Abstract
An improved and scaled-up procedure has been developed for purifying the OXA-2 plasmid-mediated beta-lactamase. This has enabled us to improve the characterization of this enzyme, including a revised determination of its amino acid composition and the sequence of the N-terminal region of the protein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON E. S., DATTA N. RESISTANCE TO PENICILLINS AND ITS TRANSFER IN ENTEROBACTERIACEAE. Lancet. 1965 Feb 20;1(7382):407–409. doi: 10.1016/s0140-6736(65)90004-8. [DOI] [PubMed] [Google Scholar]
- Ambler R. P. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- Barth P. T., Grinter N. J. Comparison of the deoxyribonucleic acid molecular weights and homologies of plasmids conferring linked resistance to streptomycin and sulfonamides. J Bacteriol. 1974 Nov;120(2):618–630. doi: 10.1128/jb.120.2.618-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
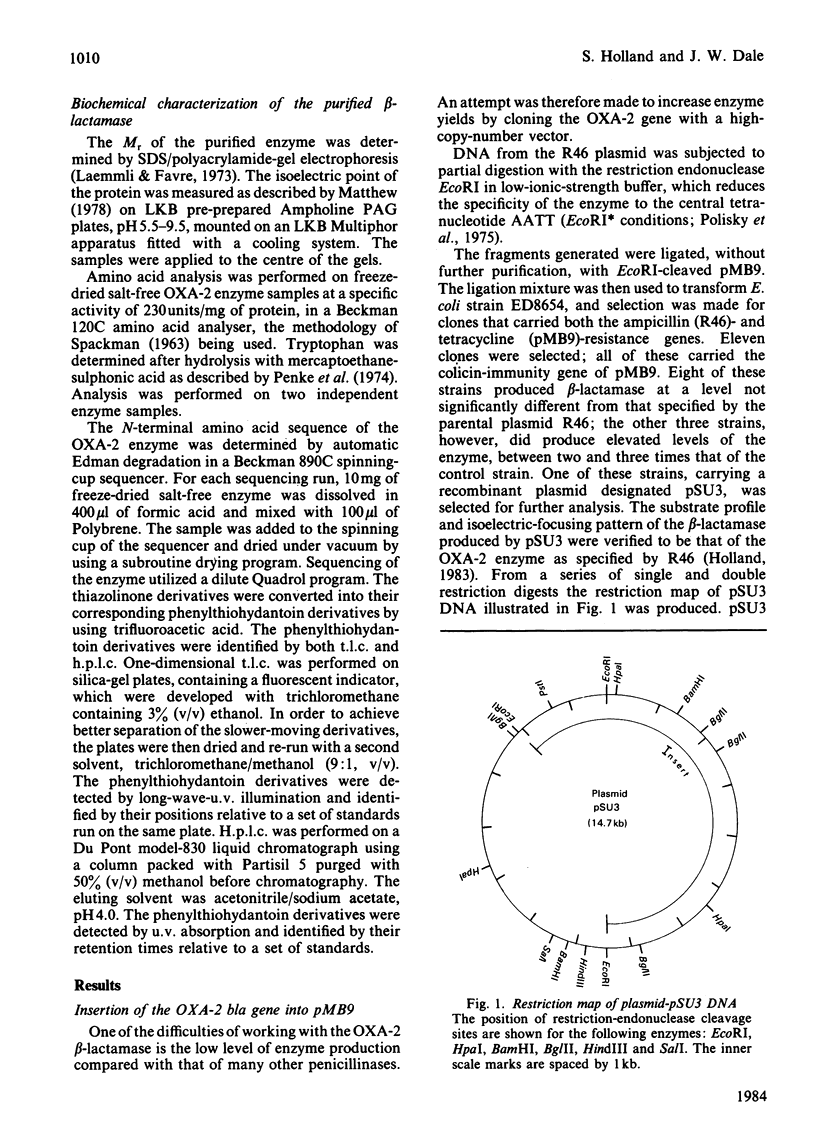
- Brown A. M., Willetts N. S. A physical and genetic map of the IncN plasmid R46. Plasmid. 1981 Mar;5(2):188–201. doi: 10.1016/0147-619x(81)90020-2. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. W. Characterization of the -lactamase specified by the resistance factor R-1818 in E. coli K12 and other Gram-negative bacteria. Biochem J. 1971 Jul;123(4):501–505. doi: 10.1042/bj1230501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. W., Smith J. T. R-factor-mediated beta-lactamases that hydrolyze oxacillin: evidence for two distinct groups. J Bacteriol. 1974 Aug;119(2):351–356. doi: 10.1128/jb.119.2.351-356.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. W., Smith J. T. The dimeric nature of an R-factor mediated beta-lactamase. Biochem Biophys Res Commun. 1976 Feb 9;68(3):1000–1005. doi: 10.1016/0006-291x(76)91245-6. [DOI] [PubMed] [Google Scholar]
- Dale J. W., Smith J. T. The purification and properties of the -lactamase specified by the resistance factor R-1818 in Escherichia coli and Proteus mirabilis. Biochem J. 1971 Jul;123(4):493–500. doi: 10.1042/bj1230493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Datta N., Kontomichalou P., Smith J. T. Molecular specificities of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1974 Jan;117(1):56–62. doi: 10.1128/jb.117.1.56-62.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- Matthew M., Hedges R. W. Analytical isoelectric focusing of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1976 Feb;125(2):713–718. doi: 10.1128/jb.125.2.713-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- Monaghan C., Holland S., Dale J. W. The interaction of anthraquinone dyes with the plasmid-mediated OXA-2 beta-lactamase. Biochem J. 1982 Aug 1;205(2):413–417. doi: 10.1042/bj2050413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K., Murray N. E. Phage lambda receptor chromosomes for DNA fragments made with restriction endonuclease III of Haemophilus influenzae and restriction endonuclease I of Escherichia coli. J Mol Biol. 1975 Nov 5;98(3):551–564. doi: 10.1016/s0022-2836(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Penke B., Ferenczi R., Kovács K. A new acid hydrolysis method for determining tryptophan in peptides and proteins. Anal Biochem. 1974 Jul;60(1):45–50. doi: 10.1016/0003-2697(74)90129-8. [DOI] [PubMed] [Google Scholar]
- Polisky B., Greene P., Garfin D. E., McCarthy B. J., Goodman H. M., Boyer H. W. Specificity of substrate recognition by the EcoRI restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3310–3314. doi: 10.1073/pnas.72.9.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuni A. A direct spectrophotometric assay and determination of Michaelis constants for the beta-lactamase reaction. Anal Biochem. 1975 Jan;63(1):17–26. doi: 10.1016/0003-2697(75)90185-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


