Abstract
Purpose
To investigate the immunomodulatory effects and potential mechanisms of human nasal mucosa-derived mesenchymal stem cells(hNMSCs) on mouse allergic rhinitis, and to compare them with human umbilical cord-derived mesenchymal stem cells (hUCMSCs).
Method
hNMSCs and hUCMSCs were isolated and cultured for identification from human nasal mucosa and umbilical cord tissues. A co-culture system of LPS-stimulated RAW264.7 cells/mouse peritoneal macrophages and MSCs was employed.Changes in inflammatory factors in RAW264.7 cells and the culture medium as well as the expression of NF-κB signaling pathway in RAW264.7 cells were detected. Forty-eight BALB/c mice were randomly divided into control, OVA, hNMSCs, and hUCMSCs groups. An allergic rhinitis (AR) model was established through ovalbumin (OVA) stimulation and treated with hNMSCs and hUCMSCs. Subsequent assessments included related symptoms, biological changes, and the expression of the NF-κB signaling pathway in the nasal mucosa of mice.
Results
MSCs can be successfully isolated from human nasal mucosa. Both hNMSCs and hUCMSCs interventions significantly reverseed the inflammation induced by LPS and suppressed the upregulation of the NF-κB signaling pathway in RAW264.7 cells. Treatment with hNMSCs and hUCMSCs alleviated mouse allergic symptoms, reduced levels of total IgE, OVA-specific IgE and IgG1 in mouse serum, TH2-type cytokines and chemokines in mouse nasal mucosa, and TH2-type cytokines in mouse spleen culture medium, while also inhibiting the expression of the NF-κB signaling pathway in the nasal mucosa of mice. moreover, the hNMSCs group showed a more significant reduction in OVA-specific IgG1 in serum and IL-4 expression levels in mouse spleen culture medium compared to the hUCMSCs group.
Conclusion
Our findings suggest that hNMSCs can ameliorate allergic rhinitis in mice, with a certain advantage in anti-inflammatory effects compared to hUCMSCs. The NF-κB pathway is likely involved in the anti-inflammatory regulation process by hNMSCs.Therefore, hNMSCs might represent a novel therapeutic approach for allergic rhinitis.
Keywords: Allergic rhinitis, Mesenchymal stem cells, Anti-inflammation, NF-κB pathway
Introduction
Allergic rhinitis is an inflammatory nasal mucosa disease mediated by immunoglobulin E (IgE) following exposure to allergens [1]. The immunological basis of AR lies in the imbalance of immune equilibrium between Th1 and Th2 cells, with the disease primarily driven by a dominant Th2 cell immune response [2]. This involves the release of various inflammatory mediators and cytokines (such as IL-4, IL-5, IL-13), among which IL-4 is a characteristic factor mainly causing Type I hypersensitivity reactions [3]. Consequently, this leads to various clinical symptoms such as sporadic sneezing, runny nose, itching in the nose, and nasal congestion, sometimes along with symptoms in the eyes [4, 5].Allergic rhinitis is among the most prevalent chronic conditions in high-income countries, whereas it is less common in low and middle-income nations, although the prevalence there is gradually increasing. Currently, the prevalence of AR in children is estimated to be between 5% and 15%, and between 10% and 40% in adults [6].The main current treatments for AR include education, allergen avoidance, pharmacotherapy, and allergen Immunotherapy (AIT) [7]. However, pharmacotherapy can only control symptoms and is unable to reverse the state of immune imbalance in patients [8].While Allergen Immunotherapy shows significant effects in desensitizing patients and preventing disease progression, factors such as lengthy treatment periods, low patient adherence, and the absence of long-term efficacy studies on large samples restrict its extensive clinical use.Furthermore, Allergen Immunotherapy is specific to certain allergens rather than being universally applicable, hence it has certain limitations in its application [9] .Due to these reasons, there is an urgent need to investigate more effective therapeutic strategies for AR.
Mesenchymal stem cells (MSCs) are multipotent stem cells derived from the mesodermal layer, They can be easily isolated from various sources and possess self-renewal and multidirectional differentiation potentials [9]. MSCs have recently gained significant attention in allergic rhinitis (AR) research. Studies show that MSCs derived from adipose tissue, bone marrow, umbilical cord, and tonsils (regardless of allogeneic or xenogeneic human sources) significantly alleviate allergy symptoms and inflammation markers in AR mouse models, This includes a decrease in nasal mucosa eosinophil infiltration, Th2 cytokines (IL-4, IL-5, IL-13), and ovalbumin-specific IgE secretion.Moreover, they do not cause cross-species graft-versus-host disease [10].The immunomodulatory function of mesenchymal stem cells is likely mediated by the release of soluble factors and direct cell-to-cell contact [11].However, the effectiveness of hNMSCs sourced from treatment-targeted tissues in AR has not been documented. Therefore, we investigated the anti-inflammatory potential and underlying mechanisms of hNMSCs using an LPS-induced macrophage inflammatory model and an OVA-induced mouse allergic rhinitis model, and compared them with hUCMSCs.
NF-κB is a central mediator of the inflammatory response [12].Normally, NF-κB is restrained by IκBα and kept in a resting state; however, when the inflammatory receptor system is activated, IκBα degrades, leading to NF-κB activation [13].It has been shown that the NF-κB pathway is activated in ovalbumin (OVA)-induced AR models, and that inhibiting NF-κB can significantly alleviate OVA-induced allergic rhinitis [14, 15].Previous research has seldom investigated the impact of mesenchymal stem cells on the NF-κB inflammation-related signaling pathway. Consequently, our study focuses on assessing the effects of hNMSCs and hUCMSCs on this pathway in both LPS-induced macrophage inflammation and OVA-induced mouse allergic rhinitis models.
Materials and methods
Experimental animals and cells
The BALB/c mice(aged 8–12 weeks, weight 15–20 g, male/female = 1:1) used in this experiment were provided by the Animal Center of Shandong University and were housed in the animal facility of the Otolaryngology Research Institute at Shandong Provincial ENT Hospital. The breeding environment was maintained at a temperature of 22.5 ± 2.5℃ and a humidity of 50 ± 10%.All animal experiments were conducted in accordance with guidelines related to animal experimentation.The nasal mucosa was obtained from the rhinology Department of Shandong Provincial Eye and ENT Hospital, collected during surgeries from patients with maxillary sinus cysts, deviated nasal septum with bubble-like middle turbinates, and middle turbinates excised during skull base surgeries, specifically from patients free of sinusitis, allergic rhinitis, or other inflammatory conditions. Umbilical cords were sourced from the Obstetrics Department of the Shandong Provincial Eye and ENT Hospital.These were collected from healthy full-term infants under aseptic conditions. The mothers had no history of infectious or genetic diseases, and the infants had no congenital disorders. This study was approved by the Hospital Ethics Committee, with the ethical approval number XYK20211206.Adequate quantities of mesenchymal stem cells were obtained and cultured from the collected human nasal mucosa and umbilical cords, and were preserved in liquid nitrogen for future use.We confirm that informed consent has been obtained from all participants and/or their legal guardians.
Isolation and culture of hNMSCs and hUCMSCs
Nasal mucosa and umbilical cords of healthy full-term infants were collected under aseptic conditions in the hospital’s operating room. The collected tissues were repeatedly rinsed with PBS containing antibiotics to remove residual blood.For nasal mucosa tissues, PBS was discarded and Dispase II(Sigma-Aldrich, Zwijndrecht, The Netherlands) was added. The tissue was gently minced using ophthalmic scissors, gently inverted to mix with the Dispase II solution, and then left in a 4 °C refrigerator overnight for digestion. The next day, the original liquid was discarded, and the tissue pieces were repeatedly washed with DMEM culture medium to terminate digestion. The selected tissue chunks were placed in T25 culture flasks, spaced appropriately, and cultured in 3 ml of medium for 3–5 days. After 3 days, the medium was replaced with mesenchymal stem cell culture medium (STEMCELL Technologies, Vancouver, BC, Canada ) until sufficient cells had proliferated for subculturing.For umbilical cord tissues, the blood remaining in the umbilical veins and arteries was squeezed out, and the cords were cut into approximately 3–4 cm segments. The surface membranes, arteries, and veins were removed using ophthalmic scissors and tweezers. The remaining Wharton’s jelly was transferred into 1.5 ml EP tubes, gently minced with ophthalmic scissors, and the tissue chunks were then placed in T75 culture flasks.They were spaced appropriately and cultured in 10 ml of DMEM/F−12 culture medium (GIBCO, CA, USA) containing 10% FBS (GIBCO, CA, US) and penicillin-streptomycin (GIBCO, CA, USA) for 3–5 days. After 5 days, the medium was changed until sufficient cells proliferated for subculturing.Subsequent experiments utilized cells from the fourth passage.
Immunophenotyping analysis
Select P4 generation hNMSCs and hUCMSCs with a confluence over 80%.Add 5 µl of FITC-labeled CD90, HLA-DR; APC-labeled CD73, CD105; and PE-labeled CD34, CD45 to each tube(antibodies were purchased from BioGems, USA, ).Establish negative and isotype controls, and incubate in darkness for 15 min.Analyze the positive expression rate of surface antigens using flow cytometry.
Multilineage differentiation of hNMSCs and hUCMSCs
Mesenchymal stem cells from P3 were used for all differentiation processes.For adipogenic differentiation, cells were cultured in complete adipogenesis medium (STEMCELL Technologies, Seattle, WA, USA), with medium changed every 3 days.After 18–21 days of differentiation, cells were fixed and stained with Oil Red O solutionn (beyotime, Shanghai, China).
For osteogenic induction, cells were cultivated in complete osteogenic differentiation culture medium (STEMCELL Technologies, Seattle, WA, USA).The medium was changed every 3–4 days until bone matrix formation (approximately 10–15 days).Osteogenic differentiation was observed through Alizarin Red staining(Cyagen, Guangzhou, China).
For chondrogenic differentiation, 0.5 × 10^6 MSCs were centrifuged at 300 g for 5–10 min to form a cell pellet.The pellet was cultured in 15 mL polypropylene tubes with complete MesenCult™-ACF Chondrogenic Differentiation Medium STEMCELL Technologies, Seattle, WA, USA), with the medium being replaced every 3 days.On day 21, when chondrocyte formation reached complete differentiation, the pellet was fixed in 4% paraformaldehyde for 30 min. Subsequently, 4 μm sections were prepared for Alcian Blue-Nuclear Fast Red staining (beyotime, Shanghai, China).
Co-culture protocol
RAW264.7 cells were seeded at 8 × 10^5 cells/well in the lower chamber of a transwell permeable support (Corning Inc., Corning, NY, USA).MSCs were seeded at 8 × 10^4 cells/well in the upper chamber (0.4 μm pore size membrane).The co-culture of cells used 1640 medium(GIBCO, CA, US), with the lower chamber containing 2.5 milliliters and the upper chamber containing 1.5 milliliters.After 24 h of co-cultivation, except for the control group where the medium in the lower chamber was replaced with 1640 medium, the medium in the lower chambers of the other groups was replaced with 5 µg/ml LPS (Sigma-Aldrich, St. Louis, MO, USA) containing medium. After 6 h of incubation, the culture supernatant and macrophages were collected and stored at−80 °C for further experiments.
Establishment of the mouse AR model
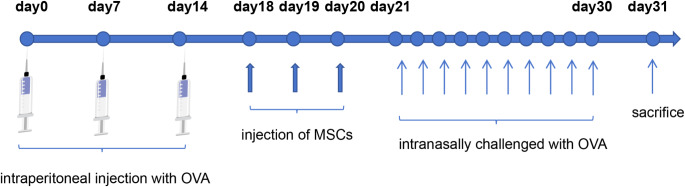
Mice were sensitized using ovalbumin (OVA) (Sigma-Aldrich, St. Louis, MO, USA ) and aluminum hydroxide (Sigma-Aldrich, St. Louis, MO, USA ). This sensitization occurred on days 0, 7, and 14 through intraperitoneal injection of 250 µl PBS containing 25 µg OVA emulsified in 2 mg aluminum hydroxide. followed by tail vein injection of mesenchymal stem cells on day 18, continued for 3 days. Starting from day 21, each mouse was administered 20 µl of 3% OVA nasally (10 µl per nostril) daily for 10 days. All mice were sacrificed 24 h following the final nasal administration, and materials were gathered for further experimentation.Forty-eight mice were randomly divided into four groups: control, OVA, hNMSCs, and hUCMSCs, with 12 mice in each group. In the control group, mice underwent sensitization, nasal administration, and treatment all using PBS. In the OVA group, mice were sensitized with OVA and aluminum hydroxide and challenged with OVA, but instead of MSC injection, PBS was injected.In the hNMSCs and hUCMSCs groups, mice were first sensitized with OVA and aluminum hydroxide. They were then administered 1*106 hNMSCs or hUCMSCs suspended in 200 µL PBS via tail vein injection, followed by an OVA challenge.(Fig. 1).
Fig. 1.
Allergic Rhinitis Model in Mice.Mice were sensitized on days 0, 7, and 14 with intraperitoneal injections of OVA and aluminum hydroxide.from days 18 to 20, The hNMSCs and hUCMSCs groups received tail vein injections of 1*106 mesenchymal stem cells suspended in 200 µL of PBS, while the control and OVA groups received tail vein injections of 200 µL of PBS.From the 21st to the 30th day, each mouse received a nasal drip of 3% OVA daily at a consistent time.All mice were euthanized 24 h after the last nasal administration
Documentation of nasal allergic symptoms and tissue preparation
Following the final OVA challenge on day 30, the frequency of sneezing and nose rubbing in mice within a 15-minute period was recorded by two blinded observers.
Nasal mucosa histological analysis
The anterior portion of the mouse head was fixed in 4% paraformaldehyde and subsequently decalcified in 0.5 M EDTA (Solarbio, Beijing, China) for a duration of 10 days. The samples were then embedded in paraffin, cut into 4 μm cross sections, and stained with hematoxylin and eosin (H&E) for histopathological evaluation.To assess the extent of allergic inflammation, the number of eosinophils in the submucosa of the nasal mucosa was counted by two blinded observers.Results were expressed as the number of eosinophils around the basal layer, counted under an optical microscope (magnification ×400).
Measurement of serum total IgE, OVA-specific IgE, and IgG1
Blood samples were obtained by the orbital blood collection method 24 h after the last OVA challenge.The levels of total IgE (neobioscience, Shenzhen, China7), OVA-specific IgE (Chondrex, Redmond, WA, USA), and IgG1 (Chondrex, Redmond, WA, USA) in mouse serum were strictly measured according to the ELISA kit instructions.
Expression of cytokines in the spleen and inflammatory factors in macrophage culture medium
24 h after the last OVA attack, the mouse spleen was removed, thoroughly ground, and lysed in red blood cell lysis solution (eBioscience, San Diego, CA, USA). The resulting cells were then cultured in a 1640 medium containing 1% penicillin-streptomycin, 10% FBS, and 100ng/ml OVA at a final concentration of 2 × 105/ml in a 96-well plate.After 48 h of culture, the cell culture medium was collected, and the concentrations of IL−4 (R&D Systems Inc., Minneapolis, MN, USA), IL−5 ((R&D Systems Inc., Minneapolis, MN, USA), IL−13 ((R&D Systems Inc., Minneapolis, MN, USA) in the spleen cell culture medium, and IL−6 (DAKEWE Biotech ,, Shenzhen, China), TNF-α (Thermo Fisher Scientific, Waltham, MA, USA) in the macrophage culture medium were measured strictly according to the ELISA kit instructions.
Real-time quantitative PCR
Total RNA was extracted from nasal mucosa tissue and macrophages using the AG RNAex pro reagent kit (Accurate Biotechnology, Guangzhou, China). Subsequently, cDNA synthesis was performed using the PrimerScriptTM RT reagent kit (TaKaRa, Dalian, China).Real-time PCR was conducted using the Mastercycler ep realplex system.The relative expression levels were calculated using the comparative 2−ΔΔCt method (Table 1).
Table 1.
Primer sequences
| Gene | Forward Sequence (5’−3’) | Reverse Sequence (5’−3’) |
|---|---|---|
| GAPDH | TGTGTCCGTCGTGGATCTGA | TTGCTGTTGAAGTCGCAGGAG |
| IL−4 | GGTCTCAACCCCCAGCTAGT | GCCGATGATCTCTCTCAAGTGAT |
| IL−5 | GCAATGAGACGATGAGGCTTC | GCCCCTGAAAGATTTCTCCAATG |
| IL−6 | CTGCAAGAGACTTCCATCCAG | AGTGGTATAGACAGGTCTGTTGG |
| IL−13 | TGAGCAACATCACACAAGACC | GGCCTTGCGGTTACAGAGG |
| CCL11 | AGCTAGTCGGGAGAGCCTAC | AAGGAAGTGACCGTGAGCAG |
| CCL24 | TCTTGCTGCACGTCCTTTATT | GCATCCAGTTTTTGTATGTGCC |
| Cxcl1 | CCCAAACCGAAGTCATAGCCA | CTCCGTTACTTGGGGACACC |
| Cxcl2 | CCAACCACCAGGCTACAGG | GCGTCACACTCAAGCTCTG |
| TNF-α | CAGGCGGTGCCTATGTCTC | CGATCACCCCGAAGTTCAGTAG |
| iNOS | ACCAGAGGACCCAGAGACAA | CCTGGCCAGATGTTCCTCTA |
| VCAM1 | CTTTATGTCAACGTTGCCCCC | AAATGCCGGAATCGTCCCTT |
| TSLP | GGAGCCTCTTCATCCTGCAA | TCCGGGCAAATGTTTTGTCG |
Western blot analysis
Harvested cells and mouse nasal mucosa tissues were fully lysed in RIPA buffer containing protease inhibitor (Sigma-Aldrich, St. Louis, MO, USA) and phosphatase inhibitor (MCE, NJ, USA, HY-K0021).Protein concentration in the extracted samples was measured using the BCA Protein Assay Kit beyotime, Shanghai, China). Samples containing 30 µg of denatured protein were separated by 10% SDS-PAGE and then transferred to a 0.22 μm PDVF membrane. The PVDF membrane was soaked in 5% skim milk and incubated at room temperature on a shaker for 1 h and 30 min.The membrane was then incubated overnight with primary antibodies at 4℃.The primary antibodies included: NF-κB p65 (D14E12) XP® Rabbit mAb (1:1000, Cell Signaling Technology, Inc., Danvers, MA, USA ), Phospho-NF-κB p65 (Ser536) (93H1) Rabbit mAb (1:800, Cell Signaling Technology, Inc., Danvers, MA, USA ), and Mouse Anti-β actin mAb (OriGene Technologies, Inc., Beijing, China ). The membrane was then washed three times with 1×TBST, followed by the addition of appropriately diluted secondary antibodies, and incubated at room temperature for 1 h. After washing the membrane three more times, it was developed using the Omni-ECL™ Femto Light Chemiluminescence Kit (epizyme Biotechnology, Shanghai, China) and the sample images were captured and saved using a gel imaging system (Alpha Innotech, San Leandro, CA, USA). The grayscale values of the bands were then analyzed using Image-Pro software.
Statistical analysis
The data obtained from the experiment were analyzed using GraphPad Prism version 9.4 (GraphPad Software, San Diego, USA), with results presented as mean ± standard deviation (SD). Comparisons between groups were made using one-way ANOVA, and data with a P-value of less than 0.05 were considered statistically significant.
Results
Surface Antigen expression of hNMSCs and hUCMSCs
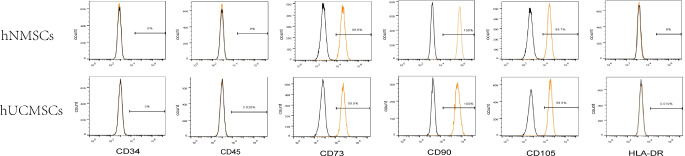
Surface antigen expression of P4 hNMSCs and hUCMSCs was analyzed using flow cytometry (Fig. 2). The results showed that the positive expression rates of surface antigens CD73, CD90, and CD105 in the fourth-generation hNMSCs were 99.6%, 100%, and 99.7% respectively, while CD34, CD45, and HLA-DR all having a 0% positive expression rate.In the fourth-generation hUCMSCs, the positive expression rates for surface antigens CD73, CD90, and CD105 were 99.5%, 100%, and 99.5% respectively, while for CD34, CD45, and HLA-DR were 0%, 0.028%, and 0.01% respectively.
Fig. 2.
Surface Antigen Expression of MSCs.Surface antigens of the fourth-generation hNMSCs and hUCMSCs were analyzed using flow cytometry. The orange peaks peaks represent the experimental antibodies. The black represent the isotype control antibodies
Induced differentiation results of hNMSCs and hUCMSCs
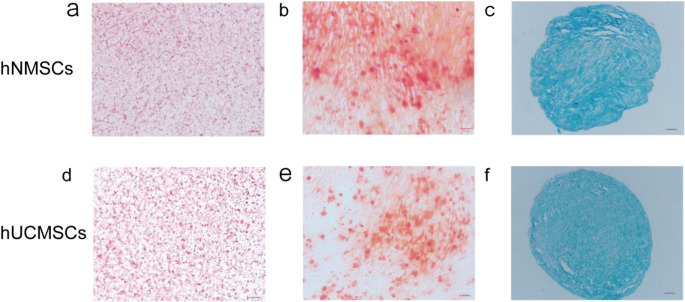
To assess the multilineage differentiation potential of hNMSCs and hUCMSCs, we stained the cells post adipogenic, osteogenic, and chondrogenic induction using specific culture media.Oil Red O staining revealed red lipid droplets within the cells (Fig. 3a, d). Alizarin Red staining dyed the calcified deposits synthesized by cells red (Fig. 3b, e). Acidic polysaccharides in cartilage tissue were distinctly blue after Alcian Blue-Hematoxylin staining (Fig. 3c, f).
Fig. 3.
Induced Differentiation Results of hNMSCs and hUCMSCs. a, d show adipogenesis induced with Oil Red O staining (× 200). d, e show osteogenesis induced with Alizarin Red staining (× 200). c, f show chondrogenesis induced with Alcian Blue-Hematoxylin staining (× 100)
MSCs exhibit anti-inflammatory effects in the LPS-stimulated RAW 264.7 cell model
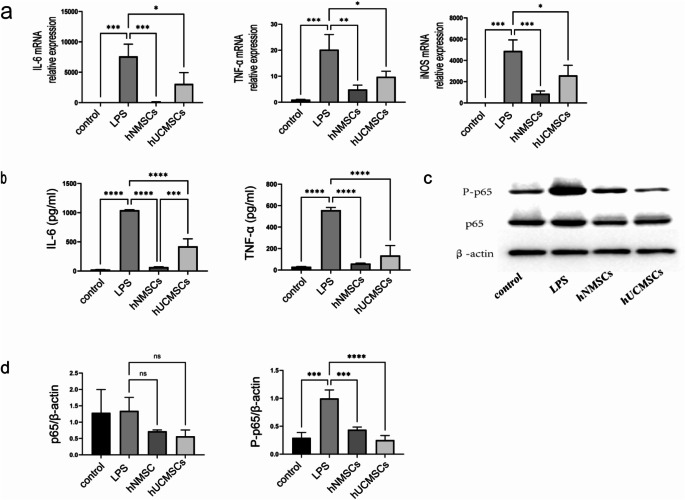
To explore the anti-inflammatory effects of MSCs, we measured the expression of IL-6, TNF-α, and iNOS in LPS-induced RAW264.7 cells.The results indicated that IL-6, TNF-α, and iNOS were significantly upregulated in macrophages under LPS stimulation (all P < 0.001), while MSCs significantly reduced the expression levels of IL-6, TNF-α, and iNOS mRNA in macrophages(Specifically, in the hNMSCs group, P < 0.001, P < 0.01, P < 0.001; in the hUCMSCs group, all were P < 0.05).And this downward trend was more pronounced in the hNMSCs group (Fig. 4a).Compared to the control group, IL-6 and TNF-α in the cell culture medium of the LPS group significantly increased (all P < 0.001), while the increase in IL-6 and TNF-α protein levels in the hNMSCs and hUCMSCs groups was effectively reversed (all P < 0.0001).Interestingly, the reduction of IL-6 was more pronounced in the hNMSCs group than in the hUCMSCs group (P < 0.001), and the downward trend of TNF-α was also more evident in the hNMSCs group (Fig. 4b).
Fig. 4.
MSCs Reduce the Expression Levels of IL-6, TNF-α, and iNOS, and the NF-κB Pathway in LPS-Induced RAW264.7 Cells.Macrophages were co-cultured with MSCs and treated with 5 µg/mL LPS for 24 h.Except for the control group, the other three experimental groups were all treated with LPS.a: The gene expressions of IL-6, TNF-α, and iNOS were measured using real-time qPCR .b: Protein levels of IL-6 and TNF-α in RAW264.7 cells were assessed using ELISA kits.c: Representative immunoblot images of protein samples.d: Expression levels of NF-κB (P-p65 and p65) in whole cell lysates were determined by protein blotting. Data shown as mean ± standard deviation (SD).*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001
MSCs inhibit the up-regulation of the NF-κB p65 pathway in LPS-stimulated RAW264.7 cells
In exploring the function of the NF-κB pathway in the MSCs response to LPS-induced macrophage inflammation, we utilized protein blotting to validate changes in protein levels in LPS-stimulated RAW264.7 cells (Fig. 4c).The results showed that LPS stimulation significantly increased the levels of phosphorylated p65 proteins in RAW264.7 cells (P < 0.001), and MSCs intervention significantly reduced the levels of phosphorylated p65 proteins.(Specifically, in the hNMSCs group, P < 0.001; in the hUCMSCs group, P < 0.0001) (Fig. 4d).
MSCs suppress symptoms and nasal mucosal inflammation in allergic mice
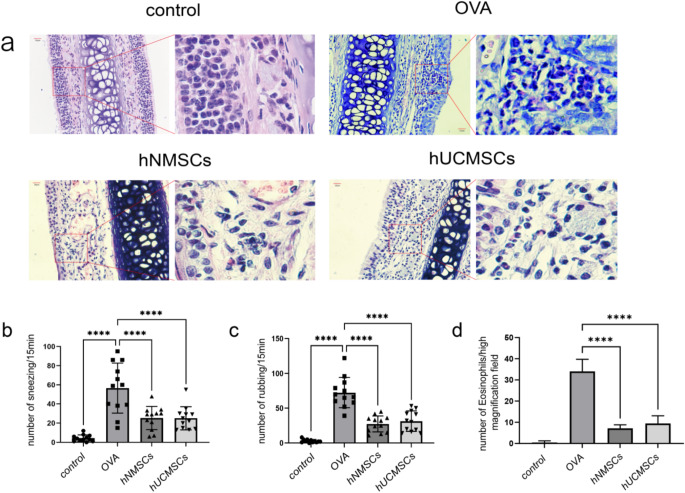
To study the therapeutic effect of hNMSCs on mouse AR and compare it with hUCMSCs, the frequency of sneezing and nose scratching was counted within 15 min after the last OVA nasal provocation.Compared to non-sensitized mice, OVA-sensitized mice exhibited allergic rhinitis symptoms, with a significant increase in sneezing and nose scratching frequency (all P < 0.0001).However, compared to the group treated with OVA, treatment with hNMSCs and hUCMSCs significantly reduced the frequency of sneezing and nose scratching (all P < 0.0001), with no statistical difference between the two groups (Fig. 5b, c).The effect of MSCs on histological changes in nasal mucosa was evaluated using H&E stainin. Notable alterations including epithelial damage, mucosal shedding, and infiltration of inflammatory cells were seen in the nasal mucosa of the AR group mice .MSC intervention reduced the damage to the nasal mucosa (Fig. 5a) and significantly decreased the infiltration of eosinophils (all P < 0.0001) (Fig. 5d). The data indicate that both hNMSCs and hUCMSCs can improve symptoms of allergic rhinitis, with no statistical difference between them.
Fig. 5.
intravenous Administration of MSCs Alleviates Nasal Mucosal Inflammation and Allergic Symptoms in Mice with Allergic Rhinitis. (a) Histological observation of mouse nasal mucosal tissue (N = 4)(magnification Χ400). (b) Frequency of sneezing(n = 12 in each group). (c) Incidence of nose rubbing(n = 12 in each group). (d) Count of eosinophils in the basal layer of mouse nasal mucosa(n = 4 in each group).*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001
MSCs suppress allergic inflammation in mice
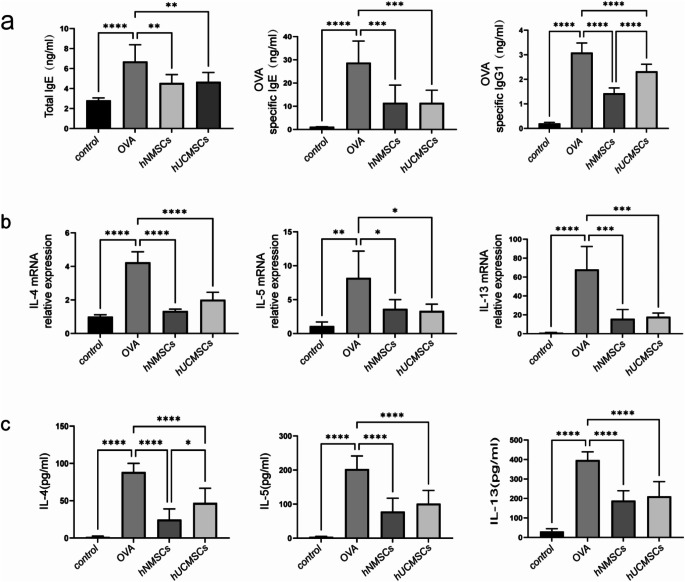
Measurement of total serum IgE, OVA-specific IgE, and IgG1 levels in mice showed that these were higher in the OVA group than in the control group (all P < 0.0001).The hNMSCs and hUCMSCs groups exhibited significantly reduced levels of total IgE, OVA-specific IgE, and IgG1 compared to the OVA group (P < 0.01, P < 0.001, and P < 0.0001 respectively).The decrease in OVA-specific IgG1 was more pronounced in the hNMSCs group compared to the hUCMSCs group(P < 0.0001), with no significant statistical difference in total serum IgE and OVA-specific IgE between the groups (Fig. 6a).
Fig. 6.
MSCs Treatment Reduces Immunoglobulins and Inflammatory Cytokines. (a) Levels of total IgE, OVA-specific IgE, and IgG1(n = 8 in each group). (b) Expression levels of cytokines IL-4, 5, 13 in nasal mucosa (n = 4 in each group). (c) Levels of cytokines IL-4, 5, 13 in spleen supernatant (n = 8 in each group). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001
To further investigate the immunomodulatory effects of hNMSCs and hUCMSCs on T cell phenotypes, we measured the mRNA expression levels of Th2 cell cytokines (IL-4, IL-5, and IL-13) in mouse nasal mucosa.In comparison with the control group, the OVA group showed significantly increased mRNA expression of IL-4, IL-5, and IL-13 (P < 0.0001; P < 0.01; P < 0.0001 respectively).After treatment with hNMSCs and hUCMSCs, the mRNA expression levels of IL-4, IL-5, and IL-13 significantly decreased (P < 0.0001; P < 0.05; P < 0.001 respecively).Although there was no statistical difference between the two MSC treatment groups, the decrease in IL-4 was more pronounced in the hNMSCs group compared to the hUCMSCs group (Fig. 6b).
In parallel with the changes in nasal mucosal cytokines, we observed a significant increase in IL-4, IL-5, and IL-13 levels in the spleen supernatant of the OVA group compared to the control group (all P < 0.0001).After hNMSCs and hUCMSCs intervention, there was a significant reduction in the levels of IL-4, IL-5, and IL-13 (all P < 0.0001), particularly IL-4, which decreased more in the hNMSCs group compared to the hUCMSCs group (P < 0.05), and no statistical differences in IL-5 and IL-13 between the groups (Fig. 6c).
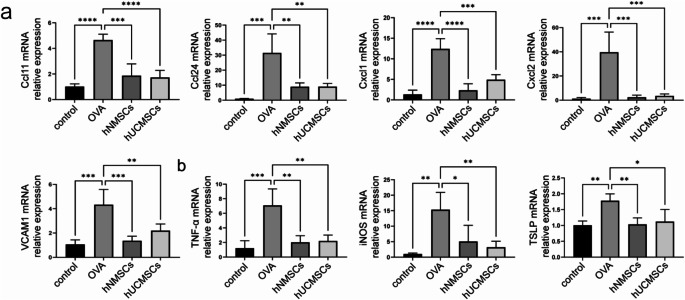
MSCs reduce levels of chemokines and pro-inflammatory factors
Further investigations were conducted on the expression levels of eosinophil chemokines(CCL11, CCL24) and neutrophil chemokines (CXCL1, CXCL2), VCAM-1, TNF-α, iNOS, and TSLP in mouse nasal mucosa.Compared to the control group, levels of CCL11, CCL24, CXCL1, CXCL2, and VCAM-1 were significantly elevated in the OVA group (P < 0.0001, P < 0.001, P < 0.0001, P < 0.001, P < 0.001) (Fig. 7a).In the hNMSCs and hUCMSCs groups, levels of CCL11, CCL24, CXCL1, CXCL2, and VCAM-1 significantly decreased(For the hNMSCs group, P < 0.001, P < 0.01, P < 0.0001, P < 0.001, P < 0.001 and for the hUCMSCs group, P < 0.0001, P < 0.01, P < 0.001, P < 0.001, P < 0.01). The expression levels of TNF-α, iNOS, and TSLP in the nasal mucosa of mice were significantly upregulated in the OVA group compared to the blank control group(P < 0.001, P < 0.01, P < 0.01 respectively).After intervention by hNMSCs and hUCMSCs, there was a significant reduction in these levels.(Specifically, in the hNMSCs group P < 0.01, P < 0.05, P < 0.01 and the hUCMSCs group P < 0.01, P < 0.01, P < 0.05),It was also found that there are no statistically significant differences in the above indices between the hNMSCs and hUCMSCs groups (Fig. 7b).
Fig. 7.
MSCs Reduce Levels of Chemokines and Pro-inflammatory Factors. (a) Expression levels of eosinophil chemokines (CCL11, CCL24), neutrophil chemokines (CXCL1, CXCL2), and VCAM-1 in nasal mucosa(n = 4 in each group). (b) Expression level of TNF-α, iNOS, TSLP in nasal mucosa(n = 4 in each group).*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001
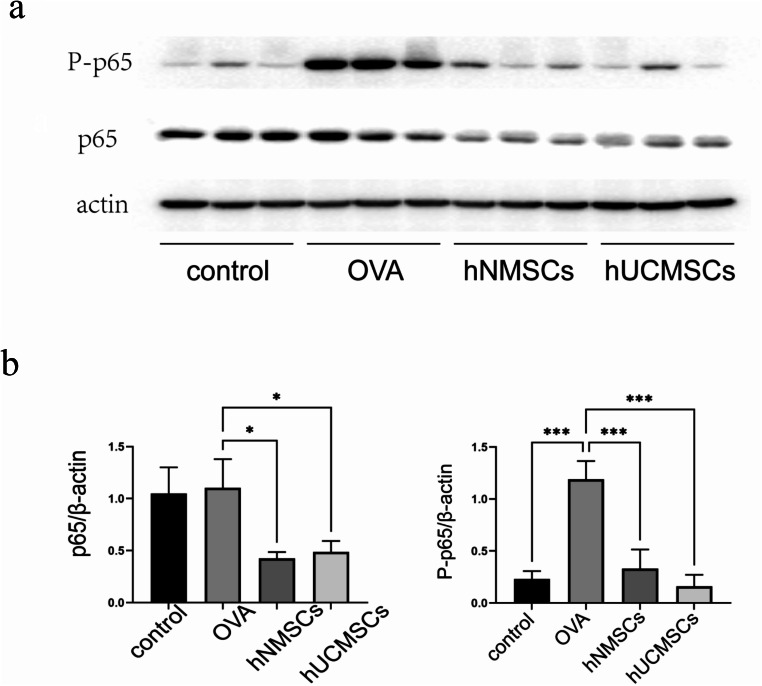
MSCs inhibit the expression of NF-κB pathway in nasal mucosa of mice with allergic rhinitis
To explore the role of the NF-κB pathway in MSCs counteracting OVA-induced allergic rhinitis in mice, we employed Western blotting to validate the changes in nasal mucosal protein levels among different groups (Fig. 8a). Consistent with cell experiments, the phosphorylated p65 protein levels in the nasal mucosa of the OVA group were significantly elevated compared to the blank group (P < 0.001). However, MSCs intervention not only significantly reduced the level of phosphorylated p65 protein (all P < 0.001) but also significantly decreased the level of p65 protein (all P < 0.05) in mouse nasal mucosa. No statistical differences were observed in the alterations of these pathways between the hNMSCs and hUCMSCs groups (Fig. 8b).
Fig. 8.
MSCs Inhibit the Expression of the NF-κB Pathway in Mouse Nasal Mucosa. (a) Representative immunoblot images of mouse nasal mucosa protein samples(n = 3 in each group). (b) Evaluation of NF-κB (P-p65 and p65) expression levels in protein extracts of mouse nasal mucosa via protein blotting.Data shown as mean ± standard deviation (SD)(n = 3 in each group).*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Discussion
Mesenchymal stem cells have been extracted from tissues including bone marrow (BM), placenta, fat, umbilical cord, and tonsils.There are slight variations in morphology and immune phenotypes among mesenchymal stem cells from various sources. The ideal source of MSCs may vary depending on the target disease or purpose [10]. This raises a pivotal question: Do MSCs isolated from the nasal mucosa, corresponding to the tissue of interest in respiratory diseases, offer enhanced specificity and affinity towards respiratory tissues? Consequently, could they potentially be more efficacious in treating respiratory ailments such as allergic rhinitis? Moreover, compared to MSCs derived from other sources, might they display superior capabilities in reconstructing and repairing damaged nasal mucosal tissue? Presently, this area remains underexplored. Addressing this gap, our research team has successfully isolated and cultured mesenchymal stem cells from human nasal mucosa. This advancement enables us to delve into their specific role and mechanism in the context of mouse allergic rhinitis, particularly in comparison with human umbilical cord MSCs (hUCMSCs).
Our research findings reveal that human nasal mucosa mesenchymal stem cells (hNMSCs) display characteristics consistent with other types of mesenchymal stem cells. These include a fibroblast-like morphology and the ability to adhere to plastic surfaces. In terms of surface antigen expression, hNMSCs show high positive rates with 99.6% for CD73, 100% for CD90, and 99.7% for CD105, while they exhibit 0% expression for hematopoietic markers CD34, CD45, and HLA-DR. Furthermore, under standard in vitro differentiation conditions, hNMSCs demonstrate the capacity to differentiate into osteoblasts, adipocytes, and chondrocytes, confirming their multipotency.
As far as we are aware, this is the inaugural study employing hNMSCs in a mouse AR model.and compares the anti-inflammatory effects of hNMSCs with hUCMSCs.Macrophages play a crucial role in the onset and progression of allergic rhinitis through various mechanisms. In the early stages of allergic rhinitis, macrophages significantly influence the production of IL-4 and class switching of immunoglobulins in B cells. Specifically, in the nasal-associated lymphoid tissue (NALT) and submandibular lymph nodes, macrophages are key cells in the production of IL-4 and IgE antibodies. [16] Additionally, they indirectly affect allergic reactions by regulating the activity of other immune cells, such as Th2 cells [17]. Therefore, in our experiments, we first used the LPS-stimulated RAW 264.7 cell model and co-cultured mesenchymal stem cells with macrophages to predict the anti-inflammatory effects of MSCs in allergic rhinitis and their potential mechanisms.MSCs can induce changes in macrophage phenotype, thereby alleviating inflammation, and can be used as a treatment strategy for sepsis [18, 19]. Consistent with these findings, our results indicate that hNMSCs and hUCMSCs significantly inhibited the upregulation of IL-6, TNF-α, and iNOS mRNA levels in LPS-stimulated macrophages, as well as the levels of IL-6 and TNF-α proteins in the culture medium.This validates the anti-inflammatory capabilities of both hNMSCs and hUCMSCs in an in vitro environment.Interestingly, the level of IL-6 protein in the culture medium was more significantly downregulated in the hNMSCs group compared to the hUCMSCs group. Although the differences in several other indicators between the two groups were not statistically significant, the downward trend was more apparent in the hNMSCs group compared to the hUCMSCs group. Overall, hNMSCs exhibited a more significant anti-inflammatory ability in an in vitro environment.
To investigate the effects of hNMSCs on mouse AR and to compare the in vivo anti-inflammatory capabilities of hNMSCs with hUCMSCs, we further developed a mouse AR model and intervened by injecting hNMSCs and hUCMSCs via the tail vein.The results showed that both hNMSCs and hUCMSCs had a significant inhibitory effect on allergic inflammation, including improving nasal symptoms in mice and reducing eosinophil infiltration in the nasal mucosa, while also significantly lowering the levels of total IgE, and OVA-specific IgE and IgG1 in serum, Concurrently, there was a decrease in the levels of TH2 cytokines IL-4, 5, 13 locally in the nasal cavity and systemically.This is consistent with other studies on AR or asthma models [20–22].
By comparing the results between the two groups, we found that hNMSCs had a stronger inhibitory effect on the secretion of IgG1 and IL-4 in AR mice than hUCMSCs.The consistency in their alterations could be attributed to IL-4 being a Th2 type cytokine, which can drive mouse IgE and IgG1 (allergen-specific IgE Ab in humans) to bind with FcγRIIB and FcγRIII on mast cells (in humans, it binds with FcεRI molecules), thus further facilitating the synthesis and release of mouse Th2 cytokines [23]. In other aspects, the two types of MSCs showed nearly identical anti-inflammatory capabilities.
Additionally, our findings indicate that hNMSCs and hUCMSCs can decrease the levels of eosinophilic cytokines CCL11, CCL24 in the mouse nasal mucosa.and the mRNA levels of vascular cell adhesion molecule-1 (VCAM-1), as well as neutrophilic cytokines CXCL-1, CXCL-2.This also explains how mesenchymal stem cell treatment reduces eosinophil infiltration in the nasal mucosa of mice .and indicates that MSCs also impact the migration of neutrophils .Moreover, we observed a significant increase in TNF-α, iNOS, TSLP in the nasal mucosa of the AR group mice relative to the control group.TNF-α is recognized as a pro-inflammatory cytokine, its essential for the generation of TH2 type cytokines like IL-4 and is also necessary for the homing of TH2 cells to sites of allergic inflammation [24]. Simultaneously, TNF-α is capable of inducing the expression of iNOS in resting cells and initiating TSLP production [25, 26]. The high expression of iNOS, producing a large amount of NO, inhibits the expression of TH1 cells and IFN-γ.thus enhancing the activation of TH2 cells and allergic inflammation [27]. TSLP successfully detects both exogenous and endogenous danger signals, and serves as an alarm by activating its downstream receptors and exacerbating allergic inflammation [28]. Interestingly, MSCs are capable of suppressing the upregulation of TNF-α, iNOS, TSLP mRNA in the nasal mucosa of mice.thereby alleviating the inflammatory response in the nasal mucosa of mice.
The mechanism of MSCs’ immunoregulatory action is complex and current studies confirm its association with cell-cell contact, extracellular vesicles (EV), and soluble factors [29]. Phospho-NFкB p65 has been shown to be overexpressed in the upper and lower airway cells of diseased mice. Natural products, such as Saikosaponin A (SSA) and Piper nigrum fruit extract, significantly alleviate AR symptoms and histopathological changes by inhibiting the activation of NF-κB p65 [30–32]. These findings propose that NF-κB p65 might play a crucial role in the pathogenesis of allergic rhinitis.Therefore, we investigated the NF-κB p65 pathway in the nasal mucosa of mice.to further analyze the anti-allergic mechanism of MSCs .In our experiments, whether it was in vitro with LPS-stimulated macrophages or in the nasal mucosa of mice stimulated with OVA, the expression of Phospho-NFкB p65 increased, indicating the activation of the NF-κB p65 signaling pathway.However, in cell experiments, the intervention of hNMSCs and hUCMSCs reduced the levels of NF-κB p65 in macrophages. Although the results were not statistically significant, the levels of Phospho-NFκB p65 were significantly reduced. In animal experiments, hNMSCs and hUCMSCs treatment significantly reduced the levels of both NF-κB p65 and Phospho-NFκB p65.suggesting that MSCs may play an anti-inflammatory role via the NF-κB signaling pathway to a certain extent.
The inhibition of NF-κB in the MSC and macrophage co-culture system is driven by several possible mechanisms. MSC-conditioned medium (MSC-CM) reduces NF-κB p65 nuclear translocation in LPS-stimulated macrophages, resulting in decreased levels of pro-inflammatory cytokines such as IL-1β and TNF-α [33]. This effect is further supported by the activation of the STAT3 pathway, which promotes M2 macrophage polarization marked by increased IL-10 and Arg-1 expression, indirectly contributing to NF-κB inhibition [34]. Additionally, soluble factors like TGF-β secreted by MSCs activate the Akt/FoxO1 pathway, which also facilitates an anti-inflammatory macrophage phenotype and further inhibits NF-κB activity [35, 36].
Intriguingly, although hNMSCs show some superiority over hUCMSCs in anti-inflammatory abilities, there is no statistically significant difference in their inhibition of the NF-κB signaling pathway. Hence, we speculate that other yet undiscovered pathways and mechanisms play a role in this process.
Conclusion
Similar to hUCMSCs, hNMSCs can ameliorate inflammation in macrophages triggered by LPS and OVA-induced allergic rhinitis in mice, NF-κB pathway might be involved in the inflammatory regulation process of hNMSCs. Compared to hUCMSCs, hNMSCs exhibit stronger anti-inflammatory effects in an in vitro environment. In vivo, hNMSCs exert a stronger inhibitory effect than hUCMSCs on the secretion of OVA-specific IgG1 and IL-4 in AR mice. while in other aspects, both showed nearly the same anti-inflammatory capabilities.Hence, hNMSCs might represent a novel therapeutic approach for allergic rhinitis.
Acknowledgements
We thank all ENT surgeons in the Department of Otolaryngology-Head and Neck Surgery, Shandong Provincial ENT Hospital, Shandong University.
Abbreviations
- hNMSCs
Human nasal mucosa-derived mesenchymal stem cells
- hUCMSCs
Human umbilical cord-derived mesenchymal stem cells
- AR
Allergic rhinitis
- OVA
Ovalbumin
Author contributions
YL conducted the laboratory experiments, data analysis, and manuscript writing. SL (Shengyang Liu) and LF contributed to the research design and data analysis.LM, JY, and TJ contributed to the animal experiments.PY and YW contributed to the collection of clinical samples.TL and YT contributed to the data collection.SL (Li Shi) and YL(Yongtian Lu) contributed to the study design and manuscript revisions.All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China(82371117);General Crosswise Tasks of Shandong University (6010420009);Shandong Provincial Traditional Chinese Medicine Project(Z-2022067);Provincial-bureau Jointly Builds Traditional Chinese Medicine Science and Technology Project(GZY-KJS-SD-2023-059).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
We provided detailed explanations of the sample collection process to all mothers who provided umbilical tissue and patients who provided nasal mucosa tissue, and all participants gave written informed consent in accordance with the Declaration of Helsinki.All procedures involving human participants and animals in this study were in accordance with the ethical standards of the institutional and/or national research committee.This study was approved by the Ethics Committee of the Shandong Provincial ENT Hospital (Approval No.: XYK20211206 and 20220241).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yongtian Lu, Email: luyongtian@263.net.
Li Shi, Email: shili126@sina.com.
References
- 1.Drazdauskaitė G, Layhadi JA, Shamji MH. Mechanisms of allergen immunotherapy in allergic rhinitis. Curr Allergy Asthma Rep. 2020;21(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen F, He D, Yan B. Apigenin attenuates allergic responses of Ovalbumin-Induced allergic rhinitis through modulation of Th1/Th2 responses in experimental mice. Dose Response. 2020;18(1):1559325820904799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura T. The roles of lipid mediators in type I hypersensitivity. J Pharmacol Sci. 2021;147(1):126–31. [DOI] [PubMed] [Google Scholar]
- 4.Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol. 2021;21(11):739–51. [DOI] [PubMed] [Google Scholar]
- 5.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bousquet J, et al. Allergic rhinitis. Nat Rev Dis Primers. 2020;6(1):95. [DOI] [PubMed] [Google Scholar]
- 7.Scadding GK, et al. BSACI guideline for the diagnosis and management of allergic and non-allergic rhinitis (revised Edition 2017; First Edition 2007). Clin Exp Allergy. 2017;47(7):p856–889. [DOI] [PubMed] [Google Scholar]
- 8.Greiner AN, et al. Allergic Rhinitis Lancet. 2011;378(9809):2112–22. [DOI] [PubMed] [Google Scholar]
- 9.Sun L, et al. Mesenchymal stem cell-based therapy for allergic Rhinitis. Stem Cells Int. 2020;2020:2367524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M, et al. Immunomodulatory properties of mesenchymal stem cells: a potential therapeutic strategy for allergic rhinitis. Allergy. 2023;78(6):1425–40. [DOI] [PubMed] [Google Scholar]
- 11.Li H, et al. Mesenchymal stem cells in allergic diseases: current status. Allergol Int. 2020;69(1):35–45. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. [DOI] [PubMed] [Google Scholar]
- 13.Cummins EP, et al. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci U S A. 2006;103(48):18154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong J, et al. Luteolin ameliorates inflammation and Th1/Th2 imbalance via regulating the TLR4/NF-κB pathway in allergic rhinitis rats. Immunopharmacol Immunotoxicol. 2021;43(3):319–27. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, et al. MiR-146a mimic attenuates murine allergic rhinitis by downregulating TLR4/TRAF6/NF-κB pathway. Immunotherapy. 2019;11(13):1095–105. [DOI] [PubMed] [Google Scholar]
- 16.Hirano M, et al. Essential role of macrophages in the initiation of allergic rhinitis in mice sensitized intranasally once with cedar pollen: regulation of class switching of immunoglobulin in B cells by controlling interleukin-4 production in T cells of submandibular lymph nodes. Microbiol Immunol. 2012;56(6):392–405. [DOI] [PubMed] [Google Scholar]
- 17.Iwasaki N, et al. Th2 cells and macrophages cooperatively induce allergic inflammation through histamine signaling. PLoS ONE. 2021;16(3):e0248158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu F, et al. MSC-secreted TGF-β regulates lipopolysaccharide-stimulated macrophage M2-like polarization via the Akt/FoxO1 pathway. Stem Cell Res Ther. 2019;10(1):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong B, et al. Exosomes from human umbilical cord mesenchymal stem cells attenuate the inflammation of severe steroid-resistant asthma by reshaping macrophage polarization. Stem Cell Res Ther. 2021;12(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samivel R, et al. Immunomodulatory effect of tonsil-derived mesenchymal stem cells in a mouse model of allergic rhinitis. Am J Rhinol Allergy. 2015;29(4):262–7. [DOI] [PubMed] [Google Scholar]
- 21.Zhao N, et al. Bone marrow-derived mesenchymal stem cells reduce immune reaction in a mouse model of allergic rhinitis. Am J Transl Res. 2016;8(12):5628–36. [PMC free article] [PubMed] [Google Scholar]
- 22.Kan XL, et al. Effect and mechanism of human umbilical cord mesenchymal stem cells in treating allergic rhinitis in mice. Sci Rep. 2020;10(1):19295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruni FM, et al. Anaphylaxis induced by Thalassophryne nattereri venom in mice is an IgE/IgG1-mediated, IL-4-dependent phenomenon. Sci Rep. 2020;10(1):584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohn L, et al. Induction of airway mucus production by T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J Exp Med. 1997;186(10):1737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuksel H, et al. Nasal mucosal expression of nitric oxide synthases in patients with allergic rhinitis and its relation to asthma. Ann Allergy Asthma Immunol. 2008;100(1):12–6. [DOI] [PubMed] [Google Scholar]
- 26.Takai T. TSLP expression: cellular sources, triggers, and regulatory mechanisms. Allergol Int. 2012;61(1):3–17. [DOI] [PubMed] [Google Scholar]
- 27.Xiong Y, et al. Inhibition of allergic airway inflammation in mice lacking nitric oxide synthase 2. J Immunol. 1999;162(1):445–52. [PubMed] [Google Scholar]
- 28.Hong H, et al. Role of IL-25, IL-33, and TSLP in triggering united airway diseases toward type 2 inflammation. Allergy. 2020;75(11):2794–804. [DOI] [PubMed] [Google Scholar]
- 29.Haddad R, Saldanha-Araujo F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: what do we know so far? Biomed Res Int. 2014;2014:216806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bui TT, et al. The protective role of Piper nigrum fruit extract in an ovalbumin-induced allergic rhinitis by targeting of NFκBp65 and STAT3 signalings. Biomed Pharmacother. 2019;109:1915–23. [DOI] [PubMed] [Google Scholar]
- 31.Chauhan PS, et al. Intranasal curcumin regulates chronic asthma in mice by modulating NF-ĸB activation and MAPK signaling. Phytomedicine. 2018;51:29–38. [DOI] [PubMed] [Google Scholar]
- 32.Piao CH, et al. Saikosaponin A ameliorates nasal inflammation by suppressing IL-6/ROR-γt/STAT3/IL-17/NF-κB pathway in OVA-induced allergic rhinitis. Chem Biol Interact. 2020;315:108874. [DOI] [PubMed] [Google Scholar]
- 33.Su VY et al. Mesenchymal stem cell-conditioned medium induces Neutrophil apoptosis Associated with inhibition of the NF-κB pathway in Endotoxin-Induced Acute Lung Injury. Int J Mol Sci, 2019. 20(9). [DOI] [PMC free article] [PubMed]
- 34.Gao S, et al. Mouse bone marrow-derived mesenchymal stem cells induce macrophage M2 polarization through the nuclear factor-κB and signal transducer and activator of transcription 3 pathways. Exp Biol Med (Maywood). 2014;239(3):366–75. [DOI] [PubMed] [Google Scholar]
- 35.Horowitz JC, et al. Activation of the pro-survival phosphatidylinositol 3-kinase/AKT pathway by transforming growth factor-beta1 in mesenchymal cells is mediated by p38 MAPK-dependent induction of an autocrine growth factor. J Biol Chem. 2004;279(2):1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin CC, et al. Transforming growth factor-beta1 stimulates heme oxygenase-1 expression via the PI3K/Akt and NF-kappaB pathways in human lung epithelial cells. Eur J Pharmacol. 2007;560(2–3):101–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.