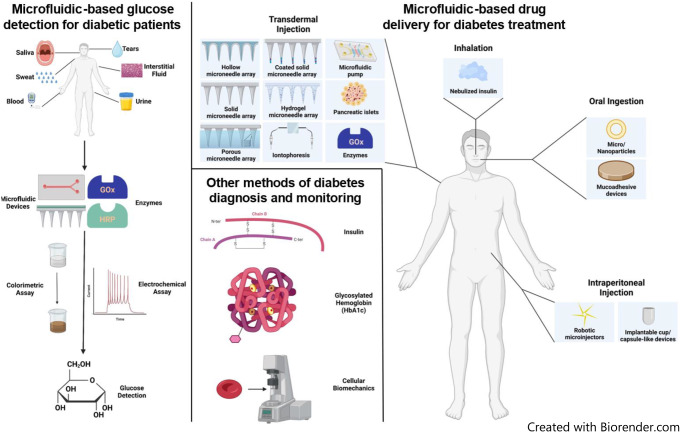
Abstract
Diabetes currently affects approximately 500 million people worldwide and is one of the most common causes of mortality in the United States. To diagnose and monitor diabetes, finger-prick blood glucose testing has long been used as the clinical gold standard. For diabetes treatment, insulin is typically delivered subcutaneously through cannula-based syringes, pens, or pumps in almost all type 1 diabetic (T1D) patients and some type 2 diabetic (T2D) patients. These painful, invasive approaches can cause non-adherence to glucose testing and insulin therapy. To address these problems, researchers have developed miniaturized blood glucose testing devices as well as microfluidic platforms for non-invasive glucose testing through other body fluids. In addition, glycated hemoglobin (HbA1c), insulin levels, and cellular biomechanics-related metrics have also been considered for microfluidic-based diabetes diagnosis. For the treatment of diabetes, insulin has been delivered transdermally through microdevices, mostly through microneedle array-based, minimally invasive injections. Researchers have also developed microfluidic platforms for oral, intraperitoneal, and inhalation-based delivery of insulin. For T2D patients, metformin, glucagon-like peptide 1 (GLP-1), and GLP-1 receptor agonists have also been delivered using microfluidic technologies. Thus far, clinical studies have been widely performed on microfluidic-based diabetes monitoring, especially glucose sensing, yet technologies for the delivery of insulin and other drugs to diabetic patients with microfluidics are still mostly in the preclinical stage. This article provides a concise review of the role of microfluidic devices in the diagnosis and monitoring of diabetes, as well as the delivery of pharmaceuticals to treat diabetes using microfluidic technologies in the recent literature.
Graphical abstract
Keywords: Microfluidics, Diabetes, Insulin, Drug delivery, Glucose monitoring, Glycated hemoglobin, Metformin, Microneedle array
Introduction
Diabetes is an extremely common condition that affects approximately 500 million people worldwide. This number is expected to grow to over 700 million by 2045 [1]. In the United States alone, approximately 37 million (11.3%) live with diabetes, and this statistic is expected to increase to 60.6 million in 2060 [2, 3]. The annual cost spent on diabetes in the United States was $327 billion in 2017, and diabetes is among the top five leading causes of death in the US [4–6]. According to Mobasseri et al. (2020), Type 1 diabetes (T1D), an autoimmune disease that causes a deficiency in insulin secretion, accounts for between 5% and 10% of all cases of diabetes. The rest of diabetic patients have type 2 diabetes (T2D), in which the body has an impaired ability to respond to insulin [7]. T1D patients typically experience more severe symptoms such as ketoacidosis, uncontrolled hyperglycemia, and hypoglycemia, as compared to T2D patients [8].
The diagnosis and monitoring of diabetes mainly relies on blood tests, most prominently finger-prick blood glucose tests [3, 9–16]. These blood tests are convenient, yet they pose limitations including pain, invasiveness, fear of needles, stress, potential infections, and non-healing of the penetrated area [3, 9, 11, 13–17]. These limitations could result in non-adherence to glucose monitoring, which in turn could cause complications including diabetic ketoacidosis, cardiovascular diseases, stroke, and blindness [10]. To eliminate these drawbacks, researchers have developed less invasive alternatives, which are already in clinical use. These alternatives include non-invasive blood glucose tests [17], as well as glucose tests based on other body fluids including interstitial fluid [18], saliva [9], sweat [19], tears [11], and urine [20]. Besides testing for glucose, researchers can also diagnose and manage diabetes based on the concentrations of glycated hemoglobin (HbA1c or simply A1c) and insulin in the blood [21–26], as well as red blood cell (RBC) and neutrophil mechanics and behavior [27–31].
Treatment of diabetes is centered around the delivery of insulin for all T1D patients because external insulin is necessary to maintain glycemic control and prevent ketoacidosis [8]. Approximately 20–30% of T2D patients are prescribed insulin, including 40% of T2D patients in the United States [32, 33]. T2D can also be treated with other peptides such as glucagon-like peptide 1 (GLP-1), as well as orally consumed small-molecule drugs such as metformin [34–39]. The majority of T1D patients and a small portion of T2D patients in the United States rely on battery-powered infusion pumps for insulin delivery, while the rest of insulin-dependent patients rely on cannula-driven insulin syringes or pens [8, 32]. All current methods of insulin delivery have associated side effects, the most obvious of which is painful cannula insertion [40–47]. In addition, embarrassment, interference with daily activity, and sometimes cost, are other limitations associated with these methods [48–53]. These limitations result in non-adherence to insulin therapy, which can cause hospitalizations and mortalities [48–51, 54, 55].
Microfluidic technologies may be key to alleviating some of the unpleasant side effects of insulin delivery that lead to non-adherence. A microfluidic device is defined as a system involving micrometer-scale channels and chambers and containing small volumes (microliters, nanoliters, or even smaller) of fluids [56–58]. Compared to conventional drug screening and delivery methods, advantages of microfluidic systems include compact size, precise dosage control, rapid and high-throughput analysis, reduced chemical waste, and reduced invasiveness [59]. In diabetes management, microfluidics has been used in preclinical and clinical studies in diagnosing and treating diabetes. In this article, we will review microfluidic technologies for diagnosing and monitoring diabetes, as well as microfluidic devices for delivering insulin and other pharmaceuticals for the treatment of diabetes.
Use of microfluidic devices in diabetes diagnosis and monitoring
Microfluidic devices have been increasingly employed in diagnosing and monitoring diabetes in the last two decades, most commonly through testing of glucose levels in different bodily fluids. Alternatively, some researchers leveraged other techniques, such as the measurement of HbA1c and insulin in blood, and the examination of mechanical deformability of RBCs. A summary of these technologies is provided in Table 1 below.
Table 1.
| Biomarker monitored | Body fluid used | Reference |
|---|---|---|
| Glucose | Blood/Plasma/Serum | [60–71] |
| Interstitial fluid | [72–79] | |
| Saliva | [66, 68, 80–86] | |
| Sweat | [60, 83, 87, 88] | |
| Tears | [83, 89, 90] | |
| Urine | [66, 68, 91–93] | |
| Glycated hemoglobin (HbA1c) | Blood | [94–96] |
| Insulin | Serum | [24, 97, 98] |
| Red blood cell deformability | Blood | [27] |
Devices for and methods of glucose detection
In miniaturized systems for glucose testing, microfluidic paper-based analytical devices (µPADs) have been the most commonly used due to their environmental friendliness, sustainability, biocompatibility, light weight, ease of transport and storage, as well as fast, easy, and inexpensive fabrication [60, 80, 81, 99]. These devices are typically fabricated with wax printing, which involves the pre-designing and patterning of molten wax or solid ink on choreographic paper, followed by the cooling of the paper to room temperature [99]. Other fabrication methods include photolithography [60], origami [61, 91], deposition with 3D pens [81], and CO2 laser cutting [89]. Besides paper-based devices, other device types include polydimethylsiloxane (PDMS) devices [72, 87], plexiglass chips [73], microfluidic thread-based electroanalytical devices (µTEDs) [66, 90], and porous microneedle arrays [74, 75].
Glucose can be detected in microfluidic devices with various approaches, including colorimetric, electrochemical, fluorescence, chemiluminescence, and nanoparticle-based characterizations. Colorimetric and electrochemical measurements have been the two most commonly used methods (Fig. 1d).
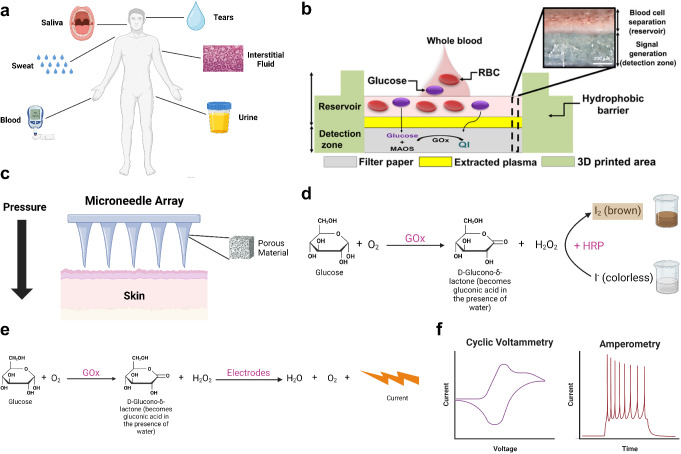
Fig. 1.
Example microfluidic devices and detection methods for glucose testing. a A schematic illustrating bodily fluids, including blood, interstitial fluid, saliva, sweat, tears, and urine, that have been explored for glucose detection. b A paper-based microfluidic blood glucose testing device with colorimetric glucose detection [64]. c Porous microneedle array-driven extraction of interstitial fluid for glucose testing (recreated from [74]). d An example chemical reaction in colorimetric glucose detection with iodide as the chromogenic agent, which is reduced to brown-colored molecular iodine in the presence of H2O2. Other chromogens such as a mixture of 4-aminoantipyrine (AAP) and 3,5-dichloro-2-hydroxybenzenesulfonic acid (DHBS) can be used in place of iodide [80]. e An example electrochemical assay for glucose detection, which generates a detectable current upon the reduction of H2O2. f Cyclic voltammetry and amperometry, two commonly used methods to characterize the electrical current generated by an electrochemical glucose detection system. (a, d, e, f) Created with Biorender. (b) Reproduced from Park C, Kim HR, Kim SK, Jeong IK, Pyun JC, Park S. Three-Dimensional Paper-Based Microfluidic Analytical Devices Integrated with a Plasma Separation Membrane for the Detection of Biomarkers in Whole Blood. ACS Appl Mater Interfaces. 2019;11:36428–36,434 [64]. Copyright permission from ACS Publications (CC License). (c) Recreated from Takeuchi K, Takama N, Kinoshita R, Okitsu T, Kim B. Flexible and porous microneedles of PDMS for continuous glucose monitoring. Biomed Microdevices. 2020;22:79 [74]
Colorimetric assays are widely used because they are known for their simplicity, stability, user-friendliness, high throughput, low cost, and instrument-free nature [62, 63, 80, 82, 92]. For colorimetric glucose detection, glucose oxidase (GOx) is typically used to oxidize glucose into D-glucono-δ-lactone (or gluconic acid in the presence of water) and hydrogen peroxide (H2O2). Horseradish peroxidase (HRP) is typically used to convert an added chromogen into a colorful state in the presence of H2O2, or vice versa [63, 64, 80, 81].
Electrochemical approaches are also often used to detect glucose in microfluidic devices. Compared to colorimetric assays, electrochemical assays are typically less user-friendly due to more complicated instruments and procedures, but they are known for an even higher sensitivity and resolution [100]. Glucose-sensing microfluidic electrochemical assays typically involve a circuit, containing electrodes and a conductive medium, that can convert the glucose concentration into an electrical current. These electrodes are usually made of carbon-based materials, such as graphite [60, 65], biochar [66], carbon nanotubes [73], or graphene [72], doped with conductive materials such as metal nanoparticles or ions [66, 72, 73], or Prussian blue [60, 67]. Silver/silver chloride (Ag/AgCl) often serves as reference electrodes, and current is generated from reactions such as the aforementioned oxidation of glucose catalyzed by GOx [65, 83, 87, 90]. Amperometry, cyclic voltammetry, and linear sweep voltammetry are general techniques to correlate the current with the glucose concentration [65, 66, 68, 73].
Bodily fluids for microfluidic glucose detection
A wide range of studies have been performed to detect glucose from various bodily fluids, including blood, interstitial fluid, saliva, sweat, tears, and urine, using microfluidic technologies (Fig. 1a). Most of these studies have clinically tested the efficacy of these custom-built, glucose-monitoring instruments. Therefore, we will mostly focus on the clinical results of these studies as well as the technologies they used for glucose detection from each bodily fluid.
Blood
As the gold standard for detecting and monitoring diabetes, blood glucose tests have been widely performed and incorporated into microfluidic devices. Traditional finger prick methods have been used to obtain blood from human subjects, and blood (or pre-processed plasma or serum) is loaded into various types of microfluidic devices (an example is shown in Fig. 1b, [64]). Numerous researchers have shown that microfluidic devices are capable of measuring blood glucose levels as accurately as or more accurately than traditional methods including colorimetry, high-performance liquid chromatography (HPLC), and commercially available blood glucose meters [60, 62, 64, 66–71]. Diabetes can also be quite accurately diagnosed using microfluidic-based glucose assays, along with microfluidic detection of other relevant chemicals such as cholesterol and triglycerides [64, 69].
Interstitial fluid
Although blood tests have been used as the clinical gold standard, they can be painful, stressful, and cause infections according to some users [3, 9, 11, 13–17]. Furthermore, subjecting fingertip tissue to chronic pricking can result in scarring and loss of finger sensation [101]. Therefore, instead of using sharp cannulas to draw blood, researchers have developed minimally invasive, microneedle-based systems for interstitial fluid extraction and glucose testing [72–79] (Fig. 1c). For example, Ribet et al. (2018) developed an integrated system consisting of a hollow silicon microneedle for drawing interstitial fluid and a microfluidic electrochemical sensing probe [76]. Takeuchi et al. (2019 and 2020) developed porous microneedle arrays using a salt leaching method that can be used to draw interstitial fluid for glucose testing [74, 75]. Although the detection of glucose from interstitial fluid is less invasive than blood glucose tests, it has an approximately 10-minute lag time attributed to the time taken for glucose to flow from the bloodstream into the interstitial fluid [76–79]. As long as there is sufficient modeling and correlation analysis, this lag time should be allowable, unless in an emergency [76].
Saliva
Because of the lag time and some invasiveness, using interstitial fluid for diabetes detection is still not ideal. Bodily fluids that can be obtained without any invasive penetration, such as saliva, urine, sweat, and tears, have also been used for glucose detection in diabetes monitoring. Thanks to the ease of obtaining saliva and its use as a well-studied diagnostic fluid, it has been most widely used of this group in the detection of glucose [102]. Numerous researchers have proposed microfluidic devices, most commonly paper-based devices, that readily detect glucose from saliva using colorimetric or electrochemical assays [66, 68, 80–86]. Salivary glucose concentration was found to be significantly higher in diabetic patients as compared to healthy subjects and can be used as an accurate indicator of hyperglycemia [80, 81, 85].
Sweat, tears, and urine
Similarly, sweat, tears, and urine have been used as alternative bodily fluids for non-invasive glucose detection, although less commonly than saliva. Sun et al. (2022), Bolat et al. (2022), and Xiao et al. (2019) successfully detected sweat glucose concentrations using custom-made microfluidic devices with electrochemical (Sun and Bolat) or colorimetric (Xiao) assays [83, 87, 88]. They found that glucose levels significantly increased upon consumption of a glucose-rich meal or solution, and Bolat et al. found that sweat glucose correlated very well with blood glucose, indicating that it is an accurate indicator of hyperglycemia. Allameh et al. (2022) and Agustini et al. (2017) measured tear glucose using µPADs with a distance-based colorimetric assay and µTEDs with an electrochemical assay, respectively [89, 90]. Agustini et al. indicated that tear glucose levels correlated very well with blood glucose levels [90]. Wei et al. (2021) suggested that similar to blood and saliva, urine glucose concentrations can be accurately measured using a hybrid microfluidic sensor [68]. Sechi et al. (2013) indicated that urine glucose was significantly increased in diabetic patients as compared to healthy subjects, which means that urine glucose can also be used as a metric for diabetes diagnosis [91]. However, most urine glucose studies are still in the preclinical stage with synthetic urine testing [84, 92, 93].
Other methods of diabetes monitoring
Although glucose quantification is the gold standard for diagnosing and monitoring diabetes, it has obvious limitations. For instance, glucose concentrations are known to have diurnal variations, and factors such as diet, exercise, stress, illness, and insulin resistance all impact glucose levels in the blood and other body fluids [24, 94–96].
HbA1c quantification
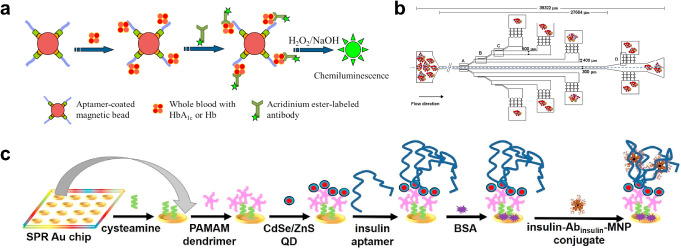
Compared to glucose tests, HbA1c tests have been suggested to be a more accurate indicator of diabetes [95, 103–105]. The most commonly used metric for HbA1c is the ratio between the concentration of HbA1c and total hemoglobin (Hb) [106–108]. The gold standard for HbA1c testing is high-performance liquid chromatography (HPLC), which is accurate but requires large-scale, costly equipment [95]. To minimize the scale and cost of HbA1c testing, Kuan et al. (2016) fabricated a polymethyl methacrylate (PMMA) microfluidic device integrated with dual complementary metal-oxide-semiconductor (CMOS) polysilicon nanowire sensors [94]. They measured total Hb and HbA1c from whole blood using a sodium lauryl sulfate hemoglobin assay and a miniaturized cation-exchange HPLC, respectively, in a time-efficient manner with a minimal amount (5 µL) of blood. Considering the peptide nature of Hb, some researchers performed immunoassays instead of HPLC to quantify HbA1c. For example, Chang et al. (2015) conducted an aptamer-antibody assay that attached Hb- and HbA1c-specific aptamers to magnetic beads loaded in a microfluidic chip (Fig. 2a) [95]. Chemiluminescence was induced upon the addition of anti-Hb or anti-HbA1c secondary antibodies labeled with acridinium ester and substrates including H2O2 and NaOH. Similarly, Wu et al. (2015) measured HbA1c with chemiluminescence in a microfluidic chip with a two-antibody assay [96].
Fig. 2.
Microfluidic diagnosis of diabetes using methods other than glucose tests. a An aptamer-antibody assay used to quantify total hemoglobin (Hb) and glycated hemoglobin (HbA1c) [95]. b A single-inlet (I), multi-outlet (O1-O9) microfluidic device for red cell deformability measurement [27]. c A surface plasmon resonance sensor array for serum insulin detection using dendrimers and aptamers [98]. (a) Reproduced from Chang KW, Li J, Yang CH, Shiesh SC, Lee GB. An integrated microfluidic system for measurement of glycated hemoglobin levels by using an aptamer-antibody assay on magnetic beads. Biosens Bioelectron. 2015;68:397–403 [95]. Copyright permission from Elsevier. (b) Recreated with BioRender from Pinho D, Faustino V, Catarino SO, Pereira AI, Minas G, Pinho FT, Lima R. Label-free multi-step microfluidic device for mechanical characterization of blood cells: Diabetes type II. Micro and Nano Engineering 2022;16:100149 [27]. Copyright permission from Elsevier. (c) Reproduced from Singh V. Ultrasensitive quantum dot-coupled-surface plasmon microfluidic aptasensor array for serum insulin detection. Talanta. 2020;219:121314 [98]. Copyright permission from Elsevier
Insulin quantification
Cohen et al. (2017) developed a real-time insulin quantification system to determine time varying demand for insulin due to the fluctuation of insulin resistance and pharmacokinetics, which cannot be addressed by glucose monitoring [24]. They detected insulin levels in serum through a custom-made microfluidic chip loaded with microspheres conjugated with streptavidin and biotinylated anti-insulin. Similarly, Furutani et al. (2018) developed a rapid enzyme-linked immunoassay (ELISA) with a six-layer disc-shaped microfluidic device to detect insulin and other glucose-regulating proteins including adiponectin and leptin [97]. Singh (2020) considered limitations of standard immunoassays, including the addition of toxic chemicals and lengthy procedures, and took a different approach. They developed a surface plasmon resonance-based insulin sensor array utilizing aptamers and quantum dots (Fig. 2c) [98].
Biomechanical testing
Besides biochemical markers, mechanical characteristics of cells can also be altered by diabetes. For instance, red blood cells (RBCs) are known to become stiffened and less deformable in diabetic patients, compared to those in healthy subjects [27–30]. Leveraging this phenomenon, Pinho et al. (2022) measured RBC deformability using a PDMS microfluidic device containing several carefully designed cross-flow filtration barriers (Fig. 2b) [27]. They found that RBC deformability was approximately 0.3 for T2D patients as compared to 0.5 for healthy subjects. This change can be used as a metric for clinical diagnosis of diabetes in the future. In addition, diabetes is also marked by impaired chemotaxis of neutrophils, which is caused by high concentrations of glucose and advanced glycation end products [31].
Use of microfluidic devices in diabetes treatment
The treatment of diabetes is centered upon insulin delivery for all T1D patients and between 20% and 30% of T2D patients [8, 32]. Using microfluidic devices, insulin can be delivered transdermally (across the skin), orally, intraperitoneally, or through inhalation. Alternatively, small molecules such as metformin, as well as other peptides such as glucagon-like peptide 1 (GLP-1) and GLP-1 receptor agonists can be used to treat diabetes. Microfluidic technologies used to treat diabetes are summarized in Table 2.
Table 2.
Summary of literature describing microfluidic technologies on the treatment of diabetes [46, 47, 109–153]
| Drug delivered | Delivery approach | Delivery tool | References |
|---|---|---|---|
| Insulin | Transdermal | Hollow microneedle arrays | [109–126] |
| Solid microneedle arrays for permeation of liquid insulin | [47, 115, 127–131] | ||
| Porous microneedle arrays | [132–134] | ||
| Iontophoresis-assisted microneedle arrays | [47, 128, 133] | ||
| Piezoelectric micropumps | [118, 122, 123, 125, 135, 136] | ||
| Membrane-driven micropumps | [120, 121, 124, 137] | ||
| Microfluidic devices with cellular and/or enzymatic components | [119, 138–140] | ||
| Oral | Micro- or nanocarriers produced by microfluidic devices | [141–143] | |
| Robotic microinjectors | [144] | ||
| Intraperitoneal | Implantable microfluidic devices | [144–146] | |
| Inhaled | Nebulizer | [147] | |
| Metformin | Transdermal | Smart sensor-integrated microneedle arrays | [148] |
| Oral | Micro- or nanocarriers produced by microfluidic devices | [149–151] | |
| GLP-1 and its receptor agonists | Transdermal | Dissolving microneedle arrays | [152] |
| Oral | Micro- or nanocarriers produced by microfluidic devices | [153] |
Insulin delivery
Current insulin delivery techniques have obvious limitations including painful cannula insertion, interference with activity, and embarrassment [42–47]. To alleviate these problems, researchers have leveraged microdevices including microfluidic chips, microneedle arrays, and nanoparticle- or microcapsule-based drug carriers. Unlike microfluidic-based diagnosis and monitoring of diabetes, the use of microfluidics and related devices for insulin delivery is still largely in the preclinical stage. Experiments with laboratory animals and in vitro examinations have been mainly used to confirm the efficacy of these products, and human subjects have been used in very few studies [113, 133, 154]. Subcutaneous injection is the most common approach for microdevice-based insulin delivery, yet other researchers seek to deliver insulin with even less invasive approaches including oral administration and inhalation. In this section, we will review recently developed microfluidics and related devices on the aforementioned approaches of insulin delivery, as well as their efficacy in glucose control.
Microfluidic devices for transdermal insulin delivery
Due to enzymatic digestion and impervious epithelia in the gastrointestinal (GI) tract, insulin is conventionally delivered through a subcutaneous rather than oral approach [41, 141, 142, 155, 156]. However, considering the non-adherence of patients due to painful cannula insertions [48–51, 54, 55], it is imperative to develop less invasive alternatives such as microneedle arrays. Various types of microneedle arrays have been developed for insulin delivery, and these microneedle arrays can be integrated with components such as microfluidic pumps, pancreatic islets or cells, and enzymes for active, controlled insulin release.
Insulin delivery using microneedle arrays
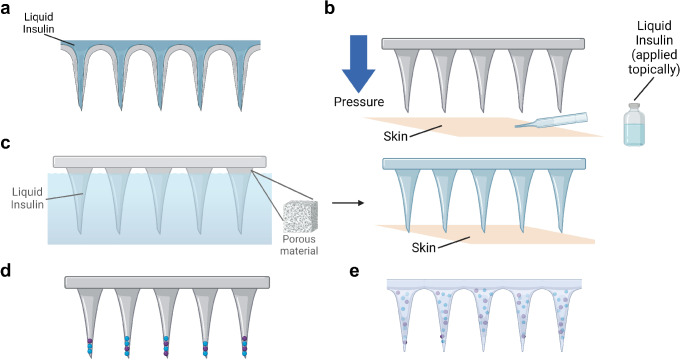
Thus far, microneedle arrays have been the most commonly explored minimally invasive tool for transdermal insulin injection, considering their slim and lightweight nature and their ability to easily penetrate the dermis without injection site reactions [117]. Various insulin-delivering microneedle arrays have been designed, including (1) hollow microneedle arrays for direct insulin injection (Fig. 3a) [109–126], (2) solid microneedle arrays for insulin permeation (Fig. 3b) [47, 115, 127–131], (3) porous microneedle arrays soaked in insulin [132–134] (Fig. 3c), (4) insulin-coated microneedle arrays [46, 157–160] (Fig. 3d), and (5) hydrogel or dissolving microneedle arrays containing insulin [161–190] (Fig. 3e).
Fig. 3.
Different types of microneedle arrays for transdermal insulin delivery. a A hollow microneedle array used to inject liquid insulin across the skin. b A solid microneedle array used to pre-permeate the skin for more efficient topical insulin treatment. c A porous microneedle array for liquid insulin absorption and injection. d A solid microneedle array coated with insulin-containing polymer. e A hydrogel/dissolving microneedle array containing insulin and glucose-sensitive enzymes. (Blue particles: micro/nanoparticles containing insulin; maroon particles: micro/nanoparticles containing enzymes). Created with Biorender
-
(I)
Hollow microneedle arrays: Hollow microneedle arrays have been widely used for transdermal insulin delivery in research due to their ability to inject fluid into the skin without the need for additional components [113]. Mechanical tests proved that most of these microneedle arrays are mechanically durable (require a higher force to bend/fracture the needles than to penetrate the skin) [109–116], while transdermal tests confirmed that they can effectively release insulin into the skin [109, 115, 116]. To study the effect of insulin delivered by these microneedle arrays on blood glucose, rodents with diabetes induced by streptozotoxin (STZ) are usually used. Vinayakumar et al. (2016), Davis et al. (2005), and Li et al. (2022) all suggested that insulin delivered by hollow microneedle arrays was able to lower the blood glucose to a normoglycemic level within several hours [111, 112, 114]. Furthermore, several studies showed that hollow microneedle arrays were able to achieve blood glucose reductions on par with subcutaneous injections (as positive controls) [109, 111, 114]. Resnik et al. [113] conducted a clinical study by delivering U-100 and U-200 insulin to a non-diabetic human subject using silicon hollow microneedle arrays. They found that the insulin-delivering microneedle arrays caused an immediate but modest glucose reduction, as opposed to a gradual but substantial drop in blood glucose caused by subcutaneous injections [113]. Moreover, as compared to subcutaneous injections, hollow microneedle arrays do not cause persistent injuries such as bleeding and erythema [117].
-
(II)
Solid microneedle arrays for liquid insulin permeation: Solid microneedle arrays are also promising tools for insulin delivery. They are typically used to pre-permeate the skin or create microchannels in the skin before insulin is applied topically [47, 127, 128, 130]. An early study by Chen et al. (2009) showed that solid stainless steel microneedle arrays induced microchannels in the skin, which enhanced the transdermal diffusion of insulin (encapsulated in nanovesicles) by approximately two orders of magnitude [128]. Similarly, Zhang et al. (2020) suggested that silicon nano-microneedles were able to enhance the diffusion of insulin across the skin by a factor of 2.5 [130]. Since solid microneedle arrays cannot act as vehicles for injection, other methods are often used in combination with these microneedle arrays to enhance the delivery of insulin across the skin. For instance, solid microneedle arrays are often coupled with electrodes to deliver insulin through iontophoresis, since electrical stimuli can drive charged particles such as insulin across the skin [47, 128]. Another commonly used method is to pre-encapsulate insulin into micro- or nanoparticles since these particles can cross the stratum corneum more easily than free insulin [115, 128]. Mechanical “press-and-release” is another method to enhance the delivery of insulin into the skin [127]. The efficacy of insulin-delivering solid microneedle arrays in lowering blood glucose levels is similar to that of hollow microneedle arrays. Yang et al. (2018), Yang et al. (2020), Zhang et al. (2020), and Chen et al. (2009) showed that insulin delivered by solid microneedle arrays was able to induce normoglycemia in diabetic rats within several hours [47, 127, 128, 130]. An additional attractive feature of this insulin delivery method is that, compared to hypodermic injections, solid microneedles do not result in sharp hypoglycemic shocks [47, 127].
-
(III)
Porous microneedle arrays: Instead of creating microchannels before insulin delivery, porous microneedle arrays can absorb liquid insulin and release insulin into the skin [132, 133]. These microneedle arrays are fabricated with special methods such as salt leaching, solid-state sintering, or the addition of porogens or surfactants [74, 75, 132–134]. Li et al. (2017) found that a titanium-based porous microneedle array increased the permeation of calcein through rabbit skin by 27 times [132]. Similar to solid microneedle arrays, porous microneedle arrays can also be coupled with iontophoresis [133]. Using an iontophoresis-driven microneedle patch, Li et al. (2021) found that the blood glucose of STZ-induced diabetic rats could be reduced to a normoglycemic level within 3 h [133]. Additionally, insulin delivered by porous microneedle arrays did not cause a hypoglycemic shock, similar to other microneedle array delivery methods [133]. The microneedle arrays could be integrated with a glucose-sensitive gating, so that insulin would only be delivered in hypoglycemic, but not normoglycemic, conditions [134].
-
(IV)
Other types of microneedle arrays: Besides the aforementioned microneedle array types, other types of microneedle arrays have been developed, including dissolving microneedle arrays, hydrogel-based microneedle arrays, and solid microneedle arrays with an insulin-containing polymer coating. Hydrogel-based and dissolving microneedle arrays are especially promising because they can achieve slow and controlled release of insulin without polymer deposition in the skin [191]. Insulin is typically incorporated in the bulk polymer of these microneedle arrays, and glucose-sensitive components (such as glucose-responsive nanovesicles) are also often added into the polymer to facilitate closed-loop insulin delivery. Similarly, solid microneedle arrays can be coated with insulin-containing polymers, which can also be released into the skin [46, 157–160]. Since this article primarily focuses on microfluidics, we will not discuss these all-solid microneedle arrays in further detail.
Other microfluidic devices for transdermal insulin delivery
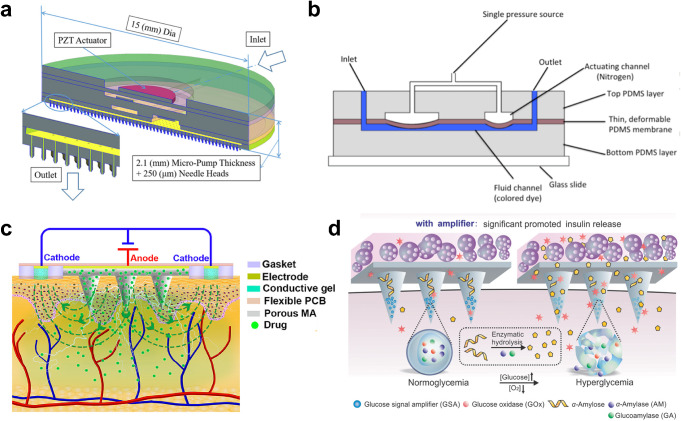
Microneedle arrays are proven to be a promising technology for insulin delivery, but they rely on passive diffusion and do not facilitate active pumping of insulin. Therefore, scientists have developed other microfluidic components, including iontophoretic devices (Fig. 4c), microfluidic pumps (Fig. 4a,b), and cellular and enzymatic components, and integrated these components with microneedle arrays to enhance the delivery of insulin (Fig. 4d).
Fig. 4.
Technologies enabling active delivery of insulin across the skin compatible with a microneedle array-based system. a. A silicon-glass-PDMS-lead zirconate titanate (PZT) piezoelectric micropump integrated with a hollow microneedle array [123]. b An insect-mimetic, pulse-driven mechanical microfluidic pump from Chatterjee et al. [192, 193]. Zhang et al. (2022) later coupled the system with a 3D-printed hollow microneedle array [120]. c A hydrogel-based iontophoretic system that helps deliver insulin across the skin coupled with a porous microneedle array [133]. d A system that contains pancreatic islets that actively secrete insulin, as well as a dissolving microneedle array containing glucose signal amplifiers (GSA) including GOx, α-amylase (AM), and glucoamylase (GA) for glucose-sensitive insulin delivery [119]. (a) Reproduced from Meshkinfam F, Rizvi G. A MEMS-Based Drug Delivery Device With Integrated Microneedle Array—Design and Simulation. J Biomech Engi. 2021;143:081010 [123]. Copyright permission from American Society of Mechanical Engineers (ASME). (b) Reproduced from Chatterjee K. Analytical and Experimental Investigation of Insect Respiratory System Inspired Microfluidics: Virginia Tech; 2018 [192]. Copyright permission from Virginia Tech Libraries. (c) Reproduced from Li Y, Yang J, Zheng Y, Ye R, Liu B, Huang Y, Zhou W, Jiang L. Iontophoresis-driven porous microneedle array patch for active transdermal drug delivery. Acta Biomater. 2021;121:349–358 [133]. Copyright permission from Elsevier. (d) Reproduced from Ye Y, Yu J, Wang C, Nguyen NY, Walker GM, Buse JB, Gu Z. Microneedles Integrated with Pancreatic Cells and Synthetic Glucose-Signal Amplifiers for Smart Insulin Delivery. Adv Mater. 2016;28:3115–3121 [119]. Copyright permission from Elsevier
-
(I)
Iontophoresis-assisted microneedle arrays: Iontophoresis drives charged particles (such as insulin) across the skin barrier using electricity [133]. Iontophoresis is typically facilitated by placing electrodes (cathode and anode) on the skin. Conductive media, such as conductive hydrogels or films, are used to complete the circuit so that charged particles like ions and insulin contained in the skin can move through the circuit (Fig. 4c) [47, 133]. Iontophoresis was used as a technique for transdermal insulin delivery as early as 1997 [194]. In this study, Haga et al. developed a series of iontophoretic devices with electroplated copper electrodes in an agar gel. Using diabetic mice, they found that the blood glucose levels could be reduced by 60% within 90 min [194]. Iontophoresis has been used in tandem with microneedle arrays for insulin delivery in later studies. In this case, microneedle arrays can act as one electrode, and the other electrode is typically composed of Ag/AgCl [47, 128, 133]. Chen et al. (2009) found that iontophoresis enhanced the transdermal permeation of insulin by factor between 3.3 and 5.3 [128]. Both Yang et al. (2020) and Li et al. (2021) found that iontophoresis was able to further reduce blood glucose in diabetic rats (but not so much as to cause hypoglycemic shock) when used with nanoparticle-encapsulated insulin whose delivery was enhanced by solid microneedle arrays. Also, iontophoresis was shown to decrease the time it takes for microneedle array-injected insulin to induce normoglycemia in diabetic rats [47, 133].
-
(II)
Microfluidic pumps: Mechanical pumping mechanisms can also facilitate the active delivery of insulin into the skin. Thus far, the overwhelming majority of microfluidic pumps used to drive transdermal insulin delivery are piezoelectric pumps [118, 122, 123, 125, 135, 136]. This is due to the ability of piezoelectric pumps to achieve a very high accuracy because they can change the pumping pressure drastically in response to a small change in voltage [171]. Briefly, piezoelectric pumps work by applying a voltage at a frequency across a piezoelectric membrane, which causes the membrane to deform, driving a fluid flow [118]. Using piezoelectric pumps, the delivery rate can be precisely controlled by fine-tuning the actuating voltage and frequency [118, 122, 123]. This makes the pumps ideal for precisely controlled insulin delivery. Besides piezoelectric pumps, numerous other types of mechanical microfluidic pumps can drive insulin delivery. Huang et al. (2007) constructed a PDMS-based micropump containing microchannels and microvalves coupled with glucose and flow sensors. Their micropump was designed to deliver insulin based on measured glucose levels and driven by the peristaltic deflection of the PDMS membranes [137]. Similarly, Chatterjee et al. (2021), Zhang (2021), and Zhang et al. (2022) developed insect-mimetic microfluidic pumps driven by periodic contractions of a PDMS membrane enabled by compressed air or the radial pulse on the human wrist [120, 121, 193] (Fig. 4b). Mishra et al. (2019) integrated hollow microneedle arrays into a Nafion membrane micropump for insulin delivery [124]. Instead of being driven directly by mechanical compressions, this micropump was actuated by a laser doppler vibrometer, which deformed the membrane as a function of the actuating voltage and frequency [124].
-
(III)
Microfluidic devices with cellular and enzymatic components: A very different type of microfluidic device used to facilitate insulin delivery is one integrated with cellular components, specifically pancreatic islets or β-cells. Instead of using commercially available insulin, researchers can leverage the ability of these cells to secrete insulin in a glucose-sensitive manner enabled by inherent feedback mechanisms in these cells. Tendulkar et al. (2011) developed a microfluidic device containing pancreatic islets immobilized in alginate microbeads. They found that the insulin secreted by the islets could increase from 0.165 ± 0.059 ng/10 islets/min in normoglycemic conditions to 0.422 ± 0.095 ng/10 islets/min in hyperglycemic conditions [138]. Similarly, Quintard et al. (2022) incorporated human islets into a two-layer, pneumatically driven microfluidic pump and found that there was a significant increase in insulin secretion when subjected to hyperglycemic stimulation [139]. Enzymatic components can also be incorporated into microfluidic devices or microneedle arrays to facilitate glucose-sensitive insulin delivery. Chen et al. (2011) constructed a glucose-sensitive microfluidic device by incorporating a membrane containing pH-sensitive nanoparticles encapsulating GOx and HRP, which resulted in insulin being released 2.4 times faster in hyperglycemia compared to normoglycemia [140]. Incorporating both pancreatic β-cells and an enzymatic glucose-sensing system in a microfluidic device, Ye et al. (2016) demonstrated that there was only a generous secretion of insulin by β-cells in hyperglycemic conditions (Fig. 4d) [119].
Microfluidic devices involved in oral insulin delivery
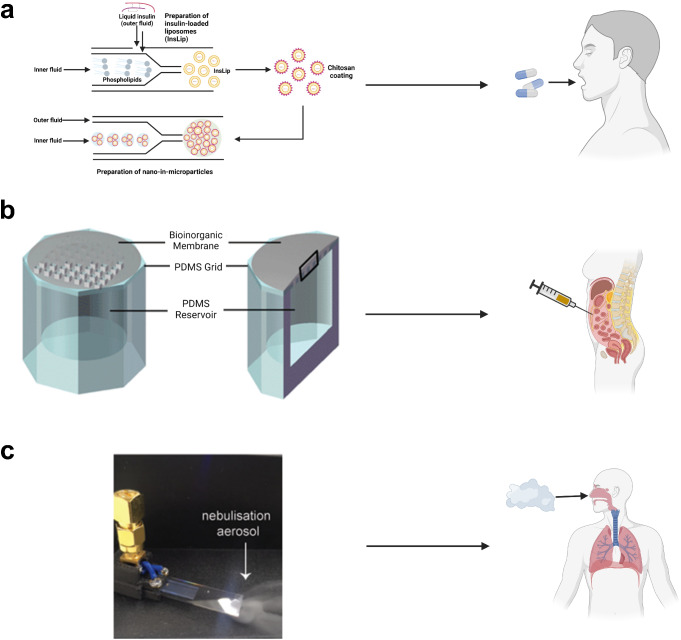
Oral delivery of insulin has advantages over transdermal routes because it increases patient compliance and can result in a more complete regulation and better re-activation of insulin-dependent glucose metabolism in the liver [141, 155]. Oral insulin delivery is challenging, however, because it is difficult for insulin to pass through the epithelium and withstand enzymatic digestion in the GI tract and acidic conditions in the stomach [142, 195, 196]. Researchers have developed numerous approaches to overcome these limitations, the most common of which is the encapsulation of insulin within micro- or nanovesicles. Microfluidic devices are commonly used to produce these carriers. Costa et al. (2020) used microfluidic devices to produce microparticles containing chitosan-coated, insulin-encapsulating liposomes, which enhanced the permeation of insulin across the intestinal wall (Fig. 5a) [141]. A similar study conducted by Ma et al. (2023) suggested that microcapsules composed of a zwitterionic copolymer produced by a microfluidic device helped insulin both survive stomach-like acidic conditions and pass through the intestinal wall. In vivo tests suggested that this oral delivery method maintained normoglycemia better than subcutaneous injections in diabetic mice [142].
Fig. 5.
Oral administration, intraperitoneal injection, and inhalation of insulin enabled by microfluidic systems. a A microfluidic device used to produce microparticles containing chitosan-coated, insulin-encapsulated nanoparticles [141]. b A PDMS microfluidic insulin reservoir integrated with a bioinorganic gel membrane [146]. c A hybrid resonant acoustics (HYDRA) microfluidic nebulizer for insulin inhalation [147]. (a) Recreated using Biorender.com from Costa C, Liu Z, Martins JP, Correia A, Figueiredo P, Rahikkala A, Li W, Seitsonen J, Ruokolainen J, Hirvonen SP, Aguiar-Ricardo A, Corvo ML, Santos HA. All-in-one microfluidic assembly of insulin-loaded pH-responsive nano-in-microparticles for oral insulin delivery. Biomater Sci. 2020;8:3270-3277 [141]. Copyright permission from RSC Publications (CC License). (b) Reproduced from Chu MK, Chen J, Gordijo CR, Chiang S, Ivovic A, Koulajian K, Giacca A, Wu XY, Sun Y. In vitro and in vivo testing of glucose-responsive insulin-delivery microdevices in diabetic rats. Lab Chip. 2012;12:2533-9 [146]. Copyright permission from RSC Publications (CC License). Modified with Biorender.com. (c) Reproduced from Nguyen EP, Lee L, Rezk AR, Sabri YM, Bhargava SK, Yeo LY. Hybrid Surface and Bulk Resonant Acoustics for Concurrent Actuation and Sensing on a Single Microfluidic Device. Anal Chem. 2018 Apr 17;90(8):5335-5342 [147]. Copyright permission from ACS Publications (CC License). Modified with Biorender
Other microfluidic devices used in service of oral insulin delivery include insulin-loaded robotic microinjectors produced by microfluidic devices intended for oral administration, as developed by Ghosh et al. (2022) [144]. These microinjectors were used to mechanically penetrate the intestinal wall with microneedle-like structures (“arms”) and therefore deliver insulin into the bloodstream. The efficacy of these microinjectors was tested with gelatin, stomach, and intestinal tissue, and it was found that the amount of insulin delivered across the tissues by these microinjectors was much higher compared to free insulin. Ghosh et al. also delivered the insulin microinjectors into diabetic rats intrarectally and found that the efficiency of insulin delivery was higher than previously developed devices for GI tract insulin delivery by an order of magnitude [144].
Microfluidic devices involved in intraperitoneal insulin delivery
Although the administration route is relatively invasive, pre-clinical and clinical tests of intraperitoneal insulin delivery have shown that this route may have advantages compared to traditional insulin delivery methods, including more stable glucose levels and less time spent in hyperglycemia and hypoglycemia in diabetic patients [197, 198]. Microfluidic platforms have been developed for intraperitoneal insulin delivery. For instance, Luo et al. (2023) used an approach in which an electrically driven microfluidic device was constructed to produce alginate- and cellulose-based droplets containing insulin-releasing β-cells [145]. These droplets were implanted into the peritoneal cavity of diabetic mice. They found that the blood glucose levels were lowered to a normoglycemic state within 2 h and stayed normoglycemic for 21 days [145]. Chu et al. (2012) developed microfluidic devices consisting of primary amine-activated PDMS and bioinorganic gel membranes containing MnO2 nanoparticles and N-isopropylacrylamide (NIPAM): methacrylic acid (MAA) hydrogel nanoparticles (Fig. 5b). The devices were intraperitoneally implanted into diabetic rats and the rats were able to maintain normoglycemia for 7 days [146].
Microfluidic devices for inhaled insulin delivery
Compared to other insulin delivery approaches, the development of microfluidic technologies for inhaled insulin is rare, possibly owing to previous finding that use of inhaled insulin is positively correlated with lung cancer [199, 200]. Nguyen et al. (2018) fabricated a hybrid resonant acoustics (HYDRA) microfluidic nebulizer that could evaporate aqueous insulin into an aerosol ready for inhalation (Fig. 5c). Briefly, this device was fabricated with lithium niobate sputtered with chromium and aluminum and patterned with interdigitated transducers. Liquid insulin was deposited into the device and nebulized using acoustic waves. The nebulizer generated aerosolized insulin droplets with a mass-median aerodynamic diameter of 2.5 μm with a geometric standard deviation of 0.2 μm, which was ideal for alveolar deposition. They also found that the chemical structure of insulin was not damaged, but studies on the control of blood glucose levels using this technology have yet to be performed [147].
Delivery of other drugs through microfluidic systems for diabetes treatment
Besides insulin, other pharmaceuticals can also lower blood glucose levels in diabetic patients, especially for type 2 diabetics [148–153, 201]. These pharmaceuticals include metformin, GLP-1, and GLP-1 receptor agonists. One advantage of these treatments over insulin therapy is that they do not tend to cause a hypoglycemic shock [152, 202].
Metformin
Metformin controls blood glucose in type 2 diabetics by increasing insulin sensitivity without stimulating insulin secretion [203, 204]. Compared to insulin, which can cause lipid accumulation in the body, metformin is not obesogenic and can cause weight loss, which is beneficial for T2D patients [205]. Due to these benefits of metformin, some researchers developed microfluidic systems for metformin delivery. Lee et al. (2016) constructed a multi-layer microfluidic device integrated with various sensors and a dissolving microneedle array for the glucose-sensitive transdermal delivery of metformin. Metformin delivered by their system significantly decreased the blood glucose level of diabetic mice to a normoglycemic state within 4 h [148]. Joshi et al. (2020) and Cesur et al. (2020 and 2021) produced metformin-encapsulated microparticles such as niosomes and microbubbles with microfluidic systems intended for oral delivery but only characterized the release profile of metformin in vitro [149–151]. While Lee et al. (2016) successfully developed their integrated device for clinical trials to examine its efficacy in monitoring the glucose levels of human subjects [148], most studies on metformin delivery using microfluidic systems have been preclinical [149–151, 201].
GLP-1 and its receptor agonists
Unlike metformin, GLP-1 and its receptor agonists can stimulate glucose-dependent insulin secretion [153]. Araujo et al. (2016) fabricated a glass-based microfluidic system used to encapsulate GLP-1 into microcapsules made of poly(lactic-co-glycolic acid) (PLGA) functionalized with chitosan and a cell-penetrating peptide (CPP) for effective penetration through the GI tract walls for oral GLP-1 delivery. They found that the microcapsules lowered the glucose levels of Type 2 diabetic rats to a normoglycemic state within 2 h and maintained normoglycemia for 6 h [153]. Chen et al. (2017) developed a microneedle array-based transdermal delivery system for a GLP-1 receptor agonist, exendin 4(Ex-4). Due to the inclusion of GOx, the delivery of Ex-4 was glucose-sensitive. The delivery of Ex-4 maintained normoglycemia in diabetic mice for 5 days without causing a hypoglycemic shock [152]. Similar to other approaches to treat diabetes with microfluidic technologies, the use of these technologies to deliver GLP-1 and its agonists remains in the preclinical stage.
Conclusions
In this paper, we reviewed technologies in use and in development for diagnosing, monitoring, and treating diabetes using microfluidic systems. The diagnostic and monitoring technologies surveyed perform glucose tests on various bodily fluids or carry out other biochemical or mechanical assays. Compared to traditional blood glucose testing with finger pricks and glucometers, microfluidic-based glucose monitoring is advantageous due to its lightweight nature, reduced sample size, and high throughput [59, 206]. Additionally, microfluidic glucose monitoring from non-blood bodily fluids including interstitial fluid, saliva, sweat, tears, and urine greatly reduces the invasiveness of glucose tests compared to traditional methods. Besides glucose, other markers of diabetes, including glycated hemoglobin, insulin, and cellular responses, have been detected using microfluidic devices. Many researchers have conducted clinical studies and confirmed that these testing schemes are efficacious in healthy and diabetic human subjects [24, 27, 60–69, 71, 76, 79–83, 85, 88, 90, 91, 94, 96–98].
The microfluidic systems reviewed here for the treatment of diabetes delivered insulin, other peptides, or metformin through transdermal or intraperitoneal injection, oral administration, and inhalation. We note that very few studies on advanced microfluidic technologies for the treatment of diabetes have entered the stage of investigating clinical efficacy in human subjects [113, 148, 154]. Most studies have focused on preclinical testing using diabetic rodents and in vitro tissue samples. Translating research involving microneedle array-based transdermal drug delivery is considered challenging because such injections involve many unknown parameters such as microneedle geometry, the force required to insert microneedle arrays into the skin, as well as issues of sterilization, immunogenicity, and flow rate accuracy, which remains a major challenge [207]. Current insulin pump technologies deliver insulin to diabetic patients at meticulously controlled rates [208, 209]. Most noninvasive insulin delivery technologies will need further development to characterize their flow rate accuracies. The oral delivery of insulin and other peptide drugs still lacks approval for clinical trials [196], possibly due to their low oral bioavailability, which is a result of acidic conditions, enzymatic digestion, and epithelial barriers in the GI tract [196, 197]. Microfluidic platforms for inhaled insulin are rare, likely due to established correlations between inhaled insulin and lung cancer [199, 200]. The delivery of metformin using microfluidic technologies, on the other hand, faces fewer barriers. Metformin has already been approved by the US Food and Drug Administration for oral consumption, so extensive clinical trials are not needed to further examine its safety [210, 211].
The emerging microfluidic technologies discussed here have the potential to greatly reduce the inconvenience and discomfort of diagnosing, monitoring, and treating diabetes. Further characterization and refinement of the technologies is needed, followed by clinical trials and other studies involving human subjects, which will ensure the safety and efficacy of these technologies for treating diabetes in human patients.
Authors’ contributions
All authors contributed to the conception and outlining of the review article. The manuscript was written by SZ and edited by AS. All authors read and approved the final manuscript.
Funding
This work was supported by the Student Engineers’ Council and the Regenerative Medicine Interdisciplinary Graduate Education Program at Virginia Tech.
Data availability
Due to the nature of this article as a review, no datasets have been generated in the work towards the completion of this article.
Statements and Declarations
Ethics approval
No human or animal studies have been performed in the work towards the completion of this article. Therefore, no ethical approvals required.
Consent to publish
No human or animal studies have been performed in the work towards the completion of this article. Therefore, no consent for publication is required. All authors agreed with the final version of this manuscript and with the current submission.
Competing interests
The authors have no financial or non-financial interests to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019;157:107843. 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.Prevention CfDCa. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States 2022 [Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html].
- 3.Villena Gonzales W, Mobashsher AT, Abbosh A. The progress of glucose monitoring—A review of invasive to minimally and non-invasive techniques, devices and sensors. Sensors. 2019;19:800. 10.3390/s19040800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Association AD. Economic costs of diabetes in the US in 2017. Diabetes Care. 2018;41:917–28. 10.2337/dci18-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stokes A, Preston SH. Deaths attributable to diabetes in the United States: comparison of data sources and estimation approaches. PLoS ONE. 2017;12:e0170219. 10.1371/journal.pone.0170219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy S, Xu J, Kochanek K, Deaths. Final data for 2010. National vital statistics reports. National Center for Health Statistics. 2013;61. [PubMed]
- 7.Mobasseri M, Shirmohammadi M, Amiri T, Vahed N, Fard HH, Ghojazadeh M. Prevalence and incidence of type 1 diabetes in the world: a systematic review and meta-analysis. Health Promot Perspect. 2020;10:98. 10.34172/hpp.2020.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umpierrez GE, Klonoff DC. Diabetes technology update: use of insulin pumps and continuous glucose monitoring in the hospital. Diabetes Care. 2018;41:1579–89. 10.2337/dci18-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta S, Sandhu SV, Bansal H, Sharma D. Comparison of salivary and serum glucose levels in diabetic patients. J Diabetes Sci Technol. 2014;9:91–6. 10.1177/1932296814552673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang L, Chang SJ, Chen C-J, Liu J-T. Non-invasive blood glucose monitoring technology: a review. Sensors. 2020;20:6925. 10.3390/s20236925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kownacka AE, Vegelyte D, Joosse M, Anton N, Toebes BJ, Lauko J, Buzzacchera I, Lipinska K, Wilson DA, Geelhoed-Duijvestijn N. Clinical evidence for use of a noninvasive biosensor for tear glucose as an alternative to painful finger-prick for diabetes management utilizing a biopolymer coating. Biomacromolecules. 2018;19:4504–11. 10.1021/acs.biomac.8b01429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr. 2018;12:181–7. 10.1016/j.dsx.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Bruen D, Delaney C, Florea L, Diamond D. Glucose sensing for diabetes monitoring: recent developments. Sensors. 2017;17:1866. 10.3390/s17081866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei T-T, Tsai H-Y, Yang C-C, Hsiao W-T, Huang K-C, editors. Noninvasive glucose evaluation by human skin oxygen saturation level. 2016 IEEE International Instrumentation and Measurement Technology Conference Proceedings; 2016: IEEE. 10.1109/I2MTC.2016.7520571.
- 15.Asaduzzaman A, Samadarsinee S, Chidella KK, editors. Simulating multisensor noninvasive blood glucose monitoring systems. SoutheastCon 2016; 2016: IEEE. 10.1109/SECON.2016.7506765.
- 16.Bolla AS, Priefer R. Blood glucose monitoring-an overview of current and future non-invasive devices. Diabetes Metabolic Syndrome: Clin Res Rev. 2020;14:739–51. 10.1016/j.dsx.2020.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Siddiqui SA, Zhang Y, Lloret J, Song H, Obradovic Z. Pain-free blood glucose monitoring using wearable sensors: recent advancements and future prospects. IEEE Rev Biomed Eng. 2018;11:21–35. 10.1109/RBME.2018.2822301. [DOI] [PubMed] [Google Scholar]
- 18.Bantle JP, Thomas W. Glucose measurement in patients with diabetes mellitus with dermal interstitial fluid. J Lab Clin Med. 1997;130. 10.1016/S0022-2143(97)90044-5.:436– 41. [DOI] [PubMed]
- 19.Karpova EV, Shcherbacheva EV, Galushin AA, Vokhmyanina DV, Karyakina EE, Karyakin AA. Noninvasive diabetes monitoring through continuous analysis of sweat using flow-through glucose biosensor. Anal Chem. 2019;91:3778–83. 10.1021/acs.analchem.8b05928. [DOI] [PubMed] [Google Scholar]
- 20.Urakami T, Morimoto S, Nitadori Y, Harada K, Owada M, Kitagawa T. Urine glucose screening program at schools in Japan to detect children with diabetes and its outcome-incidence and clinical characteristics of childhood type 2 diabetes in Japan. Pediatr Res. 2007;61:141–5. 10.1203/pdr.0b013e31802d8a69. [DOI] [PubMed] [Google Scholar]
- 21.Danese E, Montagnana M, Nouvenne A, Lippi G. Advantages and pitfalls of fructosamine and glycated albumin in the diagnosis and treatment of diabetes. J Diabetes Sci Technol. 2015;9:169–76. 10.1177/1932296814567227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lapolla A, Mosca A, Fedele D. The general use of glycated haemoglobin for the diagnosis of diabetes and other categories of glucose intolerance: still a long way to go. Nutr Metab Cardiovas Dis. 2011;21:467–75. 10.1016/j.numecd.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Sehrawat T, Jindal A, Kohli P, Thour A, Kaur J, Sachdev A, Gupta Y. Utility and limitations of glycated hemoglobin (HbA1c) in patients with liver cirrhosis as compared with oral glucose tolerance test for diagnosis of diabetes. Diabetes Ther. 2018;9:243–51. 10.1007/s13300-017-0362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen N, Sabhachandani P, Sarkar S, Kahanovitz L, Lautsch N, Russell SJ, Konry T. Microsphere based continuous-flow immunoassay in a microfluidic device for determination of clinically relevant insulin levels. Microchim Acta. 2017;184:835–41. 10.1007/s00604-017-2072-z. [Google Scholar]
- 25.Gerasimov JY, Schaefer CS, Yang W, Grout RL, Lai RY. Development of an electrochemical insulin sensor based on the insulin-linked polymorphicregion. Biosens Bioelectron. 2013;42:62–8. 10.1016/j.bios.2012.10.046. [DOI] [PubMed] [Google Scholar]
- 26.Zhu W, Xu L, Zhu C, Li B, Xiao H, Jiang H, Zhou X. Magnetically controlled electrochemical sensing membrane based on multifunctional molecularly imprinted polymers for detection of insulin. Electrochim Acta. 2016;218:91–100. 10.1016/j.electacta.2016.09.108. [Google Scholar]
- 27.Pinho D, Faustino V, Catarino SO, Pereira AI, Minas G, Pinho FT, Lima R. Label-free multi-step microfluidic device for mechanical characterization of blood cells: diabetes type II. Micro Nano Eng. 2022;16:100149. 10.1016/j.mne.2022.100149. [Google Scholar]
- 28.Chang H-Y, Li X, Karniadakis GE. Modeling of biomechanics and biorheology of red blood cells in type 2 diabetes mellitus. Biophys J. 2017;113:481–90. 10.1016/j.bpj.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moutzouri A, Athanassiou G, Dimitropoulou D, Skoutelis A, Gogos C. Severe sepsis and diabetes mellitus have additive effects on red blood cell deformability. J Infect. 2008;57:147–51. 10.1016/j.jinf.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Agrawal R, Smart T, Nobre-Cardoso J, Richards C, Bhatnagar R, Tufail A, Shima D, Jones H, Pavesio P. Assessment of red blood cell deformability in type 2 diabetes mellitus and diabetic retinopathy by dual optical tweezers stretching technique. Sci Rep. 2016;6:15873. 10.1038/srep15873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang K, Yang X, Gao C, Hua C, Hong C, Zhu L. A Novel Microfluidic device for the Neutrophil Functional phenotype analysis: effects of glucose and its derivatives AGEs. Micromachines. 2021;12:944. 10.3390/mi12080944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarkar S, Heyward J, Alexander GC, Kalyani RR. Trends in insulin types and devices used by adults with type 2 diabetes in the United States, 2016 to 2020. JAMA Netw Open. 2021;4:e2128782–e. 10.1001/jamanetworkopen.2021.28782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garg SK, Rewers AH, Akturk HK. Ever-increasing insulin-requiring patients globally. Mary Ann Liebert, Inc. 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA; 2018. p. S2-1-S2-4. 10.1089/dia.2018.0101. [DOI] [PubMed]
- 34.Ceriello A, Novials A, Ortega E, Canivell S, La Sala L, Pujadas G, Esposito K, Giugliano D, Genovese S. Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation, and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care. 2013;36:2346–50. 10.2337/dc12-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Behme MT, Dupré J, McDonald TJ. Glucagon-like peptide 1 improved glycemic control in type 1 diabetes. BMC Endocr Disord. 2003;3:1–9. 10.1186/1472-6823-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore LE, Briery CM, Clokey D, Martin RW, Williford NJ, Bofill JA, Morrison JC. Metformin and insulin in the management of gestational diabetes mellitus: preliminary results of a comparison. J Reprod Med. 2007;52:1011–5. [PubMed] [Google Scholar]
- 37.Giugliano D, Quatraro A, Consoli G, Minei A, Ceriello A, De Rosa N, D’onofrio F. Metformin for obese, insulin-treated diabetic patients: improvement in glycaemic control and reduction of metabolic risk factors. Eur J Clin Pharmacol. 1993;44:107–12. 10.1007/BF00315466. [DOI] [PubMed] [Google Scholar]
- 38.Wróbel MP, Marek B, Kajdaniuk D, Rokicka D, Szymborska-Kajanek A, Strojek K. Metformin—a new old drug. Endokrynol Pol. 2017;68:482–96. 10.5603/EP.2017.0050. [DOI] [PubMed] [Google Scholar]
- 39.Gu S, Shi J, Tang Z, Sawhney M, Hu H, Shi L, Fonseca V, Dong H. Comparison of glucose lowering effect of metformin and acarbose in type 2 diabetes mellitus: a meta-analysis. PLoS ONE. 2015;10:e0126704. 10.1371/journal.pone.0126704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo X, Wang W. Challenges and recent advances in the subcutaneous delivery of insulin. Expert Opin Drug Deliv. 2017;14:727–34. 10.1080/17425247.2016.1232247. [DOI] [PubMed] [Google Scholar]
- 41.Shah RB, Patel M, Maahs DM, Shah VN. Insulin delivery methods: past, present and future. Int J Pharm Investig. 2016;6:1. 10.4103/2230-973X.176456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu X, Zhang H, Wang Z, Shiu CYA, Gu Z. Microneedle array patches integrated with nanoparticles for therapy and diagnosis. Small Struct. 2021;2:2000097. 10.1002/sstr.202000097. [Google Scholar]
- 43.Zhang Y, Yu J, Kahkoska AR, Wang J, Buse JB, Gu Z. Advances in transdermal insulin delivery. Adv Drug Deliv Rev. 2019;139:51–70. 10.1016/j.addr.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabbagh F, Muhamad II, Niazmand R, Dikshit PK, Kim BS. Recent progress in polymeric non-invasive insulin delivery. Int J Biol Macromol. 2022. 10.1016/j.ijbiomac.2022.01.134. [DOI] [PubMed] [Google Scholar]
- 45.Darvishha S, Amiri S. (Trans) dermal insulin delivery based on polymeric systems. Int J Polym Mater Polym Biomater. 2019;68:1118–32. 10.1080/00914037.2018.1534113. [Google Scholar]
- 46.Wang Y, Wang H, Zhu X, Guan Y, Zhang Y. Smart microneedle patches for rapid, and painless transdermal insulin delivery. J Mater Chem B. 2020;8:9335–42. 10.1039/D0TB01822H. [DOI] [PubMed] [Google Scholar]
- 47.Yang J, Li Y, Ye R, Zheng Y, Li X, Chen Y, Xie X, Jiang L. Smartphone-powered iontophoresis-microneedle array patch for controlled transdermal delivery. Microsyst Nanoeng. 2020;6:112. 10.1038/s41378-020-00224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davies M, Gagliardino JJ, Gray L, Khunti K, Mohan V, Hughes R. Real-world factors affecting adherence to insulin therapy in patients with type 1 or type 2 diabetes mellitus: a systematic review. Diabet Med. 2013;30:512–24. 10.1111/dme.12128. [DOI] [PubMed] [Google Scholar]
- 49.Peyrot M, Barnett A, Meneghini L, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational global attitudes of patients and Physicians in insulin therapy study. Diabet Med. 2012;29:682–9. 10.1111/j.1464-5491.2012.03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarbacker GB, Urteaga EM. Adherence to insulin therapy. Diabetes Spectr. 2016;29:166. 10.2337/2Fdiaspect.29.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang H, Lobo JM, Kim S, Sohn M-W. Cost-related medication non-adherence among US adults with diabetes. Diabetes Res Clin Pract. 2018;143:24–33. 10.1016/j.diabres.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Payne FW, Ledden B, Lamps G. Capabilities of next-generation patch pump: improved precision, instant occlusion detection, and dual-hormone therapy. J Diabetes Sci Technol. 2019;13:49–54. 10.1177/1932296818776028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan B, DeLong B, An D, Kiourti A, Dungan K, Volakis J, Ma M, Guo L, editors. An RF-driven lightweight implantable insulin pump. 2018 International Applied Computational Electromagnetics Society Symposium (ACES); 2018: IEEE. 10.23919/ROPACES.2018.8364218.
- 54.Ho PM, Rumsfeld JS, Masoudi FA, McClure DL, Plomondon ME, Steiner JF, Magid DJ. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166:1836–41. 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 55.Farsaei S, Radfar M, Heydari Z, Abbasi F, Qorbani M. Insulin adherence in patients with diabetes: risk factors for injection omission. Prim Care Diabetes. 2014;8:338–45. 10.1016/j.pcd.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Nielsen JB, Hanson RL, Almughamsi HM, Pang C, Fish TR, Woolley AT. Microfluidics: innovations in materials and their fabrication and functionalization. Anal Chem. 2019;92:150–68. 10.1021/acs.analchem.9b04986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Domachuk P, Tsioris K, Omenetto FG, Kaplan DL. Bio-microfluidics: biomaterials and biomimetic designs. Adv Mater. 2010;22:249–60. 10.1002/adma.200900821. [DOI] [PubMed] [Google Scholar]
- 58.Niculescu A-G, Chircov C, Bîrcă AC, Grumezescu AM. Fabrication and applications of microfluidic devices: a review. Int J Mol Sci. 2021;22:2011. 10.3390/ijms22042011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rabiee M, Ghasemnia NN, Rabiee N, Bagherzadeh M. Microfluidic devices and drug delivery systems. Biomedical Applications of Microfluidic devices. Elsevier; 2021. pp. 153–. 10.1016/B978-0-12-818791-3.00013-9. 86.
- 60.Cao L, Han G-C, Xiao H, Chen Z, Fang C. A novel 3D paper-based microfluidic electrochemical glucose biosensor based on rGO-TEPA/PB sensitive film. Anal Chim Acta. 2020;1096:34–43. 10.1016/j.aca.2019.10.049. [DOI] [PubMed] [Google Scholar]
- 61.Calabria D, Zangheri M, Trozzi I, Lazzarini E, Pace A, Mirasoli M, Guardigli M. Smartphone-based chemiluminescent origami µPAD for the rapid assessment of glucose blood levels. Biosensors. 2021;11:381. 10.3390/bios11100381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li J, Sun Y, Chen C, Sheng T, Liu P, Zhang G. A smartphone-assisted microfluidic chemistry analyzer using image-based colorimetric assays for multi-index monitoring of diabetes and hyperlipidemia. Anal Chim Acta. 2019;1052:105–12. 10.1016/j.aca.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 63.Choobbari ML, Rad MB, Jahanshahi A, Ghourchian H. A sample volume independent paper microfluidic device for quantifying glucose in real human plasma. Microfluid Nanofluidics. 2020;24:1–12. 10.1007/s10404-020-02382-y. [Google Scholar]
- 64.Park C, Kim H-R, Kim S-K, Jeong I-K, Pyun J-C, Park S. Three-dimensional paper-based microfluidic analytical devices integrated with a plasma separation membrane for the detection of biomarkers in whole blood. ACS Appl Mater Interfaces. 2019;11:36428–34. 10.1021/acsami.9b13644. [DOI] [PubMed] [Google Scholar]
- 65.Nie Z, Deiss F, Liu X, Akbulut O, Whitesides GM. Integration of paper-based microfluidic devices with commercial electrochemical readers. Lab Chip. 2010;10:3163–9. 10.1039/C0LC00237B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kalinke C, Wosgrau V, Oliveira PR, Oliveira GA, Martins G, Mangrich AS, Bergamini MF, Marcolino-Junior LH. Green method for glucose determination using microfluidic device with a non-enzymatic sensor based on nickel oxyhydroxide supported at activated biochar. Talanta. 2019;200:518–25. 10.1016/j.talanta.2019.03.079. [DOI] [PubMed] [Google Scholar]
- 67.Noiphung J, Songjaroen T, Dungchai W, Henry CS, Chailapakul O, Laiwattanapaisal W. Electrochemical detection of glucose from whole blood using paper-based microfluidic devices. Anal Chim Acta. 2013;788:39–45. 10.1016/j.aca.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 68.Wei X, Guo J, Lian H, Sun X, Liu B. Cobalt metal-organic framework modified carbon cloth/paper hybrid electrochemical button-sensor for nonenzymatic glucose diagnostics. Sens Actuators B Chem. 2021;329:129205. 10.1016/j.snb.2020.129205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li J, Yang Y, Peng Z, Yang J, Li Y. A novel photoelectrochemical microfluidic chip for multi-index determination of diabetes and its complications. Biosens Bioelectron. 2022;217:114719. 10.1016/j.bios.2022.114719. [DOI] [PubMed] [Google Scholar]
- 70.Kim S, Kim D, Kim S. Simultaneous quantification of multiple biomarkers on a self-calibrating microfluidic paper-based analytic device. Anal Chim Acta. 2020;1097:120–6. 10.1016/j.aca.2019.10.068. [DOI] [PubMed] [Google Scholar]
- 71.Zhu Y, Meng X, Chen Y, Li J, Shao H, Lu Y, Pan L, Xu Y, Cheng J. Self-served and fully automated biochemical detection of finger-prick blood at home using a portable microfluidic analyzer. Sens Actuators B Chem. 2020;303:127235. 10.1016/j.snb.2019.127235. [Google Scholar]
- 72.Pu Z, Zou C, Wang R, Lai X, Yu H, Xu K, Li D. A continuous glucose monitoring device by graphene modified electrochemical sensor in microfluidic system. Biomicrofluidics. 2016;10. 10.1063/1.4942437. [DOI] [PMC free article] [PubMed]
- 73.Najmi A, Saidi MS, Shahrokhian S, Hosseini H, Hannani SK. Fabrication of a microdialysis-based nonenzymatic microfluidic sensor for regular glucose measurement. Sens Actuators B Chem. 2021;333:129569. 10.1016/j.snb.2021.129569. [Google Scholar]
- 74.Takeuchi K, Takama N, Kinoshita R, Okitsu T, Kim B. Flexible and porous microneedles of PDMS for continuous glucose monitoring. Biomed Microdevices. 2020;22:1–12. 10.1007/s10544-020-00532-1. [DOI] [PubMed] [Google Scholar]
- 75.Takeuchi K, Takama N, Kim B, Sharma K, Paul O, Ruther P. Microfluidic chip to interface porous microneedles for ISF collection. Biomed Microdevices. 2019;21:1–10. 10.1007/s10544-019-0370-4. [DOI] [PubMed] [Google Scholar]
- 76.Ribet F, Stemme G, Roxhed N. Real-time intradermal continuous glucose monitoring using a minimally invasive microneedle-based system. Biomed Microdevices. 2018;20:1–10. 10.1007/s10544-018-0349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Croce RA, Vaddiraju S, Kondo J, Wang Y, Zuo L, Zhu K, Islam SK, Burgess DJ, Papadimitrakopoulos F, Jain FC. A miniaturized transcutaneous system for continuous glucose monitoring. Biomed Microdevices. 2013;15:151–60. 10.1007/s10544-012-9708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shibata H, Heo YJ, Okitsu T, Matsunaga Y, Kawanishi T, Takeuchi S. Injectable hydrogel microbeads for fluorescence-based in vivo continuous glucose monitoring. Proc Natl Acad Sci. 2010;107:17894–8. 10.1073/pnas.1006911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nightingale AM, Leong CL, Burnish RA, Hassan S-u, Zhang Y, Clough GF, Boutelle MG, Voegeli D, Niu X. Monitoring biomolecule concentrations in tissue using a wearable droplet microfluidic-based sensor. Nat Commun. 2019;10:2741. 10.1038/s41467-019-10401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Castro LF, de Freitas SV, Duarte LC, de Souza JAC, Paixão TR, Coltro WK. Salivary diagnostics on paper microfluidic devices and their use as wearable sensors for glucose monitoring. Anal Bioanal Chem. 2019;411:4919–28. 10.1007/s00216-019-01788-0. [DOI] [PubMed] [Google Scholar]
- 81.Sousa LR, Duarte LC, Coltro WK. Instrument-free fabrication of microfluidic paper-based analytical devices through 3D pen drawing. Sens Actuators B Chem. 2020;312:128018. 10.1016/j.snb.2020.128018. [Google Scholar]
- 82.Santana-Jiménez LA, Márquez-Lucero A, Osuna V, Estrada-Moreno I, Dominguez RB. Naked-Eye detection of glucose in saliva with bienzymatic paper-based sensor. Sensors. 2018;18:1071. 10.3390/s18041071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun M, Pei X, Xin T, Liu J, Ma C, Cao M, Zhou M. A flexible microfluidic chip-based universal fully integrated nanoelectronic system with point-of-care raw sweat, tears, or saliva glucose monitoring for potential noninvasive glucose management. Anal Chem. 2022;94:1890–900. 10.1021/acs.analchem.1c05174. [DOI] [PubMed] [Google Scholar]
- 84.Klasner SA, Price AK, Hoeman KW, Wilson RS, Bell KJ, Culbertson CT. Paper-based microfluidic devices for analysis of clinically relevant analytes present in urine and saliva. Anal Bioanal Chem. 2010;397:1821–9. 10.1007/s00216-010-3718-4. [DOI] [PubMed] [Google Scholar]
- 85.Gupta S, Nayak MT, Sunitha J, Dawar G, Sinha N, Rallan NS. Correlation of salivary glucose level with blood glucose level in diabetes mellitus. J Oral Maxillofac Pathol. 2017;21:334. 10.4103/jomfp.JOMFP_222_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rossini EL, Milani MI, Lima LS, Pezza HR. Paper microfluidic device using carbon dots to detect glucose and lactate in saliva samples. Spectrochim Acta Mol Biomol Spectrosc. 2021;248:119285. 10.1016/j.saa.2020.119285. [DOI] [PubMed] [Google Scholar]
- 87.Bolat G, De la Paz E, Azeredo NF, Kartolo M, Kim J, de Loyola e Silva AN, Rueda R, Brown C, Angnes L, Wang J. Wearable soft electrochemical microfluidic device integrated with iontophoresis for sweat biosensing. Anal Bioanal Chem. 2022;414:5411–21. 10.1007/s00216-021-03865-9. [DOI] [PubMed] [Google Scholar]
- 88.Xiao G, He J, Chen X, Qiao Y, Wang F, Xia Q, Yu L, Lu Z. A wearable, cotton thread/paper-based microfluidic device coupled with smartphone for sweat glucose sensing. Cellulose. 2019;26:4553–62. 10.1007/s10570-019-02396-y. [Google Scholar]
- 89.Allameh S, Rabbani M. A distance-based microfluidic paper-based biosensor for glucose measurements in tear range. Appl Biochem Biotechnol. 2022;194:2077–92. 10.1007/s12010-022-03817-8. [DOI] [PubMed] [Google Scholar]
- 90.Agustini D, Bergamini MF, Marcolino-Junior LH. Tear glucose detection combining microfluidic thread based device, amperometric biosensor and microflow injection analysis. Biosens Bioelectron. 2017;98:161–7. 10.1016/j.bios.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 91.Sechi D, Greer B, Johnson J, Hashemi N. Three-dimensional paper-based microfluidic device for assays of protein and glucose in urine. Anal Chem. 2013;85:10733–7. 10.1021/ac4014868. [DOI] [PubMed] [Google Scholar]
- 92.Yu P, Deng M, Yang Y. New single-layered paper-based microfluidic devices for the analysis of Nitrite and glucose built via deposition of adhesive tape. Sensors. 2019;19:4082. 10.3390/s19194082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Prapaporn S, Arisara S, Wunpen C, Wijitar D. Nanocellulose films to improve the performance of distance-based glucose detection in paper-based microfluidic devices. Anal Sci. 2020;36:1447–52. 10.2116/analsci.20P168. [DOI] [PubMed] [Google Scholar]
- 94.Kuan D-H, Wang I-S, Lin J-R, Yang C-H, Huang C-H, Lin Y-H, Lin C-T, Huang N-T. A microfluidic device integrating dual CMOS polysilicon nanowire sensors for on-chip whole blood processing and simultaneous detection of multiple analytes. Lab Chip. 2016;16:3105–13. 10.1039/C6LC00410E. [DOI] [PubMed] [Google Scholar]
- 95.Chang K-W, Li J, Yang C-H, Shiesh S-C, Lee G-B. An integrated microfluidic system for measurement of glycated hemoglobin levels by using an aptamer–antibody assay on magnetic beads. Biosens Bioelectron. 2015;68:397–403. 10.1016/j.bios.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 96.Wu C-C, Lin H-I, Chang K-W, Mai JD, Shiesh S-C, Lee G-B. Measurement of glycated hemoglobin levels using an integrated microfluidic system. Microfluid Nanofluidics. 2015;18:613–21. 10.1007/s10404-014-1460-5. [Google Scholar]
- 97.Furutani S, Nishio K, Naruish N, Akazawa-Ogawa Y, Hagihara Y, Yoshida Y, Nagai H. Rapid enzyme-linked immunosorbent assays for diagnosis of diabetes in a compact disc-shaped microfluidic device. Anal Sci. 2018;34:379–82. 10.2116/analsci.34.379. [DOI] [PubMed] [Google Scholar]
- 98.Singh V. Ultrasensitive quantum dot-coupled-surface plasmon microfluidic aptasensor array for serum insulin detection. Talanta. 2020;219:121314. 10.1016/j.talanta.2020.121314. [DOI] [PubMed] [Google Scholar]
- 99.Carrilho E, Martinez AW, Whitesides GM. Understanding wax printing: a simple micropatterning process for paper-based microfluidics. Anal Chem. 2009;81:7091–5. 10.1021/ac901071p. [DOI] [PubMed] [Google Scholar]
- 100.Li X, Li C, Zhang S, Cui C, Li J, Gao Q. Simple and fast colorimetric and electrochemical methods for the ultrasensitive detection of glucose. Anal Bioanal Chem. 2021;413:5725–31. 10.1007/s00216-021-03547-6. [DOI] [PubMed] [Google Scholar]
- 101.Cha KH, Qin Y, Meyerhoff ME. Origin of Low Detection Limit and high selectivity of Roche Accu-Chek Test strips that enables measurement of tear glucose levels. Electroanalysis. 2015;27:670–6. 10.1002/elan.201400576. [Google Scholar]
- 102.Streckfus C, Bigler L. Saliva as a diagnostic fluid. Oral Dis. 2002;8:69–76. [DOI] [PubMed] [Google Scholar]
- 103.Al-Ghamdi AA. A high prevalence of depression among diabetic patients at a teaching hospital in Western Saudi Arabia. Neurosci J. 2004. 10.1034/j.1601-0825.2002.1o834.x. 9:108– 12. [PubMed] [Google Scholar]
- 104.Kilpatrick ES, Maylor PW, Keevil BG. Biological variation of glycated hemoglobin: implications for diabetes screening and monitoring. Diabetes Care. 1998;21:261–4. 10.2337/diacare.21.2.261. [DOI] [PubMed] [Google Scholar]
- 105.Farmer A. Use of HbA1c in the diagnosis of diabetes. Brit Med J. 2012. 10.1136/bmj.e7293. [DOI] [PubMed] [Google Scholar]
- 106.Roubicek M, Viñes G, Sanguineti AG, Eberhardt MS. Use of HbA (1c) in screening for diabetes/Assessing the utility of glycated hemoglobin/Response. Diabetes Care. 1998;21:1577. 10.1111/dom.12999. [PubMed] [Google Scholar]
- 107.Tankova T, Chakarova N, Dakovska L, Atanassova I. Assessment of HbA1c as a diagnostic tool in diabetes and prediabetes. Acta Diabetol. 2012;49:371–8. 10.1007/s00592-011-0334-5. [DOI] [PubMed] [Google Scholar]
- 108.Goodall I. HbA1c standardisation destination–global IFCC standardisation how, why, where and when: a tortuous pathway from Kit manufacturers, via inter-laboratory Lyophilized and whole blood comparisons to designated National Comparison Schemes. Clin Biochem Rev. 2005;26:5. [PMC free article] [PubMed] [Google Scholar]
- 109.Economidou SN, Uddin MJ, Marques MJ, Douroumis D, Sow WT, Li H, Reid A, Windmill JF, Podoleanu A. A novel 3D printed hollow microneedle microelectromechanical system for controlled, personalized transdermal drug delivery. Addit Manuf. 2021;38:101815. 10.1016/j.addma.2020.101815. [Google Scholar]
- 110.Bolton CJ, Howells O, Blayney GJ, Eng PF, Birchall JC, Gualeni B, Roberts K, Ashraf H, Guy OJ. Hollow silicon microneedle fabrication using advanced plasma etch technologies for applications in transdermal drug delivery. Lab Chip. 2020;20:2788–95. 10.1039/D0LC00567C. [DOI] [PubMed] [Google Scholar]
- 111.Vinayakumar K, Kulkarni PG, Nayak M, Dinesh N, Hegde GM, Ramachandra S, Rajanna K. A hollow stainless steel microneedle array to deliver insulin to a diabetic rat. J Micromech Microeng. 2016;26:065013. 10.1088/0960-1317/26/6/065013. [Google Scholar]
- 112.Davis SP, Martanto W, Allen MG, Prausnitz MR. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans Biomed Eng. 2005;52:909–15. 10.1109/TBME.2005.845240. [DOI] [PubMed] [Google Scholar]
- 113.Resnik D, Možek M, Pečar B, Janež A, Urbančič V, Iliescu C, Vrtačnik D. In vivo experimental study of noninvasive insulin microinjection through hollow Si microneedle array. Micromachines. 2018;9:40. 10.3390/mi9010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li R, Liu X, Yuan X, Wu S, Li L, Jiang X, Li B, Jiang X, Gou M. Fast customization of hollow microneedle patches for insulin delivery. Int J Bioprinting. 2022;8. 10.18063/2Fijb.v8i2.553. [DOI] [PMC free article] [PubMed]
- 115.Howells O, Blayney GJ, Gualeni B, Birchall JC, Eng PF, Ashraf H, Sharma S, Guy OJ. Design, fabrication, and characterisation of a silicon microneedle array for transdermal therapeutic delivery using a single step wet etch process. Eur J Pharm Biopharm. 2022;171:19–28. 10.1016/j.ejpb.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 116.Xenikakis I, Tsongas K, Tzimtzimis EK, Katsamenis OL, Demiri E, Zacharis CK, Georgiou D, Kalogianni EP, Tzetzis D, Fatouros DG. Transdermal delivery of insulin across human skin in vitro with 3D printed hollow microneedles. J Drug Deliv Sci Technol. 2022;67:102891. 10.1016/j.jddst.2021.102891. [Google Scholar]
- 117.Zhou C-P, Liu Y-L, Wang H-L, Zhang P-X, Zhang J-L. Transdermal delivery of insulin using microneedle rollers in vivo. Int J Pharm. 2010;392:127–33. 10.1016/j.ijpharm.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 118.Ma B, Liu S, Gan Z, Liu G, Cai X, Zhang H, Yang Z, editors. A PZT insulin pump integrated with a silicon micro needle array for transdermal drug delivery. 56th Electronic Components and Technology Conference 2006; 2006: IEEE. 10.1109/ECTC.2006.1645724.
- 119.Ye Y, Yu J, Wang C, Nguyen NY, Walker GM, Buse JB, Gu Z. Microneedles integrated with pancreatic cells and synthetic glucose-signal amplifiers for smart insulin delivery. Adv Mater. 2016;28:3115–21. 10.1002/adma.201506025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang S, Dunya A, Katz C, Prisbe J, Davalos R, Staples A. A slim, powerless microfluidic patch-pump for transdermal drug delivery. Bull Ame Phys Soc. 2022.
- 121.Zhang S, InsulPatch:. A Slim, Powerless Microfluidic Patch-Pump for insulin delivery. Virginia Tech; 2021.
- 122.Datta A, Biswas S, Dhar R, Bhattacharyya TK. Design and development of a piezoelectric driven micropump integrated with hollow microneedles for precise insulin delivery. J Micromech Microeng. 2023;33:075003. 10.1088/1361-6439/acd25f. [Google Scholar]
- 123.Meshkinfam F, Rizvi G. A MEMS-Based drug delivery device with Integrated Microneedle array—design and Simulation. J Biom Eng. 2021;143. 10.1115/1.4050754. [DOI] [PubMed]
- 124.Mishra R, Maiti TK, Bhattacharyya TK. Feasibility studies on nafion membrane actuated micropump integrated with hollow microneedles for insulin delivery device. J Microelectromech Syst. 2019;28:987–96. 10.1109/JMEMS.2019.2939189. [Google Scholar]
- 125.Mishra R, Bhattacharyya TK, Maity TK, editors. Design and simulation of microfluidic components towards development of a controlled drug delivery platform. 2016 29th International Conference on VLSI Design and 2016 15th International Conference on Embedded Systems (VLSID). 2016: IEEE. 10.1109/VLSID.2016.22.
- 126.Hou Z, Lin C, Zhang Q, editors. Design of a smart transdermal insulin drug delivery system. 2010 4th International Conference on Bioinformatics and Biomedical Engineering; 2010: IEEE. 10.1109/ICBBE.2010.5517141.
- 127.Yang J, Chen Z, Ye R, Li J, Lin Y, Gao J, Ren L, Liu B, Jiang L. Touch-actuated microneedle array patch for closed-loop transdermal drug delivery. Drug Deliv. 2018;25:1728–39. 10.1080/10717544.2018.1507060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen H, Zhu H, Zheng J, Mou D, Wan J, Zhang J, Shi T, Zhao Y, Xu H, Yang X. Iontophoresis-driven penetration of nanovesicles through microneedle-induced skin microchannels for enhancing transdermal delivery of insulin. J Control Release. 2009;139:63–72. 10.1016/j.jconrel.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 129.Leeladurga V, Teja UC, Sultana SA, Sudeep K, Anusha VSS, Han T, Nalluri BN, Das DB. Application of microneedle arrays for enhancement of transdermal permeation of insulin: in vitro experiments, scaling analyses and numerical simulations. AAPS PharmSciTech. 2016;17:915–22. 10.1208/s12249-015-0416-8. [DOI] [PubMed] [Google Scholar]
- 130.Zhang P, Zhang Y, Liu C-G. Polymeric nanoparticles based on carboxymethyl chitosan in combination with painless microneedle therapy systems for enhancing transdermal insulin delivery. RSC Adv. 2020;10:24319–29. 10.1039/D0RA04460A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu Y, Gao Y, Qin G, Zhang S, Qiu Y, Li F, Xu B. Sustained release of insulin through skin by intradermal microdelivery system. Biomed Microdevices. 2010;12:665–71. 10.1007/s10544-010-9419-0. [DOI] [PubMed] [Google Scholar]
- 132.Li J, Liu B, Zhou Y, Chen Z, Jiang L, Yuan W, Liang L. Fabrication of a Ti porous microneedle array by metal injection molding for transdermal drug delivery. PLoS ONE. 2017;12:e0172043. 10.1371/journal.pone.0172043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li Y, Yang J, Zheng Y, Ye R, Liu B, Huang Y, Zhou W, Jiang L. Iontophoresis-driven porous microneedle array patch for active transdermal drug delivery. Acta Biomater. 2021;121:349–58. 10.1016/j.actbio.2020.12.023. [DOI] [PubMed] [Google Scholar]
- 134.Gholami S, Zarkesh I, Ghanian M-H, Hajizadeh-Saffar E, Hassan-Aghaei F, Mohebi M-M, Baharvand H. Dynamically capped hierarchically porous microneedles enable post-fabrication loading and self-regulated transdermal delivery of insulin. Chem Eng J. 2021;421:127823. 10.1016/j.cej.2020.127823. [Google Scholar]
- 135.Revathi S, Padmanabhan R. Study of the effect on flow rate for planar type polymer-based diaphragm piezoelectric actuated valveless micropump for insulin delivery. Adv Mater Proc Technol. 2016;2:31–43. 10.1080/2374068X.2016.1147766. [Google Scholar]
- 136.Yang Z, Dong L, Wang M, Liu G, Li X, Li Y. A wearable insulin delivery system based on a piezoelectric micropump. Sens Actuators Phys. 2022;347:113909. 10.1016/j.sna.2022.113909. [Google Scholar]
- 137.Huang C-J, Chen Y-H, Wang C-H, Chou T-C, Lee G-B. Integrated microfluidic systems for automatic glucose sensing and insulin injection. Sens Actuators B Chem. 2007;122:461–8. 10.1016/j.snb.2006.06.015. [Google Scholar]
- 138.Tendulkar S, McQuilling J, Childers C, Pareta R, Opara E, Ramasubramanian M, editors. A scalable microfluidic device for the mass production of microencapsulated islets. Transplant Proc. Elsevier; 2011. 10.1016/j.transproceed.2011.10.023. [DOI] [PMC free article] [PubMed]
- 139.Quintard C, Tubbs E, Achard J-L, Navarro F, Gidrol X, Fouillet Y. Microfluidic device integrating a network of hyper-elastic valves for automated glucose stimulation and insulin secretion collection from a single pancreatic islet. Biosens Bioelectron. 2022;202:113967. 10.1016/j.bios.2022.113967. [DOI] [PubMed] [Google Scholar]
- 140.Chen J, Gordijo CR, Chu M, Wu XY, Sun Y, editors. A glucose-responsive insulin delivery micro device embedded with nanohydrogel particles as smart valves. 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference; 2011: IEEE. 10.1109/TRANSDUCERS.2011.5969367.
- 141.Costa C, Liu Z, Martins JP, Correia A, Figueiredo P, Rahikkala A, Li W, Seitsonen J, Ruokolainen J, Hirvonen S-P. All-in-one microfluidic assembly of insulin-loaded pH-responsive nano-in-microparticles for oral insulin delivery. Biomater Sci. 2020;8:3270–7. 10.1039/D0BM00743A. [DOI] [PubMed] [Google Scholar]
- 142.Ma Y, Li Q, Yang J, Cheng Y, Li C, Zhao C, Chen W, Huang D, Qian H. Crosslinked zwitterionic microcapsules to overcome gastrointestinal barriers for oral insulin delivery. Biomater Sci. 2023. 10.1039/D2BM01606K. [DOI] [PubMed] [Google Scholar]
- 143.Yang Y, Wang X, Yuan X, Zhu Q, Chen S, Xia D. Glucose-activatable insulin delivery with charge-conversional polyelectrolyte multilayers for diabetes care. Front Bioeng Biotechnol. 2022;10:996763. 10.3389/fbioe.2022.996763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ghosh A, Liu W, Li L, Pahapale GJ, Choi SY, Xu L, Huang Q, Zhang R, Zhong Z, Selaru FM. Autonomous untethered microinjectors for gastrointestinal delivery of insulin. ACS Nano. 2022;16:16211–20. 10.1021/acsnano.2c05098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Luo Z, Dong Y, Yu M, Fu X, Qiu Y, Sun X, Chu X. A novel insulin delivery system by β cells encapsulated in microcapsules. Front Chem. 2023;10:1104979. 10.3389/fchem.2022.1104979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chu MK, Chen J, Gordijo CR, Chiang S, Ivovic A, Koulajian K, Giacca A, Wu XY, Sun Y. In vitro and in vivo testing of glucose-responsive insulin-delivery microdevices in diabetic rats. Lab Chip. 2012;12:2533–9. 10.1039/C2LC40139H. [DOI] [PubMed] [Google Scholar]
- 147.Nguyen EP, Lee L, Rezk AR, Sabri YM, Bhargava SK, Yeo LY. Hybrid surface and bulk resonant acoustics for concurrent actuation and sensing on a single microfluidic device. Anal Chem. 2018;90:5335–42. 10.1021/acs.analchem.8b00466. [DOI] [PubMed] [Google Scholar]
- 148.Lee H, Choi TK, Lee YB, Cho HR, Ghaffari R, Wang L, Choi HJ, Chung TD, Lu N, Hyeon T. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat Nanotechnol. 2016;11:566–72. 10.1038/nnano.2016.38. [DOI] [PubMed] [Google Scholar]
- 149.Cesur S, Cam ME, Sayın FS, Su S, Gunduz O, editors. Controlled release of metformin loaded polyvinyl alcohol (PVA) microbubble/nanoparticles using microfluidic device for the treatment of type 2 diabetes mellitus. Bioinformatics and Biomedical Engineering: 8th International Work-Conference, IWBBIO 2020, Granada, Spain, May 6–8, 2020, Proceedings 8; 2020: Springer. 10.1007/978-3-030-45385-5_17.
- 150.Cesur S, Cam ME, Sayın FS, Su S, Harker A, Edirisinghe M, Gunduz O. Metformin-loaded polymer-based microbubbles/nanoparticles generated for the treatment of type 2 diabetes mellitus. Langmuir. 2021;38:5040–51. 10.1021/acs.langmuir.1c00587. [DOI] [PubMed] [Google Scholar]
- 151.Joshi S, White R, Sahu R, Dennis VA, Singh SR. Comprehensive screening of drug encapsulation and co-encapsulation into niosomes produced using a microfluidic device. Processes. 2020;8:535. 10.3390/pr8050535. [Google Scholar]
- 152.Chen W, Tian R, Xu C, Yung BC, Wang G, Liu Y, Ni Q, Zhang F, Zhou Z, Wang J. Microneedle-array patches loaded with dual mineralized protein/peptide particles for type 2 diabetes therapy. Nat Commun. 2017;8:1777. 10.1038/s41467-017-01764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Araujo F, Shrestha N, Gomes MJ, Herranz-Blanco B, Liu D, Hirvonen J, Granja PL, Santos HA, Sarmento B. In vivo dual-delivery of glucagon like peptide-1 (GLP-1) and dipeptidyl peptidase-4 (DPP4) inhibitor through composites prepared by microfluidics for diabetes therapy. Nanoscale. 2016;8:10706–13. 10.1039/C6NR00294C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yuzhakov VV. The AdminPen™ microneedle device for painless & convenient drug delivery. Drug Deliv Technol. 2010;10:32–6. [Google Scholar]
- 155.Whitehead K, Shen Z, Mitragotri S. Oral delivery of macromolecules using intestinal patches: applications for insulin delivery. J Control Release. 2004;98:37–45. 10.1016/j.jconrel.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 156.Wang L-Y, Gu Y-H, Su Z-G, Ma G-H. Preparation and improvement of release behavior of chitosan microspheres containing insulin. Int J Pharm. 2006;311:187–95. 10.1016/j.ijpharm.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 157.Ye R, Yang J, Li Y, Zheng Y, Yang J, Li Y, Liu B, Jiang L. Fabrication of tip-hollow and tip-dissolvable microneedle arrays for transdermal drug delivery. ACS Biomater Sci Eng. 2020;6:2487–94. 10.1021/acsbiomaterials.0c00120. [DOI] [PubMed] [Google Scholar]
- 158.Mizuno Y, Takasawa K, Hanada T, Nakamura K, Yamada K, Tsubaki H, Hara M, Tashiro Y, Matsuo M, Ito T. Fabrication of novel-shaped microneedles to overcome the disadvantages of solid microneedles for the transdermal delivery of insulin. Biomed Microdevices. 2021;23:1–8. 10.1007/s10544-021-00576-x. [DOI] [PubMed] [Google Scholar]
- 159.Economidou SN, Pere CPP, Reid A, Uddin MJ, Windmill JF, Lamprou DA, Douroumis D. 3D printed microneedle patches using stereolithography (SLA) for intradermal insulin delivery. Mater Sci Eng C. 2019;102:743–55. 10.1016/j.msec.2019.04.063. [DOI] [PubMed] [Google Scholar]
- 160.Seong K-Y, Seo M-S, Hwang DY, O’Cearbhaill ED, Sreenan S, Karp JM, Yang SY. A self-adherent, bullet-shaped microneedle patch for controlled transdermal delivery of insulin. J Control Release. 2017;265:48–56. 10.1016/j.jconrel.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 161.Sun X, Ji W, Zhang B, Ma L, Fu W, Qian W, Zhang X, Li J, Sheng E, Tao Y. A theranostic microneedle array patch for integrated glycemia sensing and self-regulated release of insulin. Biomater Sci. 2022;10:1209–16. 10.1039/D1BM01834E. [DOI] [PubMed] [Google Scholar]
- 162.Yu J, Qian C, Zhang Y, Cui Z, Zhu Y, Shen Q, Ligler FS, Buse JB, Gu Z. Hypoxia and H2O2 dual-sensitive vesicles for enhanced glucose-responsive insulin delivery. Nano Lett. 2017;17:733–9. 10.1021/acs.nanolett.6b03848. [DOI] [PubMed] [Google Scholar]
- 163.Yu J, Zhang Y, Gu Z. Glucose-responsive insulin delivery by microneedle-array patches loaded with hypoxia-sensitive vesicles. Biomedical Nanotechnology: Methods Protoc. 2017;251–9. 10.1007/978-1-4939-6840-4_17. [DOI] [PubMed]
- 164.Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, Ligler FS, Buse JB, Gu Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci. 2015;112:8260–5. 10.1073/pnas.1505405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hu X, Yu J, Qian C, Lu Y, Kahkoska AR, Xie Z, Jing X, Buse JB, Gu Z. H2O2-responsive vesicles integrated with transcutaneous patches for glucose-mediated insulin delivery. ACS Nano. 2017;11:613–20. 10.1021/acsnano.6b06892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Luo F-Q, Chen G, Xu W, Zhou D, Li J-X, Huang Y-C, Lin R, Gu Z, Du J-Z. Microneedle-array patch with pH-sensitive formulation for glucose-responsive insulin delivery. Nano Res. 2021;14:2689–96. 10.1007/s12274-020-3273-z. [Google Scholar]
- 167.Liu S, Jin M-n, Quan Y-s, Kamiyama F, Katsumi H, Sakane T, Yamamoto A. The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. J Control Release. 2012;161:933–41. 10.1016/j.jconrel.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 168.Chen S, Matsumoto H, Moro-oka Y, Tanaka M, Miyahara Y, Suganami T, Matsumoto A. Microneedle‐array patch fabricated with enzyme‐free polymeric components capable of on‐demand insulin delivery. Adv Funct Mater. 2019;29:1807369. 10.1002/adfm.201807369. [Google Scholar]
- 169.Ye Z, Xiang Y, Monroe T, Yu S, Dong P, Xian S, Webber MJ. Polymeric microneedle arrays with glucose-sensing dynamic-covalent bonding for insulin delivery. Biomacromolecules. 2022;23:4401–11. 10.1021/acs.biomac.2c00878. [DOI] [PubMed] [Google Scholar]
- 170.Migalska K, Morrow DI, Garland MJ, Thakur R, Woolfson AD, Donnelly RF. Laser-engineered dissolving microneedle arrays for transdermal macromolecular drug delivery. Pharm Res. 2011;28:1919–30. 10.1007/s11095-011-0419-4. [DOI] [PubMed] [Google Scholar]
- 171.Ling M-H, Chen M-C. Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater. 2013;9:8952–61. 10.1016/j.actbio.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 172.Yu W, Jiang G, Zhang Y, Liu D, Xu B, Zhou J. Polymer microneedles fabricated from alginate and hyaluronate for transdermal delivery of insulin. Mater Sci Eng C. 2017;80:187–96. 10.1016/j.msec.2017.05.143. [DOI] [PubMed] [Google Scholar]
- 173.Vora LK, Courtenay AJ, Tekko IA, Larrañeta E, Donnelly RF. Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules. Int J Biol Macromol. 2020;146:290–8. 10.1016/j.ijbiomac.2019.12.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lin Y, Hu W, Bai X, Ju Y, Cao C, Zou S, Tong Z, Cen C, Jiang G, Kong X. Glucose-and pH-Responsive Supramolecular Polymer vesicles based on host–Guest Interaction for Transcutaneous Delivery of insulin. ACS Appl Bio Mater. 2020;3:6376–83. 10.1021/acsabm.0c00813. [DOI] [PubMed] [Google Scholar]
- 175.Liu D, Yu B, Jiang G, Yu W, Zhang Y, Xu B. Fabrication of composite microneedles integrated with insulin-loaded CaCO3 microparticles and PVP for transdermal delivery in diabetic rats. Mater Sci Eng C. 2018;90:180–8. 10.1016/j.msec.2018.04.055. [DOI] [PubMed] [Google Scholar]
- 176.Hong X, Wu Z, Chen L, Wu F, Wei L, Yuan W. Hydrogel microneedle arrays for transdermal drug delivery. Nano-Micro Lett. 2014;6:191–9. 10.1007/BF03353783. [Google Scholar]
- 177.Wang J, Ye Y, Yu J, Kahkoska AR, Zhang X, Wang C, Sun W, Corder RD, Chen Z, Khan SA. Core–shell microneedle gel for self-regulated insulin delivery. ACS Nano. 2018;12:2466–73. 10.1021/acsnano.7b08152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Donnelly RF, Singh TRR, Garland MJ, Migalska K, Majithiya R, McCrudden CM, Kole PL, Mahmood TMT, McCarthy HO, Woolfson AD. Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery. Adv Funct Mater. 2012;22:4879–90. 10.1002/adfm.201200864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang Y, Wang J, Yu J, Wen D, Kahkoska AR, Lu Y, Zhang X, Buse JB, Gu Z. Bioresponsive microneedles with a sheath structure for H2O2 and pH cascade-triggered insulin delivery. Small. 2018;14:1704181. 10.1002/smll.201704181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yu W, Jiang G, Liu D, Li L, Chen H, Liu Y, Huang Q, Tong Z, Yao J, Kong X. Fabrication of biodegradable composite microneedles based on calcium sulfate and gelatin for transdermal delivery of insulin. Mater Sci Eng C. 2017;71:725–34. 10.1016/j.msec.2016.10.063. [DOI] [PubMed] [Google Scholar]
- 181.Chen Q, Xiao Z, Wang C, Chen G, Zhang Y, Zhang X, Han X, Wang J, Ye X, Prausnitz MR. Microneedle patches loaded with Nanovesicles for glucose transporter-mediated insulin delivery. ACS Nano. 2022;16:18223–31. 10.1021/acsnano.2c05687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Donnelly RF, Majithiya R, Singh TRR, Morrow DI, Garland MJ, Demir YK, Migalska K, Ryan E, Gillen D, Scott CJ. Design, optimization and characterisation of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique. Pharm Res. 2011;28:41–57. 10.1007/s11095-010-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liu S, Jin M-n, Quan Y-s, Kamiyama F, Kusamori K, Katsumi H, Sakane T, Yamamoto A. Transdermal delivery of relatively high molecular weight drugs using novel self-dissolving microneedle arrays fabricated from hyaluronic acid and their characteristics and safety after application to the skin. Eur J Pharm Biopharm. 2014;86:267–76. 10.1016/j.ejpb.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 184.Ito Y, Hirono M, Fukushima K, Sugioka N, Takada K. Two-layered dissolving microneedles formulated with intermediate-acting insulin. Int J Pharm. 2012;436:387–93. 10.1016/j.ijpharm.2012.06.047. [DOI] [PubMed] [Google Scholar]
- 185.Chen BZ, Zhang LQ, Xia YY, Zhang XP, Guo XD. A basal-bolus insulin regimen integrated microneedle patch for intraday postprandial glucose control. Sci Adv. 2020;6:eaba7260. 10.1126/sciadv.aba7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jiang G, Xu B, Zhu J, Zhang Y, Liu T, Song G. Polymer microneedles integrated with glucose-responsive mesoporous bioactive glass nanoparticles for transdermal delivery of insulin. Biomed Phys Eng Express. 2019;5:045038. 10.1088/2057-1976/ab3202. [Google Scholar]
- 187.Tong Z, Zhou J, Zhong J, Tang Q, Lei Z, Luo H, Ma P, Liu X. Glucose-and H2O2-responsive polymeric vesicles integrated with microneedle patches for glucose-sensitive transcutaneous delivery of insulin in diabetic rats. ACS Appl Mater Interfaces. 2018;10:20014–24. 10.1021/acsami.8b04484. [DOI] [PubMed] [Google Scholar]
- 188.Xu B, Cao Q, Zhang Y, Yu W, Zhu J, Liu D, Jiang G. Microneedles integrated with ZnO quantum-dot-capped mesoporous bioactive glasses for glucose-mediated insulin delivery. ACS Biomater Sci Eng. 2018;4:2473–83. 10.1021/acsbiomaterials.8b00626. [DOI] [PubMed] [Google Scholar]
- 189.Xu B, Jiang G, Yu W, Liu D, Zhang Y, Zhou J, Sun S, Liu Y. H2O2-responsive mesoporous silica nanoparticles integrated with microneedle patches for the glucose-monitored transdermal delivery of insulin. J Mater Chem B. 2017;5:8200–8. 10.1039/C7TB02082A. [DOI] [PubMed] [Google Scholar]
- 190.Zhu M, Liu Y, Jiang F, Cao J, Kundu SC, Lu S. Combined silk fibroin microneedles for insulin delivery. ACS Biomater Sci Eng. 2020;6:3422–9. 10.1021/acsbiomaterials.0c00273. [DOI] [PubMed] [Google Scholar]
- 191.Zhao J, Xu G, Yao X, Zhou H, Lyu B, Pei S, Wen P. Microneedle-based insulin transdermal delivery system: current status and translation challenges. Drug Deliv Transl Res. 2022:1–25. 10.1007/s13346-021-01077-3. [DOI] [PMC free article] [PubMed]
- 192.Chatterjee K. Analytical and Experimental Investigation of Insect Respiratory System inspired Microfluidics. Virginia Tech; 2018.
- 193.Chatterjee K, Graybill PM, Socha JJ, Davalos RV, Staples AE. Frequency-specific, valveless flow control in insect-mimetic microfluidic devices. Bioinspir Biomim. 2021;16:036004. 10.1088/1748-3190/abe4bc. [DOI] [PubMed] [Google Scholar]
- 194.Haga M, Akatani M, Kikuchi J, Ueno Y, Hayashi M. Transdermal iontophoretic delivery of insulin using a photoetched microdevice. J Control Release. 1997;43:139–49. 10.1016/S0168-3659(96)01476-9. [Google Scholar]
- 195.Xiao Y, Tang Z, Wang J, Liu C, Kong N, Farokhzad OC, Tao W. Oral insulin delivery platforms: strategies to address the biological barriers. Angew Chem Int Ed. 2020;59:19787–95. 10.1002/anie.202008879. [DOI] [PubMed] [Google Scholar]
- 196.Wang M, Wang C, Ren S, Pan J, Wang Y, Shen Y, Zeng Z, Cui H, Zhao X. Versatile oral insulin delivery nanosystems: from materials to nanostructures. Int J Mol Sci. 2022;23:3362. 10.3390/ijms23063362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dassau E, Renard E, Place J, Farret A, Pelletier MJ, Lee J, Huyett LM, Chakrabarty A, Doyle FJ III, Zisser HC. Intraperitoneal insulin delivery provides superior glycaemic regulation to subcutaneous insulin delivery in model predictive control-based fully‐automated artificial pancreas in patients with type 1 diabetes: a pilot study. Diabetes Obes Metab. 2017;19:1698–705. 10.1111/dom.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dirnena-Fusini I, Åm MK, Fougner AL, Carlsen SM, Christiansen SC. Physiological effects of intraperitoneal versus subcutaneous insulin infusion in patients with diabetes mellitus type 1: a systematic review and meta-analysis. PLoS ONE. 2021;16:e0249611. 10.1371/journal.pone.0249611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Klonoff DC. Afrezza inhaled insulin: the fastest-acting FDA-approved insulin on the market has favorable properties. Los Angeles, CA: SAGE Publications Sage CA; 2014. pp. 1071–3. 10.1177/1932296814555820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mitri J, Pittas AG. Inhaled insulin—what went wrong. Nat Clin Pract Endocrinol Metab. 2009;5:24–5. 10.1038/ncpendmet1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liu T, Jiang G, Song G, Zhu J, Yang Y. Fabrication of separable microneedles with phase change coating for NIR-triggered transdermal delivery of metformin on diabetic rats. Biomed Microdevices. 2020;22:1–11. 10.1007/s10544-019-0468-8. [DOI] [PubMed] [Google Scholar]
- 202.Nasri H, Rafieian-Kopaei M. Metformin: current knowledge. J Res Med Sci. 2014;19:658. [PMC free article] [PubMed] [Google Scholar]
- 203.Cusi K, DeFronzo R. Metformin: a review of its metabolic effects. Diabetes Rev. 1998;6:89–131. [Google Scholar]
- 204.Ou H-Y, Cheng J-T, Yu E, Wu T-J. Metformin increases insulin sensitivity and plasma β-endorphin in human subjects. Horm Metab Res. 2006;38:106–11. 10.1055/s-2006-925128. [DOI] [PubMed] [Google Scholar]
- 205.Yerevanian A, Soukas AA. Metformin: mechanisms in human obesity and weight loss. Curr Obes Rep. 2019;8:156–64. 10.1007/s13679-019-00335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hébert M, Huissoon J, Ren CL. A perspective of active microfluidic platforms as an enabling tool for applications in other fields. J Micromech Microeng. 2022;32:043001. 10.1088/1361-6439/ac545f. [Google Scholar]
- 207.Avcil M, Çelik A. Microneedles in drug delivery: progress and challenges. Micromachines. 2021;12:1321. 10.3390/mi12111321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kamecke U, Waldenmaier D, Haug C, Ziegler R, Freckmann G. Establishing methods to determine clinically relevant bolus and basal rate delivery accuracy of insulin pumps. J Diabetes Sci Technol. 2019;13:60–7. 10.1177/1932296818788349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Freckmann G, Kamecke U, Waldenmaier D, Haug C, Ziegler R. Accuracy of bolus and basal rate delivery of different insulin pump systems. Diabetes Technol Ther. 2019;21:201–8. 10.1089/dia.2018.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Prabhakaran S, Thirumal D, Gimbun J, Ranganathan B. Metformin-A Panacea Pharmaceutical Agent through convergence revolution initiative. J Nat Remedies. 2017;69–79. 10.18311/jnr/2017/17938.
- 211.Dahlén AD, Dashi G, Maslov I, Attwood MM, Jonsson J, Trukhan V, Schiöth HB. Trends in antidiabetic drug discovery: FDA approved drugs, new drugs in clinical trials and global sales. Front Pharmacol. 2022;12:807548. 10.3389/fphar.2021.807548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to the nature of this article as a review, no datasets have been generated in the work towards the completion of this article.