Abstract
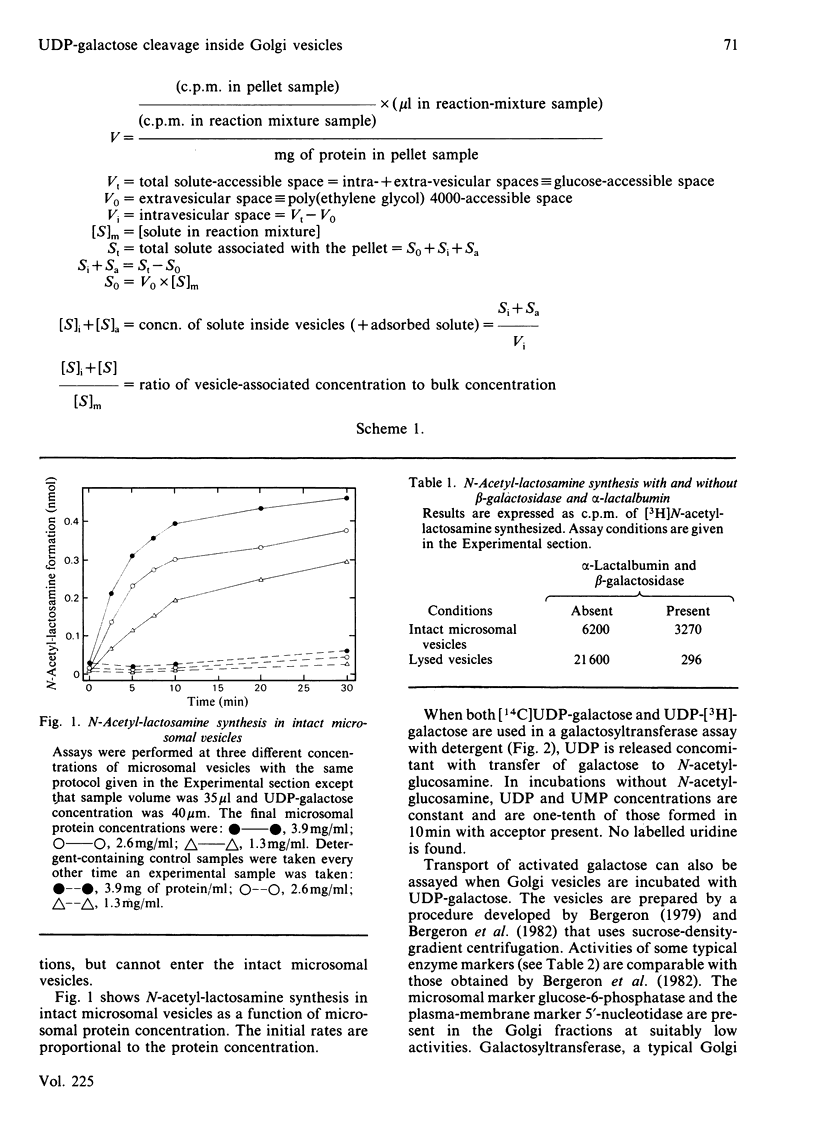
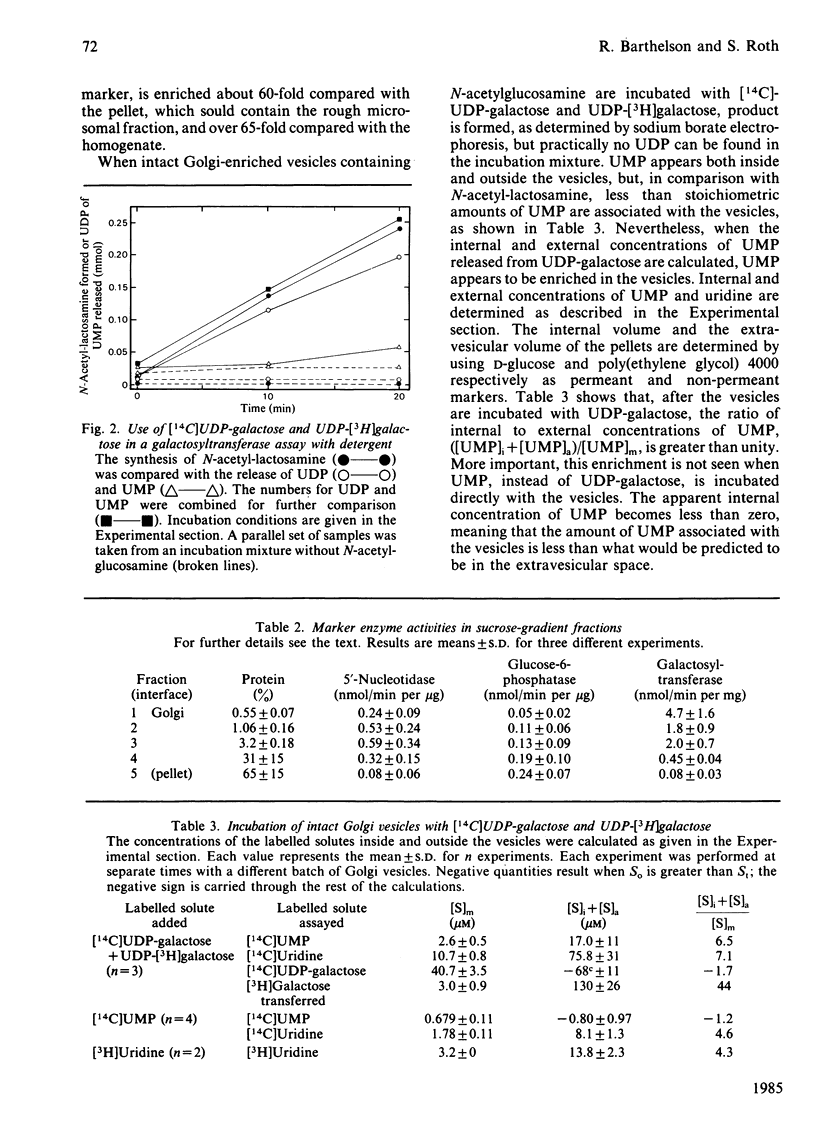
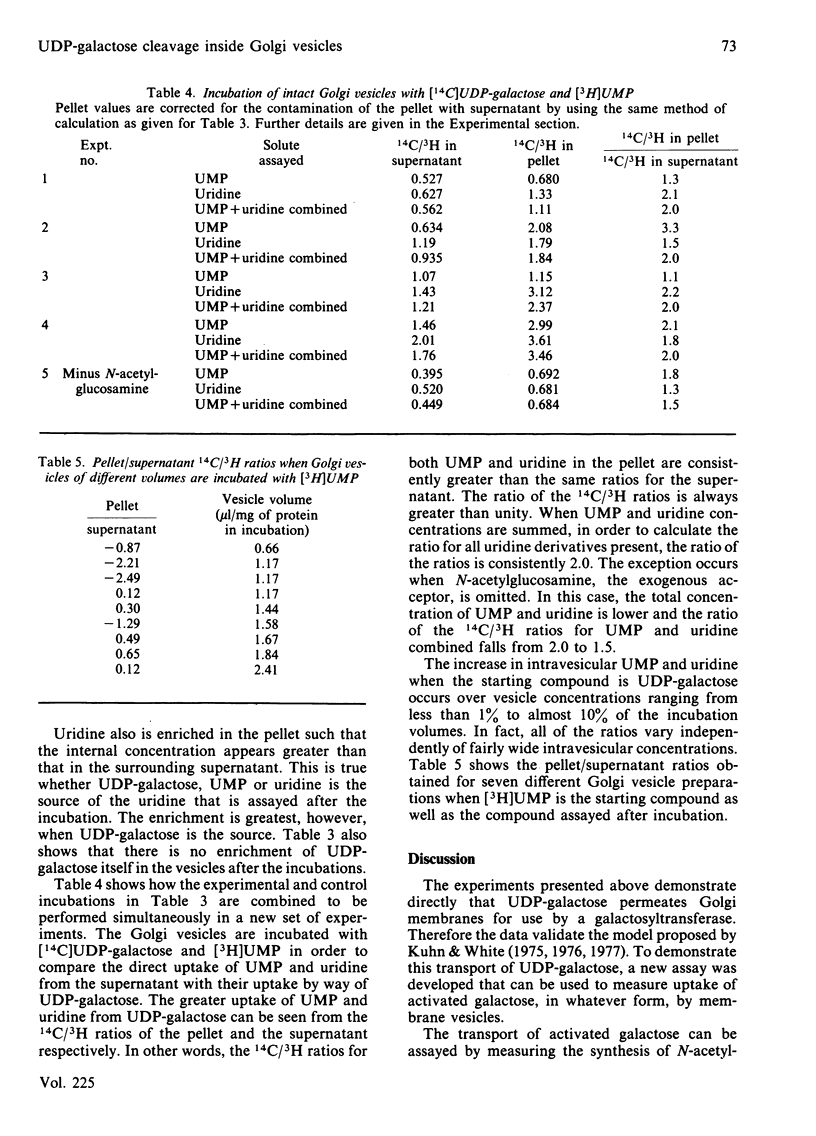
UDP-galactose appears to be produced on one side of a membrane barrier, opposite the galactosyltransferases that use it as a sugar donor. The translocation of activated galactose across membranes was studied in rat submaxillary-gland microsomal vesicles and in rat liver Golgi vesicles. When these intact vesicles containing the acceptor, N-acetylglucosamine, were incubated in the presence of UDP-galactose and two inhibitors of galactosyltransferase activity, the product, N-acetyl-lactosamine, formed within the vesicles. Thus at least the galactose moiety of UDP-galactose crossed the membranes. When intact Golgi vesicles were incubated with UDP-galactose labelled in both the uridine and the galactose moieties, labelled N-acetyllactosamine was again produced in the vesicles, but less than stoichiometric amounts of the uridine label was found there. Calculation of internal and external concentrations of UMP, a major product released from the cleaved uridine moiety, showed that the vesicles were actually enriched in UMP. When free UMP was incubated with the vesicles, this enrichment did not occur. This result was direct evidence for facilitated transport of UDP-galactose into the Golgi for use by galactosyltransferase.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson D. R., Rodewald R. Evidence for the sorting of endocytic vesicle contents during the receptor-mediated transport of IgG across the newborn rat intestine. J Cell Biol. 1981 Oct;91(1):270–280. doi: 10.1083/jcb.91.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo S., De Luca L. M., Silverman-Jones C. S., Yuspa S. H. Mode of action of retinol. Involvement in glycosylation reactions of cultured mouse epidermal cells. J Biol Chem. 1979 May 10;254(9):3279–3287. [PubMed] [Google Scholar]
- Ballas L. M., Arion W. J. Measurement of glucose 6-phosphate penetration into liver microsomes. Confirmation of substrate transport in the glucose-6-phosphatase system. J Biol Chem. 1977 Dec 10;252(23):8512–8518. [PubMed] [Google Scholar]
- Bergeron J. J. Golgi fractions from livers of control and ethanol-intoxicated rats. Enzymic and morphologic properties following rapid isolation. Biochim Biophys Acta. 1979 Aug 23;555(3):493–503. doi: 10.1016/0005-2736(79)90402-4. [DOI] [PubMed] [Google Scholar]
- Bergeron J. J., Rachubinski R. A., Sikstrom R. A., Posner B. I., Paiement J. Galactose transfer to endogenous acceptors within Golgi fractions of rat liver. J Cell Biol. 1982 Jan;92(1):139–146. doi: 10.1083/jcb.92.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandan E., Fleischer B. Orientation and role of nucleosidediphosphatase and 5'-nucleotidase in Golgi vesicles from rat liver. Biochemistry. 1982 Sep 14;21(19):4640–4645. doi: 10.1021/bi00262a019. [DOI] [PubMed] [Google Scholar]
- Carey D. J., Hirschberg C. B. Kinetics of glycosylation and intracellular transport of sialoglycoproteins in mouse liver. J Biol Chem. 1980 May 10;255(9):4348–4354. [PubMed] [Google Scholar]
- Carey D. J., Sommers L. W., Hirschberg C. B. CMP-N-acetylneuraminic acid: isolation from and penetration into mouse liver microsomes. Cell. 1980 Mar;19(3):597–605. doi: 10.1016/s0092-8674(80)80036-5. [DOI] [PubMed] [Google Scholar]
- De Luca L. M., Frot-Coutaz J. P., Silverman-Jones C. S., Roller P. R. Chemical synthesis of phosphorylated retinoids. Their mannosyl acceptor activity in rat liver membranes. J Biol Chem. 1977 Apr 25;252(8):2575–2579. [PubMed] [Google Scholar]
- Fleischer B. Isolation and characterization of Golgi apparatus and membranes from rat liver. Methods Enzymol. 1974;31:180–191. doi: 10.1016/0076-6879(74)31020-8. [DOI] [PubMed] [Google Scholar]
- Fleischer B. Orientation of glycoprotein galactosyltransferase and sialyltransferase enzymes in vesicles derived from rat liver Golgi apparatus. J Cell Biol. 1981 May;89(2):246–255. doi: 10.1083/jcb.89.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J. Transmembrane assembly of N-linked glycoproteins. Studies on the topology of saccharide synthesis. J Biol Chem. 1982 Mar 25;257(6):2787–2794. [PubMed] [Google Scholar]
- Haselbeck A., Tanner W. Dolichyl phosphate-mediated mannosyl transfer through liposomal membranes. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1520–1524. doi: 10.1073/pnas.79.5.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J., White A. Evidence for specific transport of uridine diphosphate galactose across the Golgi membrane of rat mammary gland. Biochem J. 1976 Jan 15;154(1):243–244. doi: 10.1042/bj1540243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J., White A. The role of nucleoside diphosphatase in a uridine nucleotide cycle associated with lactose synthesis in rat mammary-gland Golgi apparatus. Biochem J. 1977 Dec 15;168(3):423–433. doi: 10.1042/bj1680423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J., White A. The topography of lactose synthesis. Biochem J. 1975 Apr;148(1):77–84. doi: 10.1042/bj1480077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUFELD E. F., GINSBURG V., PUTMAN E. W., FANSHIER D., HASSID W. Z. Formation and interconversion of sugar nucleotides by plant extracts. Arch Biochem Biophys. 1957 Jul;69:602–616. doi: 10.1016/0003-9861(57)90524-6. [DOI] [PubMed] [Google Scholar]
- Peterson P. A., Rask L., Helting T., Ostberg L., Fernstedt Y. Formation and properties of retinylphosphate galactose. J Biol Chem. 1976 Aug 25;251(16):4986–4995. [PubMed] [Google Scholar]
- Pierce M., Turley E. A., Roth S. Cell surface glycosyltransferase activities. Int Rev Cytol. 1980;65:1–47. doi: 10.1016/s0074-7696(08)61958-0. [DOI] [PubMed] [Google Scholar]
- Roth J., Berger E. G. Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J Cell Biol. 1982 Apr;93(1):223–229. doi: 10.1083/jcb.93.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S., McGuire E. J., Roseman S. Evidence for cell-surface glycosyltransferases. Their potential role in cellular recognition. J Cell Biol. 1971 Nov;51(21):536–547. doi: 10.1083/jcb.51.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider M. D., Rogers O. C. Transmembrane movement of oligosaccharide-lipids during glycoprotein synthesis. Cell. 1984 Mar;36(3):753–761. doi: 10.1016/0092-8674(84)90355-6. [DOI] [PubMed] [Google Scholar]
- Snider M. D., Sultzman L. A., Robbins P. W. Transmembrane location of oligosaccharide-lipid synthesis in microsomal vesicles. Cell. 1980 Sep;21(2):385–392. doi: 10.1016/0092-8674(80)90475-4. [DOI] [PubMed] [Google Scholar]
- Sommers L. W., Hirschberg C. B. Transport of sugar nucleotides into rat liver Golgi. A new Golgi marker activity. J Biol Chem. 1982 Sep 25;257(18):10811–10817. [PubMed] [Google Scholar]
- Zatta P., Zakim D., Vessey D. A. The transfer of galactose from UDP-galactose to endogenous lipid acceptors in liver microsomes. Biochim Biophys Acta. 1975 Jun 12;392(2):361–365. doi: 10.1016/0304-4165(75)90018-5. [DOI] [PubMed] [Google Scholar]
- de Luca L. M., Bhat P. V., Sasak W., Adamo S. Biosynthesis of phosphoryl and glycosyl phosphoryl derivatives of vitamin A in biological membranes. Fed Proc. 1979 Oct;38(11):2535–2539. [PubMed] [Google Scholar]


