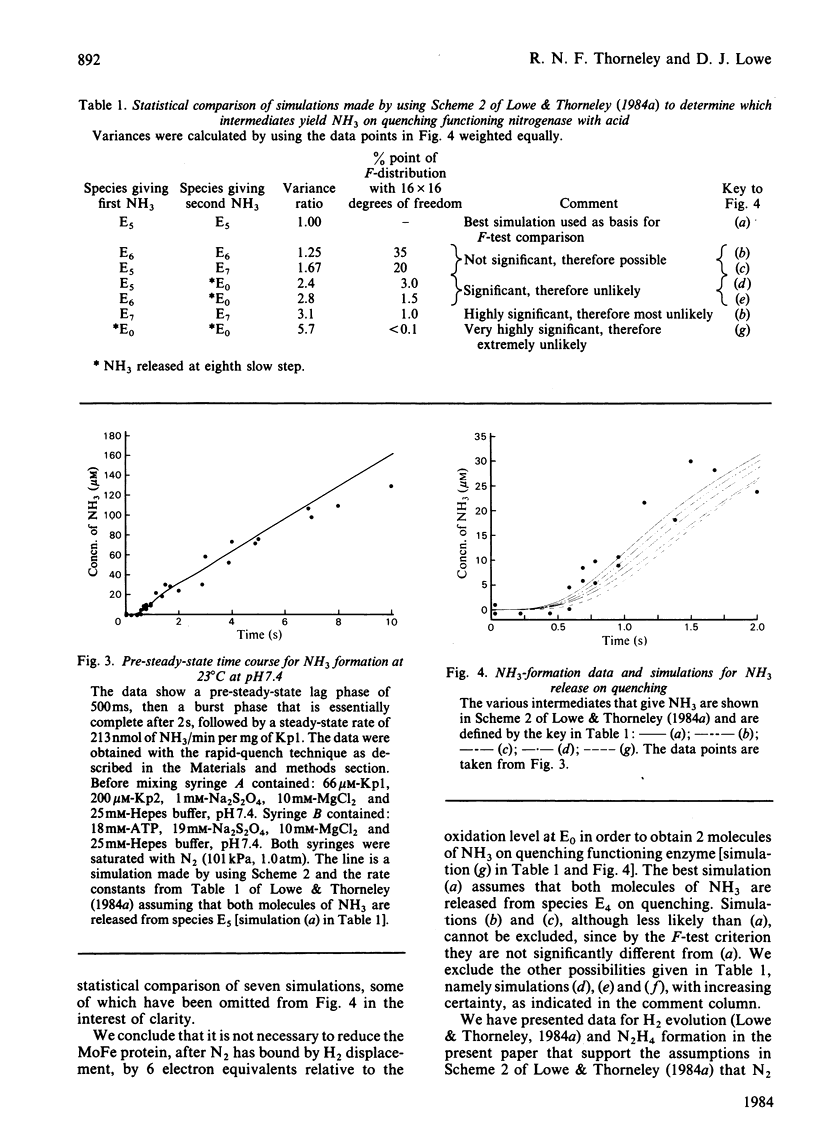
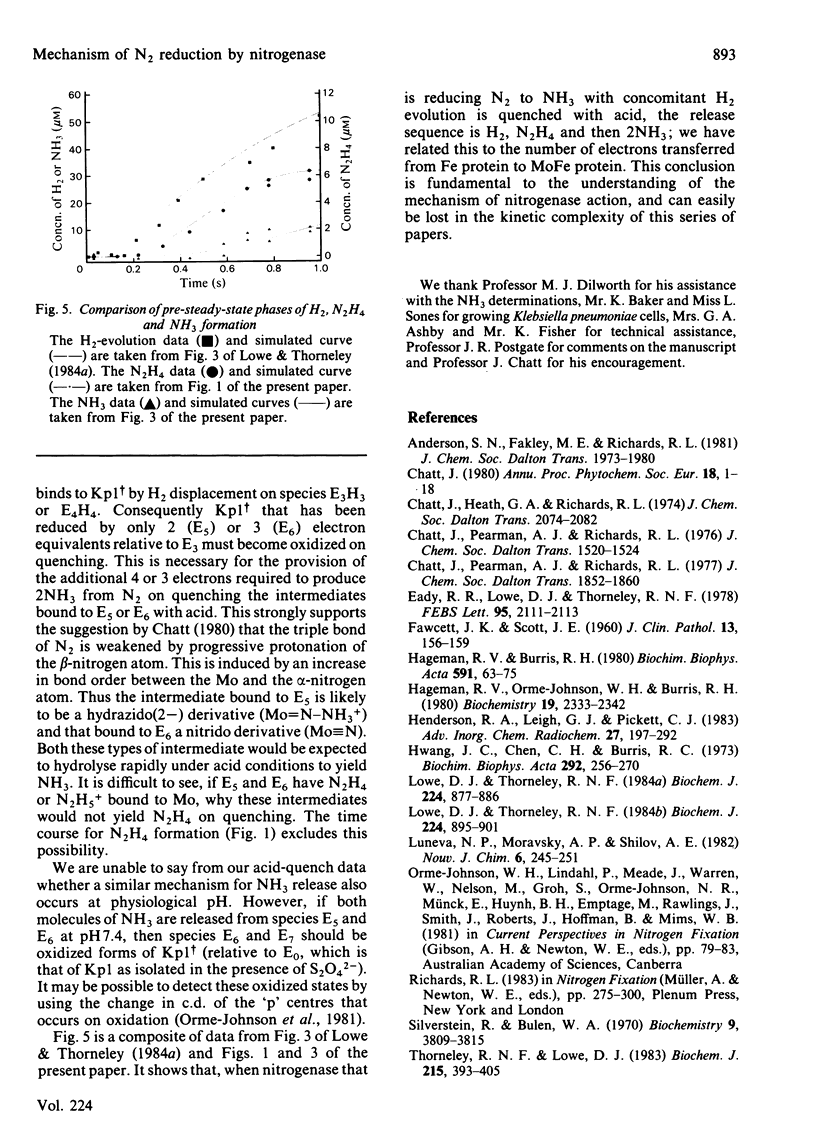
The reduction of N2 to 2NH3 by Klebsiella pneumoniae nitrogenase was studied by a rapid-quench technique. The pre-steady-state time course for N2H4, formed on quenching by the acid-induced hydrolysis of an enzyme-bound intermediate in N2 reduction, showed a 230 ms lag followed by a damped oscillatory approach to a constant concentration in the steady state. The pre-steady-state time course for NH3 formation exhibited a lag of 500 ms and a burst phase that was essentially complete at 1.5s, before a steady-state rate was achieved. These time courses have been simulated by using a previously described kinetic model for the mechanism of nitrogenase action [Lowe & Thorneley (1984) Biochem. J. 224, 877-886]. A hydrazido(2-) structure (=N-NH2) is favoured for the intermediate that yields N2H4 on quenching. The NH3-formation data indicate enzyme-bound metallo-nitrido (identical to N) or -imido (=NH) intermediates formed after N-N bond cleavage to produce the first molecule of NH3 and which subsequently give the second molecule of NH3 by hydrolysis on quenching. The simulations require stoichiometric reduction of one N2 molecule at each Mo and the displacement of one H2 when N2 binds to the MoFe protein. Inhibition by H2 of N2-reduction activity occurs before the formation of the proposed hydrazido(2-) species, and is explained by H2 displacement of N2 at the active site.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FAWCETT J. K., SCOTT J. E. A rapid and precise method for the determination of urea. J Clin Pathol. 1960 Mar;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman R. V., Burris R. H. Electron allocation to alternative substrates of Azotobacter nitrogenase is controlled by the electron flux through dinitrogenase. Biochim Biophys Acta. 1980 Jun 10;591(1):63–75. doi: 10.1016/0005-2728(80)90220-0. [DOI] [PubMed] [Google Scholar]
- Hageman R. V., Orme-Johnson W. H., Burris R. H. Role of magnesium adenosine 5'-triphosphate in the hydrogen evolution reaction catalyzed by nitrogenase from Azotobacter vinelandii. Biochemistry. 1980 May 27;19(11):2333–2342. doi: 10.1021/bi00552a009. [DOI] [PubMed] [Google Scholar]
- Hwang J. C., Chen C. H., Burris R. H. Inhibition of nitrogenase-catalyzed reductions. Biochim Biophys Acta. 1973 Jan 18;292(1):256–270. doi: 10.1016/0005-2728(73)90270-3. [DOI] [PubMed] [Google Scholar]
- Lowe D. J., Thorneley R. N. The mechanism of Klebsiella pneumoniae nitrogenase action. Pre-steady-state kinetics of H2 formation. Biochem J. 1984 Dec 15;224(3):877–886. doi: 10.1042/bj2240877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. J., Thorneley R. N. The mechanism of Klebsiella pneumoniae nitrogenase action. The determination of rate constants required for the simulation of the kinetics of N2 reduction and H2 evolution. Biochem J. 1984 Dec 15;224(3):895–901. doi: 10.1042/bj2240895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein R., Bulen W. A. Kinetic studies of the nitrogense-catalyzed hydrogen volution and nitrogen reduction reactions. Biochemistry. 1970 Sep 15;9(19):3809–3815. doi: 10.1021/bi00821a021. [DOI] [PubMed] [Google Scholar]
- Thorneley R. N., Lowe D. J. Nitrogenase of Klebsiella pneumoniae. Kinetics of the dissociation of oxidized iron protein from molybdenum-iron protein: identification of the rate-limiting step for substrate reduction. Biochem J. 1983 Nov 1;215(2):393–403. doi: 10.1042/bj2150393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Lowe D. J. The mechanism of Klebsiella pneumoniae nitrogenase action. Simulation of the dependences of H2-evolution rate on component-protein concentration and ratio and sodium dithionite concentration. Biochem J. 1984 Dec 15;224(3):903–909. doi: 10.1042/bj2240903. [DOI] [PMC free article] [PubMed] [Google Scholar]


