Abstract
The diagnosis and awareness of transthyretin amyloidosis cardiomyopathy (ATTR-CM) in heart failure with left ventricular ejection fraction (LVEF) > 40% remains under-recognized. This study aimed to investigate the prevalence and characteristics of ATTR-CM in patients with heart failure with LVEF > 40%. Patients with LVEF > 40% and maximal left ventricular wall thickness (MWT) > 10 mm who underwent bone scintigraphy were retrospectively investigated. Patients with a definite cause of heart failure were excluded. ATTR-CM was diagnosed when grade 2 or 3 myocardial uptake was observed on scintigraphy. Among 97 patients (male, 62.5%; median age, 69 years), 13 (13.4%) were diagnosed with ATTR-CM (wild type, 69.2%; hereditary type, 30.8%). Age or biomarker levels did not differ significantly; however, all patients with ATTR-CM were male. The ATTR-CM group had a significantly higher prevalence of polyneuropathy or carpal tunnel syndrome than the non-ATTR-CM group, accompanied by a longer PR interval, thicker MWT, larger left atrial volume index, and higher E/e′. Accordingly, ATTR was present in a substantial number, particularly among men. Clinicians should suspect ATTR when a male patient exhibits neurologic symptoms, diastolic dysfunction, and a long PR interval.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-74191-0.
Subject terms: Cardiology, Diagnosis
Introduction
Heart failure with preserved ejection fraction (HFpEF) and heart failure with mildly reduced ejection fraction (HFmrEF) account for > 50% of all heart failure (HF) cases, associated with high mortality and morbidity rates with a gradually increasing prevalence1,2. As HFpEF and HFmrEF exhibit multiple cardiac and extra-cardiac pathophysiology and heterogeneous and complex characteristics, standardised diagnosis and treatment approaches may be inadequate3–6. Recently, patients with HFpEF and HFmrEF have been shown to benefit from sodium–glucose cotransporter-2 inhibitors; however, identification of HFpEF and HFmrEF aetiology and targeted therapeutic strategies for its subtypes are lacking6–10. Transthyretin amyloid cardiomyopathy (ATTR-CM), in which amyloid fibrils infiltrate the myocardium, is one of the etiologies of HFpEF and HFmrEF, especially among older adults11–14. Endomyocardial biopsy (EMB) is the gold standard method for ATTR-CM diagnosis. However, in recent studies, scintigraphy revealed an almost 100% positive predictive value, allowing the non-invasive diagnosis of ATTR-CM14–16. Patients with ATTR-CM can progress to advanced heart failure and often have arrhythmias or conduction disturbances17,18. Tafamidis, a novel drug that inhibits amyloid deposition, was found to improve clinical outcomes in these patients and is known to be more effective when treatment is initiated at an early stage of ATTR-CM19. Therefore, early diagnosis and treatment of ATTR-CM in patients with HFpEF or HFmrEF is essential; however, the significance of awareness and ATTR-CM diagnosis remains poorly recognised among physicians. Recently, several studies have reported ATTR-CM prevalence and characteristics in patients with HF with left ventricular hypertrophy (LVH)20,21; however, studies among Asians are lacking. In the current study, we aimed to determine the prevalence and characteristics of ATTR-CM in Korean patients with HFpEF or HFmrEF.
Results
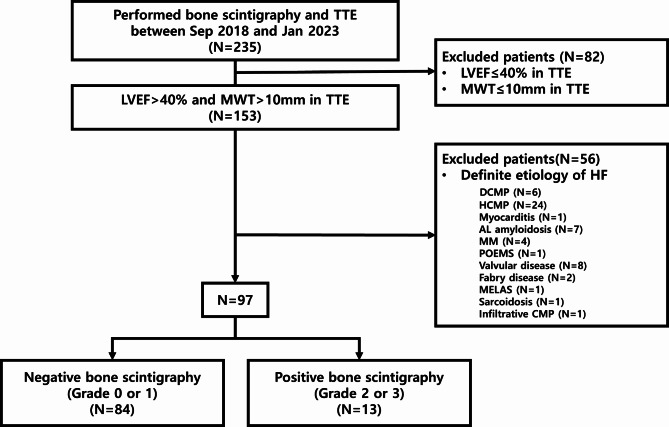
During the study period, 153 patients met the inclusion criteria (Fig. 1). Of these, 56 patients with HF attributed to a definite cause were excluded (6 DCMP, 24 HCMP, 1 myocarditis, 7 AL amyloidosis, 4 MM, etc.). Finally, 97 patients (mean age, 72 years; 60 [62.5%] male; median H2FPEF score, 3) were analyzed. Among the study participants, 13 (13.4%) were diagnosed with ATTR-CM based on grade 2 or 3 myocardial uptake on bone scintigraphy, whereas 84 (86.6%) had grade 0 or 1 myocardial uptakes on bone scintigraphy. Of the patients diagnosed with ATTR-CM, 11 (84.6%) were confirmed using EMB and genetic testing; 7 were treated with tafamidis, 3 with double-stranded small interfering RNA, and 3 were untreated. The representative case of ATTR-CM patient was shown in Fig. 2.
Fig. 1.
Flow chart of the study population. CMP cardiomyopathy, DCMP dilated cardiomyopathy, HCMP hypertrophic cardiomyopathy, HF heart failure, LVEF left ventricular ejection fraction, MELAS Mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes, MM multiple myeloma, MWT maximal LV wall thickness, POEMS polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes, TTE transthoracic echocardiography.
Fig. 2.
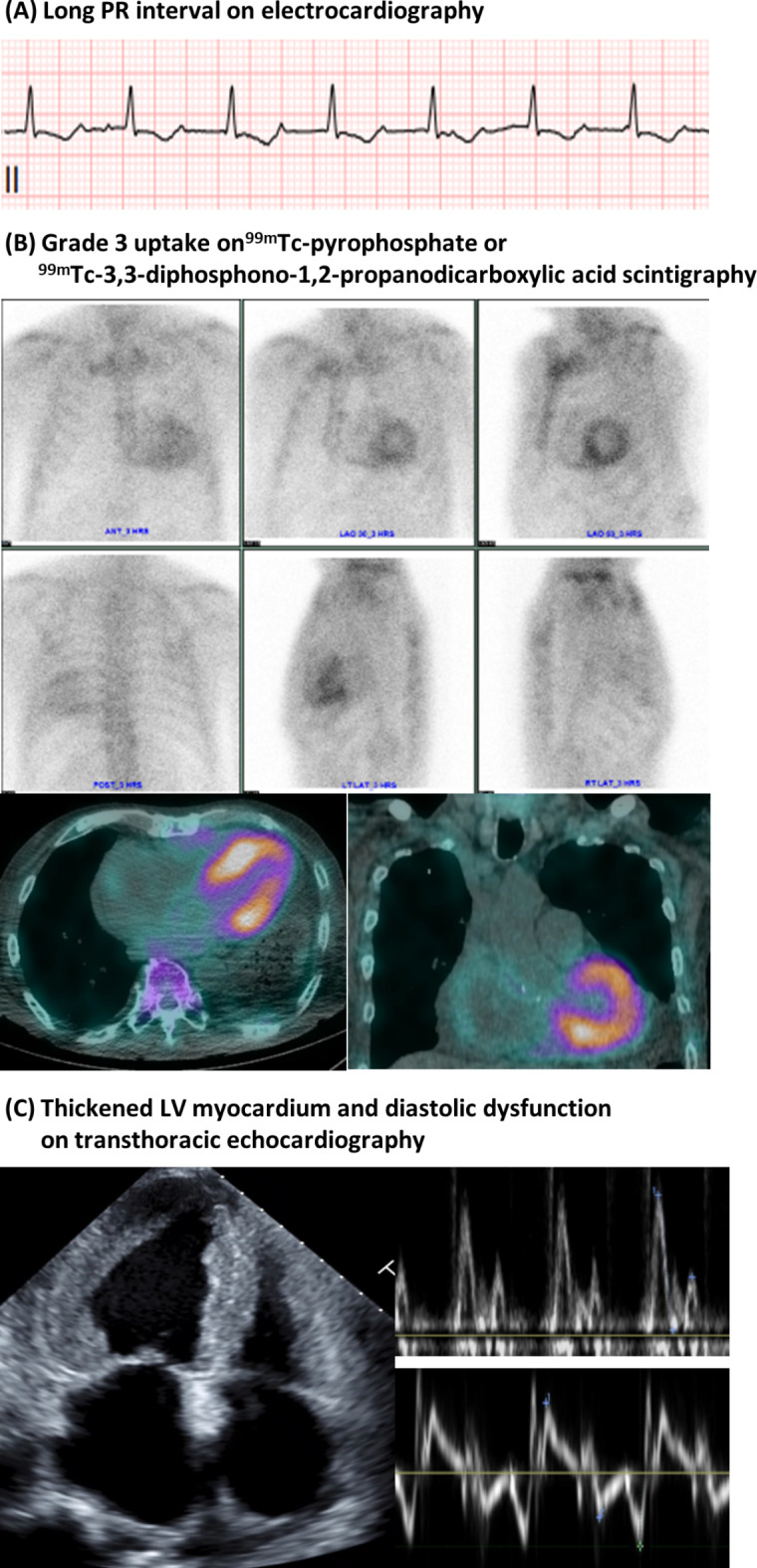
The representative case of ATTR-CM patient. A 67-year-old male patient was diagnosed with ATTR-CM. (A) PR interval was prolonged to 280 ms on electrocardiography. (B) Grade 3 uptake in the left ventricular myocardium was showed in bone scintigraphy. (C) Transthoracic echocardiography showed advanced diastolic dysfunction with left and right ventricular myocardial wall thickening.
The baseline clinical characteristics of the study population are shown in Table 1. Of the ATTR-CM patients, nine were diagnosed with wild type (69.2%), and four were diagnosed with a hereditary type (30.8%). The ATTR-CM and non-ATTR-CM groups did not differ significantly in terms of age, the prevalence of hypertension, diabetes mellitus, atrial fibrillation, coronary artery disease, H2FPEF score, and serum biomarker levels (N-terminal pro-B-type natriuretic peptide or high sensitive troponin) between. However, all patients in the ATTR-CM group were male. The prevalence of red flag signs, such as polyneuropathy (61.5% vs. 11.9%, P < 0.001) or carpal tunnel syndrome (53.8% vs. 1.2%, P < 0.001), and estimated glomerular filtration rates (71.5 ± 16.3 vs. 53.6 ± 30.3 mL/min/1.73 m2, P = 0.004) were significantly higher in the ATTR-CM group; however, the mean body mass index (22.6 ± 3.7 vs. 25.8 ± 5.2 kg/m2, P = 0.036) of the ATTR-CM group was significantly lower than that in the non-ATTR-CM group. There was no significant difference in medication history except calcium channel blocker prescription rates.
Table 1.
Baseline characteristics.
| Total (n = 97) | ATTR-CM (n = 13) | Non-ATTR-CM (n = 84) | P-value | |
|---|---|---|---|---|
| Demographics and characteristics | ||||
| Age, years | 72 (62–79) | 78 (73–82) | 69 (60–78) | 0.052 |
| Male gender, n (%) | 60 (62.5%) | 13 (100.0%) | 47 (56.0%) | 0.006 |
| Height, cm | 162.5 ± 9.6 | 163.4 ± 5.8 | 162.3 ± 10.0 | 0.581 |
| Weight, kg | 66.7 ± 15.1 | 60.1 ± 10.1 | 67.7 ± 15.4 | 0.090 |
| BMI, kg/m2 | 25.3 ± 5.2 | 22.6 ± 3.7 | 25.8 ± 5.2 | 0.036 |
| Hypertension, n(%) | 65 (67.7%) | 6 (46.2%) | 60 (71.4%) | 0.134 |
| Diabetes mellitus, n(%) | 41 (42.7%) | 2 (15.4%) | 39 (46.4%) | 0.071 |
| Atrial fibrillation, n (%) | 22 (22.9%) | 4 (30.8%) | 18 (21.4%) | 0.695 |
| Coronary artery disease, n (%) | 19 (19.8%) | 2 (15.4%) | 17 (20.2%) | 0.972 |
| H2FPEF score | 3 (2–5) | 3 (2–5) | 3 (2–5) | 0.802 |
| Red flag signs | ||||
| Polyneuropathy, n (%) | 18 (18.8%) | 8 (61.5%) | 10 (11.9%) | < 0.001 |
| Carpal tunnel syndrome, n (%) | 8 (8.3%) | 7 (53.8%) | 1 (1.2%) | < 0.001 |
| Lumbar spinal stenosis, n (%) | 16 (16.7%) | 3 (23.1%) | 13 (15.5%) | 0.775 |
| Autonomic dysfunction, n (%) | 18 (18.8%) | 8 (61.5%) | 10 (11.9%) | < 0.001 |
| Laboratory test | ||||
| Hb, g/dL | 12.3 ± 2.3 | 12.9 ± 2.3 | 12.2 ± 2.3 | 0.356 |
| eGFR, ml/min/1.73 m2 | 56.1 ± 29.4 | 71.5 ± 16.3 | 53.6 ± 30.3 | 0.004 |
| Albumin, g/dL | 4.2 (3.8–4.5) | 4.2 (3.7–4.2) | 4.3 (3.8–4.5) | 0.186 |
| Troponin T, pg/mL | 38.0 (20.5–57.5) | 55.0 (46.0–57.3) | 35.0 (15.8–60.5) | 0.112 |
| NT-proBNP, pg/mL | 1102 (272–4163) | 1534 (865–2340) | 1036 (244–4175) | 0.447 |
| Medication | ||||
| ACEI/ARB | 47 (49.0%) | 4 (30.8%) | 43 (51.2%) | 0.283 |
| Beta-blocker | 40 (41.7%) | 5 (38.5%) | 35 (41.7%) | 1.000 |
| CCB | 37 (38.5%) | 1 (7.7%) | 36 (42.9%) | 0.034 |
| MRA | 11 (11.5%) | 2 (15.4%) | 9 (10.7%) | 0.981 |
| Diuretics | 45 (46.9%) | 7 (53.8%) | 38 (45.2%) | 0.779 |
| SGLT2 inhibitor | 8 (8.3%) | 0 (0.0%) | 8 (9.5%) | 0.535 |
| Statin | 58 (60.4%) | 6 (46.2%) | 52 (61.9%) | 0.439 |
Values are mean (standard deviation), n (%, percentage), median (interquartile range).
ACEI angiotensin converting enzyme inhibitor, ARB angiotensin receptor blocker, ATTR-CM transthyretin amyloid cardiomyopathy, BMI body mass index, CCB calcium channel blocker, eGFR estimated glomerular filtration rate, Hb hemoglobin, MRA mineralocorticoid receptor antagonist, NT-proBNP N-terminal pro–B-type natriuretic peptide, SGLT2 sodium-glucose-cotransporter 2.
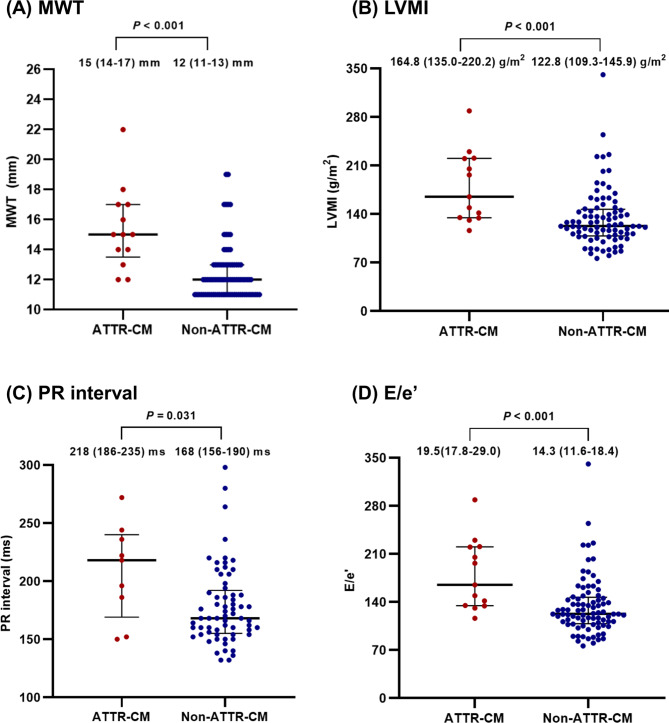
Table 2 presents the ECG and echocardiographic characteristics of patients with ATTR-CM. Based on the ECG, the ATTR-CM group showed a higher prevalence of low-voltage QRS (41.7% vs 6.0%, P = 0.001) and longer PR interval (218 vs. 168 ms, P = 0.031) than the non-ATTR-CM group. Regarding TTE parameters, the mean LVEF of patients with ATTR-CM was lower (57.4% vs. 64.2%, P = 0.021) than that of non-ATTR-CM patients. Additionally, patients in the ATTR-CM group had greater interventricular septal wall thickness (15 vs. 12 mm, P < 0.001), posterior wall thickness (14 vs. 11 mm, P < 0.001), and MWT (15 vs. 12 mm, P < 0.001) than patients in the non-ATTR-CM groups. In addition, LV mass index (LVMI, 164.8 vs. 122.8 g/m2, P < 0.001) and relative wall thickness (0.63 vs. 0.48, P < 0.001) values were also greater in patients with ATTR-CM. Patients with ATTR-CM had higher values of E/e’ ratio (19.5 vs. 14.3, P < 0.001), E’ velocity (4 vs. 5 m/s, P = 0.001), and left atrial volume index (53.7 vs. 39.2 ml/m2, P = 0.044) than non-ATTR-CM patients; however, right ventricular systolic pressure and LV end-diastolic dimension did not differ between two groups.
Table 2.
Electrocardiographic and echocardiographic parameters.
| Total (n = 97) | ATTR-CM (n = 13) | Non-ATTR-CM (n = 84) | P-value | |
|---|---|---|---|---|
| Electrocardiography | ||||
| PR interval, ms | 171 (156–197) | 218 (186–236) | 168 (156–190) | 0.031 |
| QRS voltage, mV | 2.0 (1.3–2.6) | 1.7 (0.9–2.4) | 2.0 (1.4–2.6) | 0.215 |
| Left ventricular hypertrophy criteria, n (%) | 11 (11.7%) | 1 (8.3%) | 10 (12.0%) | 1.000 |
| Low QRS voltage criteria, n (%) | 10 (10.6%) | 5 (41.7%) | 5 (6.0%) | 0.001 |
| QRS duration, ms | 95 (83–108) | 99 (81–142) | 94 (84–108) | 0.567 |
| Voltage-to-mass ratio, mV/(cm2/m2) | 0.15 (0.10–0.21) | 0.10 (0.05–0.18) | 0.16 (0.11–0.21) | 0.049 |
| Any type of AV block, n (%) | 21 (21.6%) | 5 (38.5%) | 16 (20.1%) | 0.072 |
| Echocardiography | ||||
| LVEF, % | 63.4 ± 10.1 | 57.4 ± 11.1 | 64.2 ± 9.6 | 0.021 |
| E/e′ ratio | 15.0 (11.9–19.2) | 19.5 (17.8–29.0) | 14.3 (11.6–18.4) | < 0.001 |
| e′, cm/s | 4 (4–6) | 4 (3–4) | 5 (4–6) | 0.001 |
| Deceleration time, ms | 206.5 ± 57.3 | 179.2 ± 44.1 | 210.6 ± 57.8 | 0.064 |
| RV systolic pressure, mmHg | 28 (22–37) | 33 (26–37) | 28 (22–37) | 0.452 |
| LV end-diastolic dimension, mm | 49 (45–52) | 48 (44–49) | 50 (45–52) | 0.204 |
| LA volume index, ml/m2 | 39.6 (31.7–53.8) | 53.7 (43.4–60.6) | 39.2 (31.1–48.5) | 0.044 |
| LV mass index, g/m2 |
128.8 (112.6-154.8) |
164.8 (135.0–220.2) |
122.8 (109.3–145.9) |
< 0.001 |
| Maximum LV wall thickness, mm | 12 (11–13) | 15 (14–17) | 12 (11–13) | < 0.001 |
| Interventricular septal wall thickness, mm | 12 (11–13) | 15 (14–17) | 12 (11–13) | < 0.001 |
| Posterior wall thickness, mm | 12 (11–13) | 14 (13–15) | 11 (11–12) | < 0.001 |
| Relative LV wall thickness | 0.49 (0.44–0.57) | 0.63 (0.55–0.67) | 0.48 (0.43–0.55) | < 0.001 |
| Pericardial effusion, n (%) | 16 (16.7%) | 4 (30.8%) | 12 (14.3%) | 0.276 |
| Bone scintigraphy | ||||
| Grade 0 | 70 (72.9%) | 0 (0.0%) | 70 (83.3%) | |
| Grade 1 | 13 (13.5%) | 0 (0.0%) | 14 (16.7%) | |
| Grade 2 | 1 (1.1%) | 1(1.1%) | 0 (0.0%) | |
| Grade 3 | 12 (12.5%) | 12 (12.5%) | 0 (0.0%) | |
Values are mean (standard deviation), n (%, percentage), median (interquartile range).
ATTR-CM transthyretin amyloid cardiomyopathy, AV atrioventricular, LA left atrium, LV left ventricle, LVEF left ventricular ejection fraction, RV right ventricle.
The prevalence of ATTR-CM differed according to MWT, PR interval, E/e’, and LVMI. A significantly larger proportion of ATTR-CM was detected among patients with an MWT > 12 mm (84.6% vs. 33.6%, P = 0.002) than among those with an MWT ≤ 12 mm group. However, two patients with ATTR-CM (15.4%) were in the MWT ≤ 12 mm group, and they were all wild-type ATTR-CMs (Supplementary Fig. S1). Furthermore, PR interval > 200 ms (55.6% vs. 19.0%, P = 0.046), E/e’ > 14 (92.3% vs. 49.4%, P = 0.010), and LVMI > 115 g/m2 (100% vs 67.5%, P = 0.036) were also clinical features of patients with ATTR-CM (Supplementary Fig. S2). Figure 3 shows the distribution of MWT, LVMI, PR interval, and E/e’ in ATTR-CM and non-ATTR-CM groups.
Fig. 3.
The distribution of MWT, LVMI, PR interval, and E/e′ in ATTR-CM and non-ATTR-CM. In analysis for PR interval, values from 9 patients in the ATTR-CM group and 64 patients in the non-ATTR-CM group were used in the analysis, excluding patients with no values; in analysis for MWT, LVMI, E/e′, values from all patients were used in the analysis. MWT maximal wall thickness, LVMI left ventricular mass index.
Discussion
In this cohort study, the number of patients with scintigraphy-diagnosed ATTR-CM accounted for 13.4% of patients with HFpEF or HFmrEF. Patients with ATTR-CM were all male, often presenting red flag signs such as carpal tunnel syndrome, and showed abnormal findings on ECG and TTE.
ATTR-CM is induced by the deposition of misfolded and transthyretin (TTR) protein aggregates in tissues, particularly in the heart, eventually leading to organ dysfunction17,22. It is divided into two subtypes, i.e., hereditary ATTR-CM with TTR gene mutation and wild-type ATTR-CM without mutation, and the proportion of wild-type ATTR-CM is higher among older adults11,12. The prevalence of ATTR-CM continues to increase gradually, and the prevalence of ATTR-CM in patients with HFpEF was found to range from 5 to 17%11,23. Consistent with previous studies, the current study revealed a prevalence of approximately 13%.
Patients with ATTR-CM are known to have the worst prognosis and substantially lower quality of life than patients with other types of cardiomyopathy24,25. ATTR-CM initially appears as HFpEF or HFmrEF; however, as the disease progresses, it eventually presents as heart failure with reduced ejection fraction in the late stage26. Accordingly, early identification and diagnosis of ATTR-CM are crucial when patients are in HFpEF or HFmrEF27. Early detection of ATTR-CM can be achieved by scintigraphy before ECG, TTE, or biomarker changes14,16,28. Therefore, it is reasonable to perform scintigraphy to determine the cause of HFpEF or HFmrEF, even in the absence of changes in the other tests. In addition to grade 2 or 3 uptake on bone scintigraphy, non-invasive features suggestive of ATTR-CM include low-voltage QRS on ECG, and increased LV wall thickness or apical sparing patter of LV strain image on TTE17,29. In our study, a low voltage was observed in less than 50% of patients with ATTR-CM only, which is less than that reported previously30; however, a PR interval of ≥ 200 ms in electrocardiography was identified as an important clinical feature of ATTR-CM in a Korean population. Thus, we believe that diagnosing ATTR-CM by serial monitoring the PR interval would be helpful. Furthermore, 15.4% of the patients in this study were diagnosed with ATTR-CM by scintigraphy despite having an MWT of ≤ 12 mm. Furthermore, a recent study has revealed that ATTR-CM was confirmed using scintigraphy in 5% of patients even without LVH; these patients all had wild-type ATTR-CM23. Similarly, in our study, approximately 3.5% of patients with an MWT of ≤ 12 mm were diagnosed with wild-type ATTR-CM. The fact that ATTR-CM can be diagnosed even in patients with LV wall thickness ≤ 12 mm represents the limitations of the current guidelines for suspecting and testing ATTR-CM in patients with LV wall thickness > 12 mm in HFpEF or HFmrEF31,32. In addition, given that female individuals have thinner LV walls than males, certain females may experience ATTR-CM even if the LV wall thickness does not exceed 12 mm33. Therefore, in patients with HFpEF or HFmrEF of unknown cause, ATTR-CM should be suspected, and active diagnosis using scintigraphy, a non-invasive test, is needed.
Unlike Western populations, data on Asians with ATTR-CM are limited. In a recently published Japanese multi-centre registry, 14.2% of patients with HFpEF who underwent bone scintigraphy regardless of LV wall thickness were diagnosed with ATTR-CM34. Meanwhile, in data from China, the prevalence of ATTR-CM in patients with HFpEF or HFmrEF with an LV wall thickness > 12 mm was approximately 5.3%, which was significantly lower than that reported among Western populations35. Among Asian populations, only one multinational study identified the common genetic variation and phenotypic characteristics of hereditary TTR amyloidosis in a Southeast Asian cohort. However, the cohort size was markedly limited, comprising less than 30 patients36. Therefore, continued research on ATTR-CM in Asians is necessary, and future large-scale multinational studies are required to further advance our understanding of this disease.
This study had some limitations. First, given that this study was conducted in a single centre with a small study population, it is difficult to generalize the findings, and a larger study is warranted. Second, as this was a retrospective study, scintigraphy was performed at the clinicians’ discretion rather than in all patients with HFpEF or HFmrEF presenting with LV wall thickness greater than 10 mm, potentially introducing a selection bias in the study population. Nevertheless, to the best of our knowledge, the current study represents the largest investigation of scintigraphy among a Korean population with HFpEF and HFmrEF. Third, data on the prognosis of ATTR-CM are lacking. Because ATTR-CM can progress to advanced heart failure, its diagnosis and treatment remain crucial. Given that the current study focused only on diagnosis, the prognosis of Korean patients with ATTR-CM needs to be examined in future investigations. However, a key strength of this study is that it was conducted on patients with a definitive diagnosis of HFpEF or HFmrEF, where research results related to ATTR-CM in patients with HFpEF or HFmrEF are rare.
In conclusion, to the best of our knowledge, this is the first study to present the prevalence and characteristics of ATTR-CM in Korean patients with HFpEF or HFmrEF diagnosed using scintigraphy. Therefore, the findings of this study suggest that ATTR-CM should be suspected in patients with HFpEF or HFmrEF, particularly in specific subgroups, such as males with neurologic symptoms, diastolic dysfunction, long PR interval, and/or low-voltage QRS, in the Korean population.
Methods
Study design and population
Between September 2018 and January 2023, patients who underwent transthoracic echocardiography (TTE) and 99mTc-pyrophosphate (PYP) or 99mTc-3,3-diphosphono-1,2-propanodicarboxylic (DPD) acid scintigraphy in Severance hospital were retrospectively investigated. Among them, patients with a left ventricular (LV) ejection fraction (LVEF) > 40% and maximal LV wall thickness (MWT) > 10 mm were included (Fig. 1). Patients with the following definite causes of heart failure were excluded: dilated cardiomyopathy (DCMP), hypertrophic cardiomyopathy (HCMP), myocarditis, AL amyloidosis, multiple myeloma (MM), valvular disease, sarcoidosis, other infiltrative cardiomyopathy (CMP), Fabry disease, ‘Polyneuropathy, Organomegaly, Endocrinopathy, Monoclonal protein, Skin changes’ (POEMS) disease, or ‘Mitochondrial Encephalopathy, Lactic Acidosis, and Stroke-like episodes’ (MELAS). The present study was reviewed and approved by the Institutional Review Board of Yonsei University College of Medicine (Approval number: 4-2022-1550) and adheres to the principles outlined in the Declaration of Helsinki. The requirement for informed consent was waived due to the retrospective study design.
Baseline and data collection
Laboratory and clinical data were collected from electronic medical records. Laboratory results within the nearest 3 months of TTE and bone scintigraphy data were collected. The H2FPEF score was calculated to assess simple clinical features of HFpEF37. Polyneuropathy, carpal tunnel syndrome, lumbar spinal stenosis, and autonomic dysfunction were identified as red flag signs that are typical clinical features of patients with ATTR-CM and were defined as diagnosed by nerve conduction velocity, autonomic function, or imaging tests.
Echocardiographic parameters
TTE was performed by an expert using Vivid 7 or Vivid E9 (GE Healthcare, Chicago, IL, USA) and iE33 or Epiq7 (Phillips Healthcare, Best, The Netherlands) with the 2.5-MHz transducer. Standard two-dimensional, Doppler, and tissue Doppler imaging parameters were measured according to the recommendations of the current American Society of Echocardiography38,39. Left atrial volume and LVEF were assessed using the Simpson biplane method from the apical 4- and 2-chamber views. LV wall thickness was measured in the parasternal short-axis view. LV mass and relative wall thickness were calculated using the recommended formula. Diastolic function was assessed using mitral inflow pulsed-wave and mitral annular tissue Doppler. Right ventricular systolic pressure was determined using tricuspid velocity and inferior vena cava diameter. All measurements were digitally stored. MWT was defined as the thickest interventricular septal wall thickness and posterior wall thickness.
Electrocardiogram parameters
All (ECGs) were reviewed and measured manually. For the LV hypertrophy criteria, the Sokolow criteria was used40, and the voltage-to-mass ratio obtained by dividing the Sokolow index by the LV mass index was also evaluated30. The low voltage QRS was defined as when the QRS voltage was < 5 mm in the limb leads or < 1 mV in all precordial leads.
99mTc-pyrophosphate or 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy
PYP or DPD scintigraphy was performed using GE Medical Systems single-photon emission computed tomography/computed tomography (SPECT/CT) (Discovery NM/CT 670) with a standardized protocol41. The patients were administrated 740 MBq PYP or DPD intravenously and scanned at 1 h when using PYP and 3 h when using DPD after injection. An additional 3-h scan was obtained in the PYP scan if significant blood pool activity was noted in the 1-h scan. Two experienced nuclear medicine physicians evaluated the results to confirm the presence and degree of myocardial uptake and distribution, rating it from grade 0 to 3 as follows: grade 0, no myocardial uptake; grade 1, less myocardial uptake than ribs; grade 2, myocardial uptake equal to ribs; grade 3, greater myocardial uptake than ribs with weak or absent rib uptake. Cases with grade 2 or 3 myocardial uptake were confirmed positive and diagnosed with ATTR-CM.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation or median (inter-quartile range [IQR]), and categorical variables as percentages. The differences between the ATTR-CM and non-ATTR CM groups were compared using the Student’s t-test, Chi-square test, or Fisher’s exact test. Because t-tests was primarily used to test our hypotheses, a t-test-based method was implemented to estimate the sample size. In detail, the “pwrss.t.2means” function in pwrss package was utilized with 0.8 of Cohen’s D and 0.13 of disease/sample ratio. With a power of 0.8 and a significance level of 0.05, the sample size to test the hypothesis was estimated to be 124 (Supplementary Fig. S3). Statistical significance was set at a two-sided P < 0.05. Statistical analyses were conducted using the R software (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
S.-E.K.: data acquisition, data analysis, data interpretation, and manuscript drafting and review; S.-H.L., C.J.L., S.H.H., W.J.K., S.-M.K.: data acquisition, data interpretation, and manuscript review; J.O.: study concept and design, data analysis, data interpretation, manuscript review, and study supervision.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1A2C1093325).
Data availability
The datasets generated and/or analysed during the current study are not publicly available due privacy/ethical restrictions but are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jaewon Oh, Email: ericjcoh@yuhs.ac.
Won Jun Kang, Email: mdkwj@yuhs.ac.
References
- 1.Park, J. J. et al. Heart failure statistics in Korea, 2020: a report from the Korean Society of Heart failure. Int. J. Heart Fail.3, 224–236. 10.36628/ijhf.2021.0023 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seferović, P. M. et al. The heart failure Association Atlas: heart failure epidemiology and management statistics 2019. Eur. J. Heart Fail.23, 906–914. 10.1002/ejhf.2143 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Redfield, M. M. Heart failure with preserved ejection fraction. N. Engl. J. Med.376, 897. 10.1056/NEJMc1615918 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Dunlay, S. M., Roger, V. L. & Redfield, M. M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol.14, 591–602. 10.1038/nrcardio.2017.65 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Lee, H. H. et al. Korea Heart Disease fact sheet 2020: analysis of Nationwide Data. Korean Circ. J.51, 495–503. 10.4070/kcj.2021.0097 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borlaug, B. A. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol.17, 559–573. 10.1038/s41569-020-0363-2 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Vaduganathan, M. et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet400, 757–767. 10.1016/s0140-6736(22)01429-5 (2022). [DOI] [PubMed] [Google Scholar]
- 8.Oh, J., Lee, S. H., Lee, C. J. & Kang, S. M. Sodium-glucose co-transporter 2 inhibitors: a new path for heart failure treatment. Korean Circ. J.51, 399–408. 10.4070/kcj.2021.0070 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anker, S. D. et al. Empagliflozin in Heart failure with a preserved ejection fraction. N. Engl. J. Med.385, 1451–1461. 10.1056/NEJMoa2107038 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Solomon, S. D. et al. Dapagliflozin in Heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med.387, 1089–1098. 10.1056/NEJMoa2206286 (2022). [DOI] [PubMed] [Google Scholar]
- 11.González-López, E. et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur. Heart J.36, 2585–2594. 10.1093/eurheartj/ehv338 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Shiozaki, T., Sato, N., Hayashi, T., Kobayashi, K. & Asamura, H. Wild-type ATTR amyloidosis may be associated with unexpected death among the elderly. Leg. Med. (Tokyo)41, 101634. 10.1016/j.legalmed.2019.101634 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Tanskanen, M. et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann. Med.40, 232–239. 10.1080/07853890701842988 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Tahara, N. et al. (99m) technetium-pyrophosphate scintigraphy: a practical guide for early diagnosis of transthyretin amyloid cardiomyopathy. ESC Heart Fail.9, 251–262. 10.1002/ehf2.13693 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillmore, J. D. et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation133, 2404–2412. 10.1161/circulationaha.116.021612 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Maurer, M. S. & Ruberg, F. L. Cardiac scintigraphy and screening for transthyretin cardiac amyloidosis: caveat emptor. Circulation144, 1005–1007. 10.1161/circulationaha.121.055517 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruberg, F. L., Grogan, M., Hanna, M., Kelly, J. W. & Maurer, M. S. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J. Am. Coll. Cardiol.73, 2872–2891. 10.1016/j.jacc.2019.04.003 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnappa, D. et al. Atrial fibrillation in the elderly: the role of sub-clinical isolated cardiac amyloidosis. Sci. Rep.9, 16584. 10.1038/s41598-019-53119-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maurer, M. S. et al. Tafamidis Treatment for patients with transthyretin amyloid cardiomyopathy. N. Engl. J. Med.379, 1007–1016. 10.1056/NEJMoa1805689 (2018). [DOI] [PubMed] [Google Scholar]
- 20.AbouEzzeddine, O. F. et al. Prevalence of transthyretin amyloid cardiomyopathy in heart failure with preserved ejection fraction. JAMA Cardiol.6, 1267–1274. 10.1001/jamacardio.2021.3070 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goland, S. et al. Wild-type TTR amyloidosis among patients with unexplained heart failure and systolic LV dysfunction. PLoS ONE16, e0254104. 10.1371/journal.pone.0254104 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westermark, P., Sletten, K., Johansson, B. & Cornwell, G. G. Fibril in senile systemic amyloidosis is derived from normal transthyretin. Proc. Natl. Acad. Sci. U.S.A.87, 2843–2845. 10.1073/pnas.87.7.2843 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devesa, A. et al. Prevalence of transthyretin amyloidosis in patients with heart failure and no left ventricular hypertrophy. ESC Heart Fail.8, 2856–2865. 10.1002/ehf2.13360 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kocher, F. et al. Heart failure from ATTRwt amyloid cardiomyopathy is associated with poor prognosis. ESC Heart Fail.7, 3919–3928. 10.1002/ehf2.12986 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lane, T. et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation140, 16–26. 10.1161/circulationaha.118.038169 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Seferović, P. M. et al. Heart failure in cardiomyopathies: a position paper from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail.21, 553–576. 10.1002/ejhf.1461 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Ioannou, A. et al. Impact of earlier diagnosis in cardiac ATTR amyloidosis over the course of 20 years. Circulation146, 1657–1670. 10.1161/circulationaha.122.060852 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minutoli, F. et al. Serial scanning with (99m)Tc-3, 3-diphosphono-1, 2-propanodicarboxylic acid ((99m)Tc-DPD) for early detection of cardiac amyloid deposition and prediction of clinical worsening in subjects carrying a transthyretin gene mutation. J. Nucl. Cardiol.28, 1949–1957. 10.1007/s12350-019-01950-2 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Damy, T. et al. Clinical, ECG and echocardiographic clues to the diagnosis of TTR-related cardiomyopathy. Open. Heart3, e000289. 10.1136/openhrt-2015-000289 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rapezzi, C. et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation120, 1203–1212. 10.1161/circulationaha.108.843334 (2009). [DOI] [PubMed] [Google Scholar]
- 31.McDonagh, T. A. et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J.42, 3599–3726. 10.1093/eurheartj/ehab368 (2021). [DOI] [PubMed]
- 32.Kitaoka, H. et al. JCS 2020 Guideline on diagnosis and treatment of cardiac amyloidosis. Circ. J.84, 1610–1671. 10.1253/circj.CJ-20-0110 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Patel, R. K. et al. Sex differences among patients with transthyretin amyloid cardiomyopathy—from diagnosis to prognosis. Eur. J. Heart Fail.24, 2355–2363. 10.1002/ejhf.2646 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naito, T. et al. Prevalence of transthyretin amyloidosis among heart failure patients with preserved ejection fraction in Japan. ESC Heart Fail.10, 1896–1906. 10.1002/ehf2.14364 (2023). [Google Scholar]
- 35.Yang, H. et al. An echo score raises the suspicion of cardiac amyloidosis in Chinese with heart failure with preserved ejection fraction. ESC Heart Fail.9, 4280–4290. 10.1002/ehf2.14164 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen, Z. et al. Hereditary transthyretin amyloidosis—Clinical and genetic characteristics of a Multiracial South-East Asian Cohort in Singapore. J. Neuromuscul. Dis.8, 723–733. 10.3233/jnd-210656 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Reddy, Y. N. V., Carter, R. E., Obokata, M., Redfield, M. M. & Borlaug, B. A. A simple, evidence-based Approach to help Guide diagnosis of heart failure with preserved ejection fraction. Circulation138, 861–870. 10.1161/circulationaha.118.034646 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging16, 233–270. 10.1093/ehjci/jev014 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Blume, G. G. et al. Left atrial function: physiology, assessment, and clinical implications. Eur. J. Echocardiogr.12, 421–430. 10.1093/ejechocard/jeq175 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Sokolow, M. & Lyon, T. P. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am. Heart J.37, 161–186. 10.1016/0002-8703(49)90562-1 (1949). [DOI] [PubMed] [Google Scholar]
- 41.Dorbala, S. et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: part 1 of 2-evidence base and standardized methods of imaging. J. Nucl. Cardiol.26, 2065–2123. 10.1007/s12350-019-01760-6 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due privacy/ethical restrictions but are available from the corresponding author on reasonable request.