Abstract
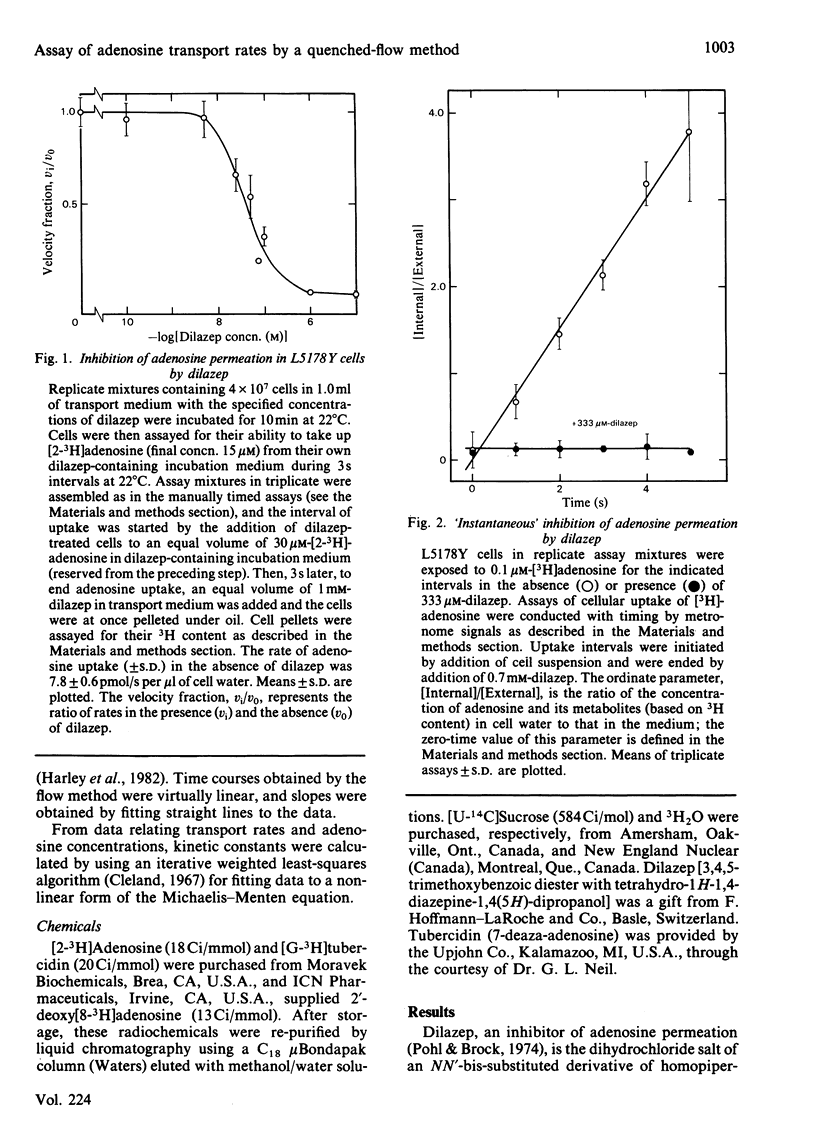
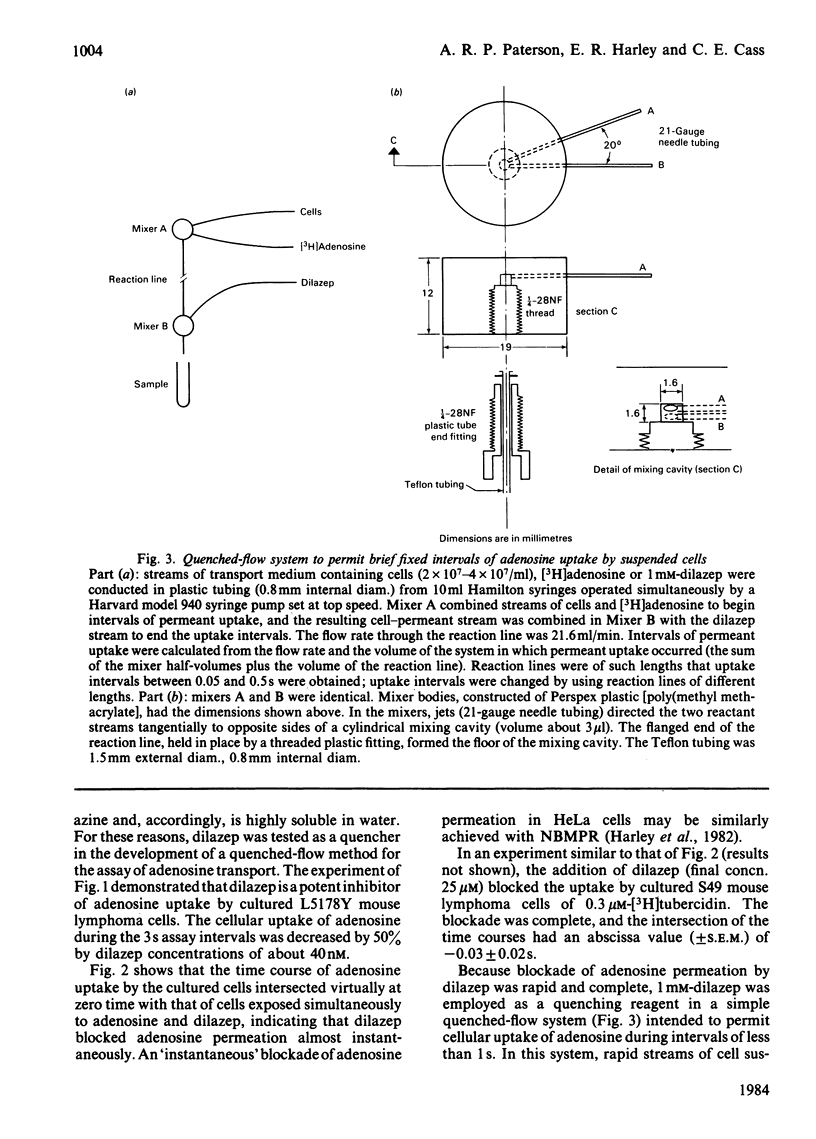
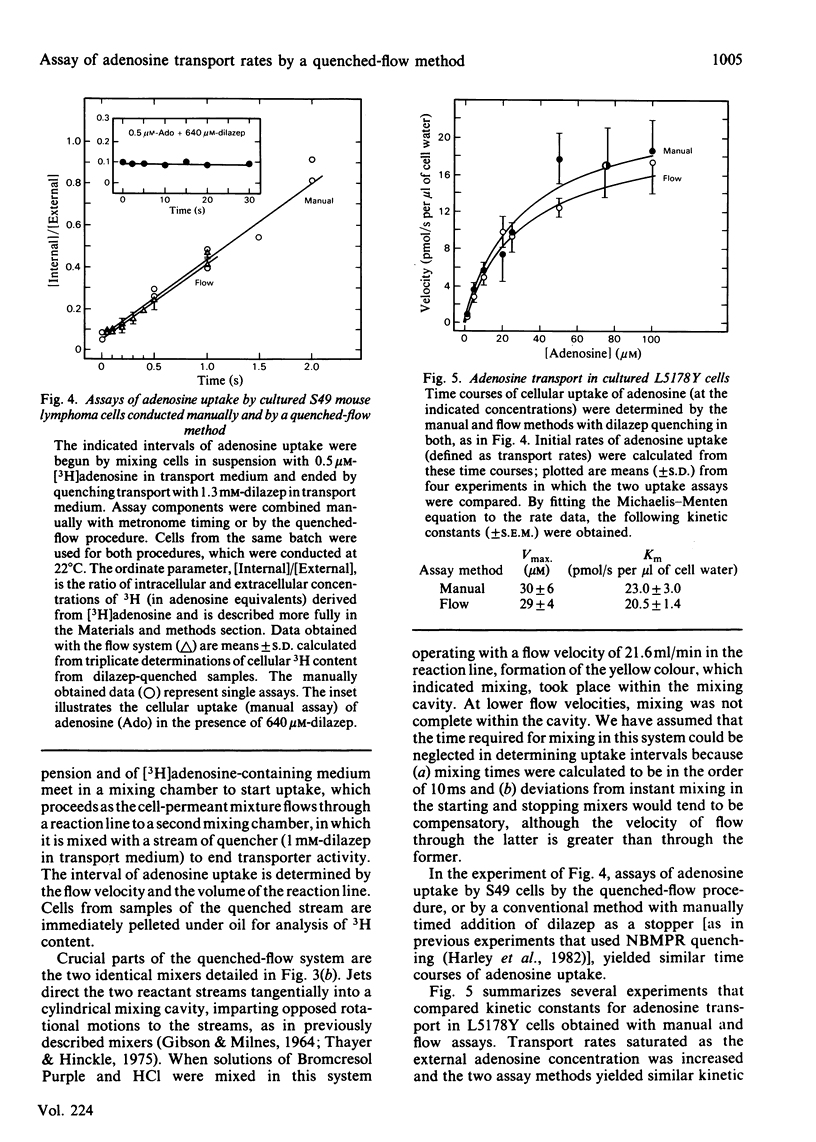
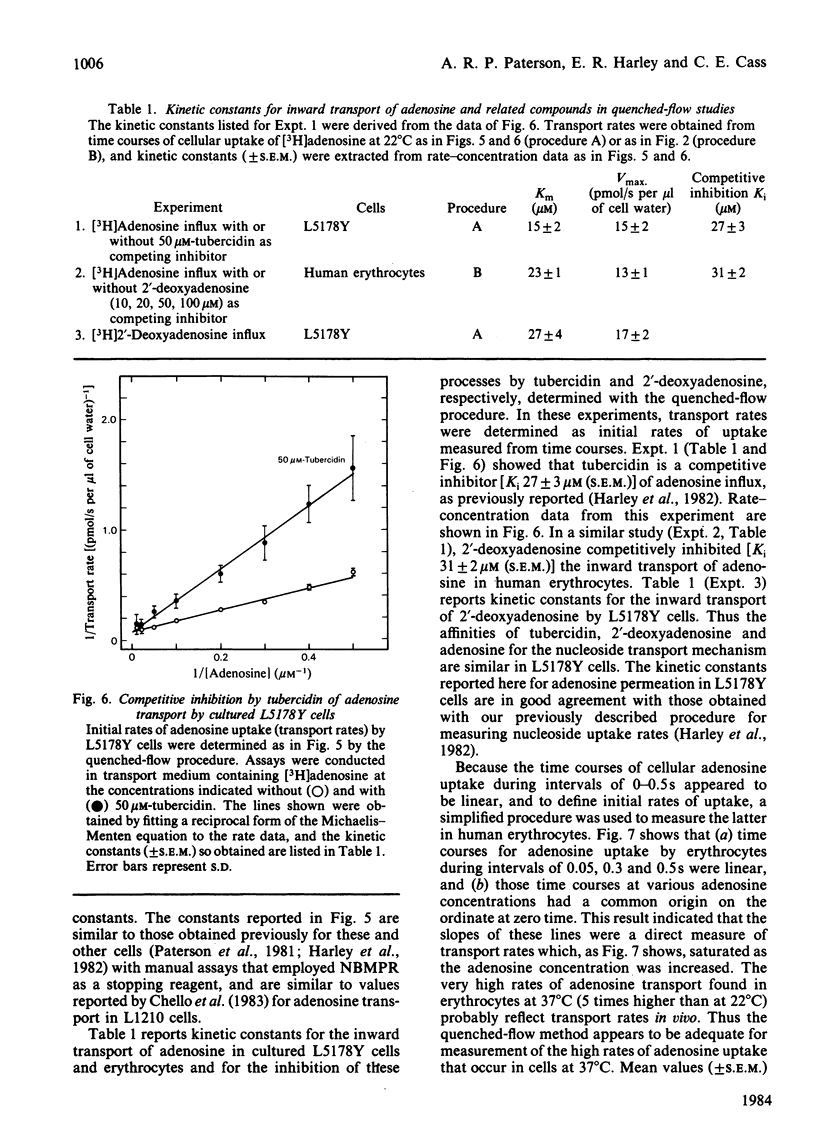
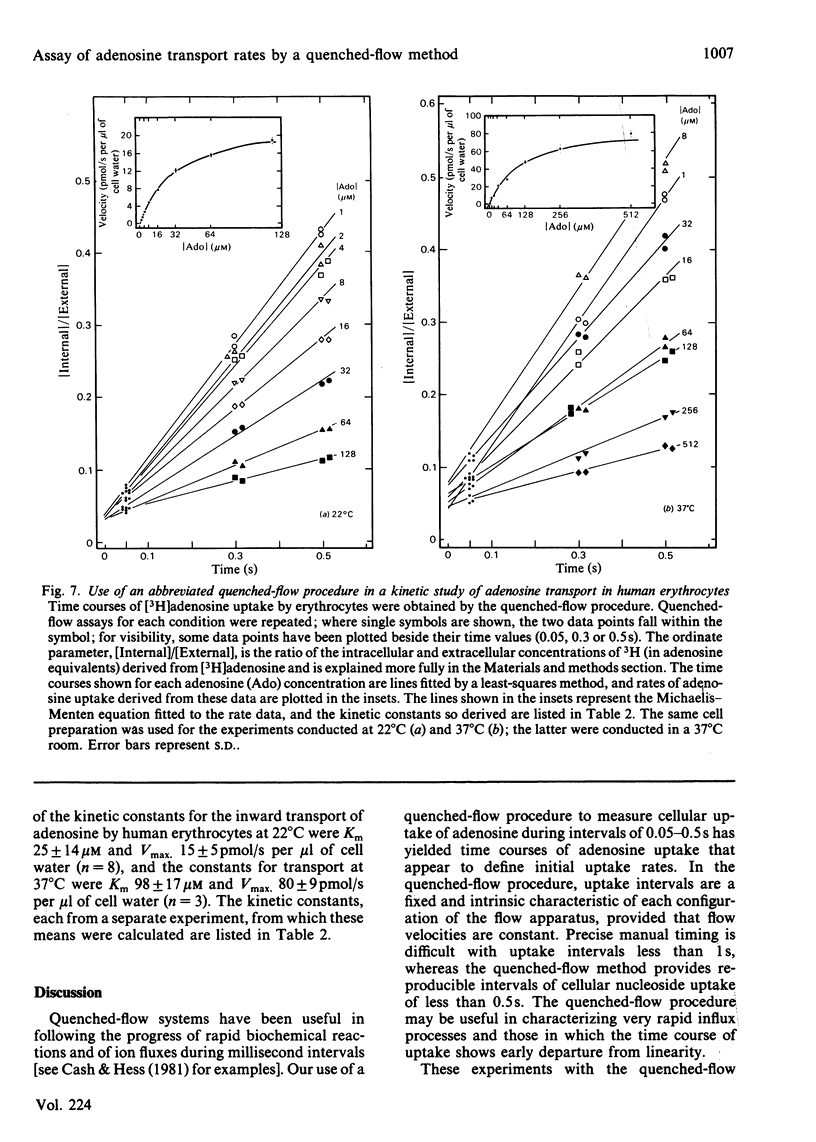
Dilazep, a vasodilator previously recognized as an inhibitor of adenosine permeation, very rapidly blocked the uptake of adenosine by cultured L5178Y cells, and accordingly was used as a quencher in a simple quenched-flow system for measuring cellular uptake of nucleosides during very short intervals. Time courses of cellular uptake of adenosine, assayed during intervals between 0.05 and 0.5s with the quenched-flow system, were linear and defined initial rates of adenosine uptake. The latter are rates of inward transport of adenosine. Kinetic constants for that process in cultured S49 cells determined with the quenched-flow procedure were similar to those determined with an assay dependent on manual timing. In studies of adenosine uptake kinetics in human erythrocytes at 22 degrees C and 37 degrees C in which the quenched-flow procedure was used, time courses of adenosine uptake were linear at both temperatures and defined initial uptake rates; kinetic constants (means +/- S.E.M.) at 22 degrees C (n = 8) were Km 25 +/- 14 microM and Vmax. 15 +/- 5 pmol/s per microliter of cell water and at 37 degrees C (n = 3) were Km 98 +/- 17 microM and Vmax. 80 +/- 9 pmol/s per microliter of cell water.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belt J. A. Heterogeneity of nucleoside transport in mammalian cells. Two types of transport activity in L1210 and other cultured neoplastic cells. Mol Pharmacol. 1983 Nov;24(3):479–484. [PubMed] [Google Scholar]
- Bowen D., Diasio R. B., Goldman I. D. Distinguishing between membrane transport and intracellular metabolism of fluorodeoxyuridine in Ehrlich ascites tumor cells by application of kinetic and high performance liquid chromatographic techniques. J Biol Chem. 1979 Jun 25;254(12):5333–5339. [PubMed] [Google Scholar]
- Cabantchik Z. I., Ginsburg H. Transport of uridine in human red blood cells. Demonstration of a simple carrier-mediated process. J Gen Physiol. 1977 Jan;69(1):75–96. doi: 10.1085/jgp.69.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash D. J., Hess G. P. Quenched flow technique with plasma membrane vesicles: acetylcholine receptor-mediated transmembrane ion flux. Anal Biochem. 1981 Mar 15;112(1):39–51. doi: 10.1016/0003-2697(81)90257-8. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Gaudette L. A., Paterson A. R. Mediated transport of nucleosides in human erythrocytes. Specific binding of the inhibitor nitrobenzylthioinosine to nucleoside transport sites in the erythrocyte membrane. Biochim Biophys Acta. 1974 Apr 12;345(1):1–10. doi: 10.1016/0005-2736(74)90239-9. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Paterson A. R. Mediated transport of nucleosides in human erythrocytes. Accelerative exchange diffusion of uridine and thymidine and specificity toward pyrimidine nucleosides as permeants. J Biol Chem. 1972 May 25;247(10):3314–3320. [PubMed] [Google Scholar]
- Chello P. L., Sirotnak F. M., Dorick D. M., Yang C. H., Montgomery J. A. Initial rate kinetics and evidence for duality of mediated transport of adenosine, related purine nucleosides, and nucleoside analogues in L1210 cells. Cancer Res. 1983 Jan;43(1):97–103. [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson Q. H., Milnes L. Apparatus for rapid and sensitive spectrophotometry. Biochem J. 1964 Apr;91(1):161–171. doi: 10.1042/bj0910161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley E. R., Paterson A. R., Cass C. E. Initial rate kinetics of the transport of adenosine and 4-amino-7-(beta-D-ribofuranosyl)pyrrolo[2,3-d]pyrimidine (tubercidin) in cultured cells. Cancer Res. 1982 Apr;42(4):1289–1295. [PubMed] [Google Scholar]
- Heichal O., Ish-Shalom D., Koren R., Stein W. D. The kinetic dissection of transport from metabolic trapping during substrate uptake by intact cells. Uridine uptake by quiescent and serum-activated Nil 8 hamster cells and their murine sarcoma virus-transformed counterparts. Biochim Biophys Acta. 1979 Feb 20;551(1):169–186. doi: 10.1016/0005-2736(79)90363-8. [DOI] [PubMed] [Google Scholar]
- Kolassa N., Stengg R., Turnheim K. Influence of hexobendine, dipyridamole, dilazep, lidoflazine, inosine and purine riboside on adenosine uptake by the isolated epithelium of guinea pig jejunum. Pharmacology. 1978;16(1):54–60. doi: 10.1159/000136747. [DOI] [PubMed] [Google Scholar]
- Lum C. T., Marz R., Plagemann P. G., Wohlhueter R. M. Adenosine transport and metabolism in mouse leukemia cells and in canine thymocytes and peripheral blood leukocytes. J Cell Physiol. 1979 Nov;101(2):173–200. doi: 10.1002/jcp.1041010202. [DOI] [PubMed] [Google Scholar]
- Pande S. V. Liquid scintillation counting of aqueous samples using triton-containing scintillants. Anal Biochem. 1976 Jul;74(1):25–34. doi: 10.1016/0003-2697(76)90306-7. [DOI] [PubMed] [Google Scholar]
- Paterson A. R., Lau E. Y., Dahlig E., Cass C. E. A common basis for inhibition of nucleoside transport by dipyridamole and nitrobenzylthioinosine? Mol Pharmacol. 1980 Jul;18(1):40–44. [PubMed] [Google Scholar]
- Plagemann P. G., Marz R., Wohlhueter R. M. Uridine transport in Novikoff rat hepatoma cells and other cell lines and its relationship to uridine phosphorylation and phosphorolysis. J Cell Physiol. 1978 Oct;97(1):49–72. doi: 10.1002/jcp.1040970107. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Wohlhueter R. M. Nucleoside transport in cultured mammalian cells. Multiple forms with different sensitivity to inhibition by nitrobenzylthioinosine or hypoxanthine. Biochim Biophys Acta. 1984 Jun 13;773(1):39–52. doi: 10.1016/0005-2736(84)90548-0. [DOI] [PubMed] [Google Scholar]
- Pohl V. P., Brock N. Vergleichende Untersuchungen zur Hemmung des Adenosinabbaus in vitro durch Dilazep. Arzneimittelforschung. 1974 Nov;24(11A):1901–1905. [PubMed] [Google Scholar]
- Strauss P. R., Sheehan J. M., Taylor J. Plasma membrane mediated thymidine transport in AKR spleen cells. Can J Biochem. 1980 Dec;58(12):1405–1413. doi: 10.1139/o80-190. [DOI] [PubMed] [Google Scholar]
- Thayer W. S., Hinkle P. C. Kinetics of adenosine triphosphate synthesis in bovine heart submitochondrial particles. J Biol Chem. 1975 Jul 25;250(14):5336–5336. [PubMed] [Google Scholar]
- Wohlhueter R. M., Marz R., Graff J. C., Plagemann P. G. A rapid-mixing technique to measure transport in suspended animal cells: applications to nucleoside transport in Novikoff rat hepatoma cells. Methods Cell Biol. 1978;20:211–236. doi: 10.1016/s0091-679x(08)62020-8. [DOI] [PubMed] [Google Scholar]
- Young J. D., Jarvis S. M. Nucleoside transport in animal cells. Biosci Rep. 1983 Apr;3(4):309–322. doi: 10.1007/BF01122895. [DOI] [PubMed] [Google Scholar]


