Abstract
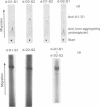
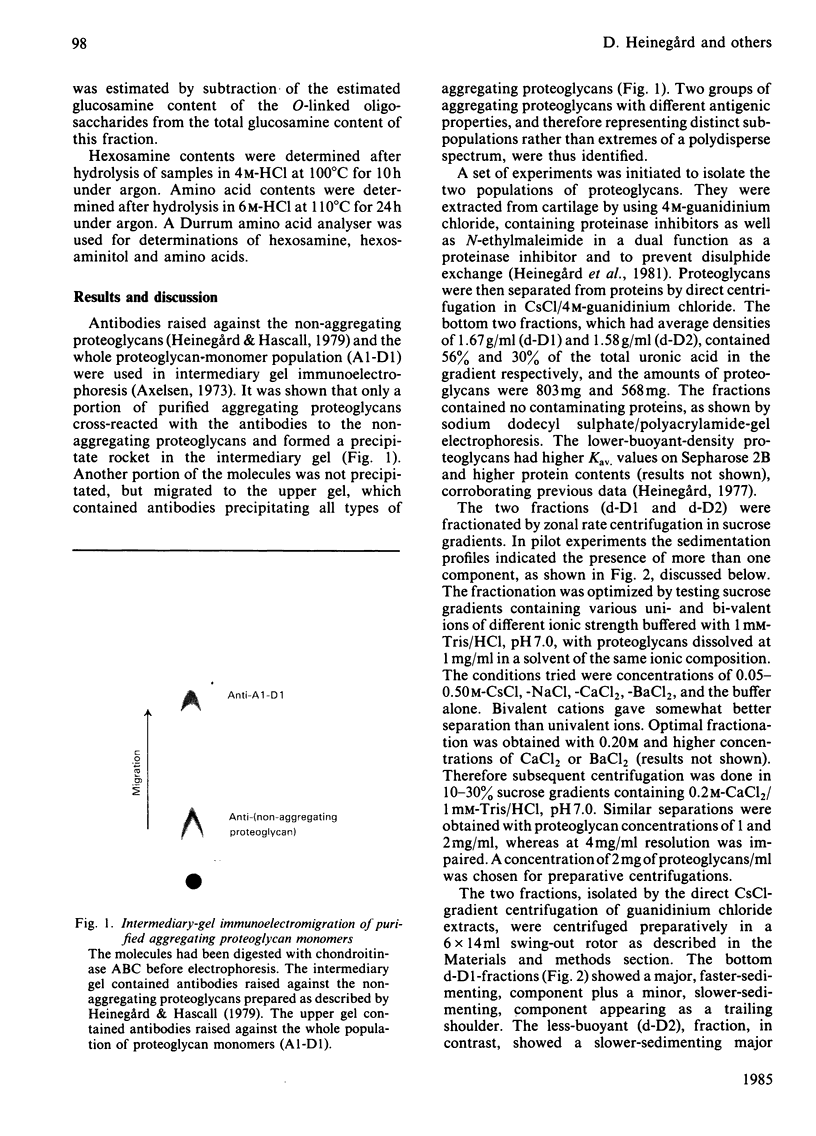
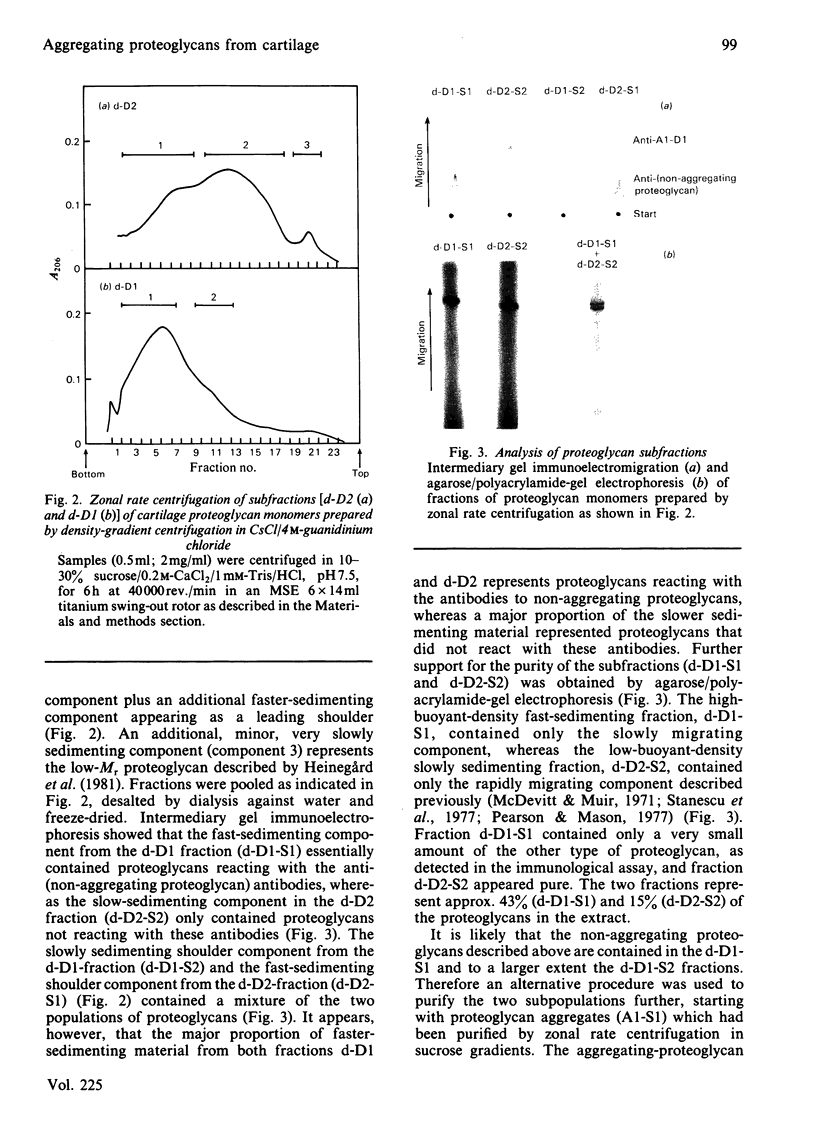
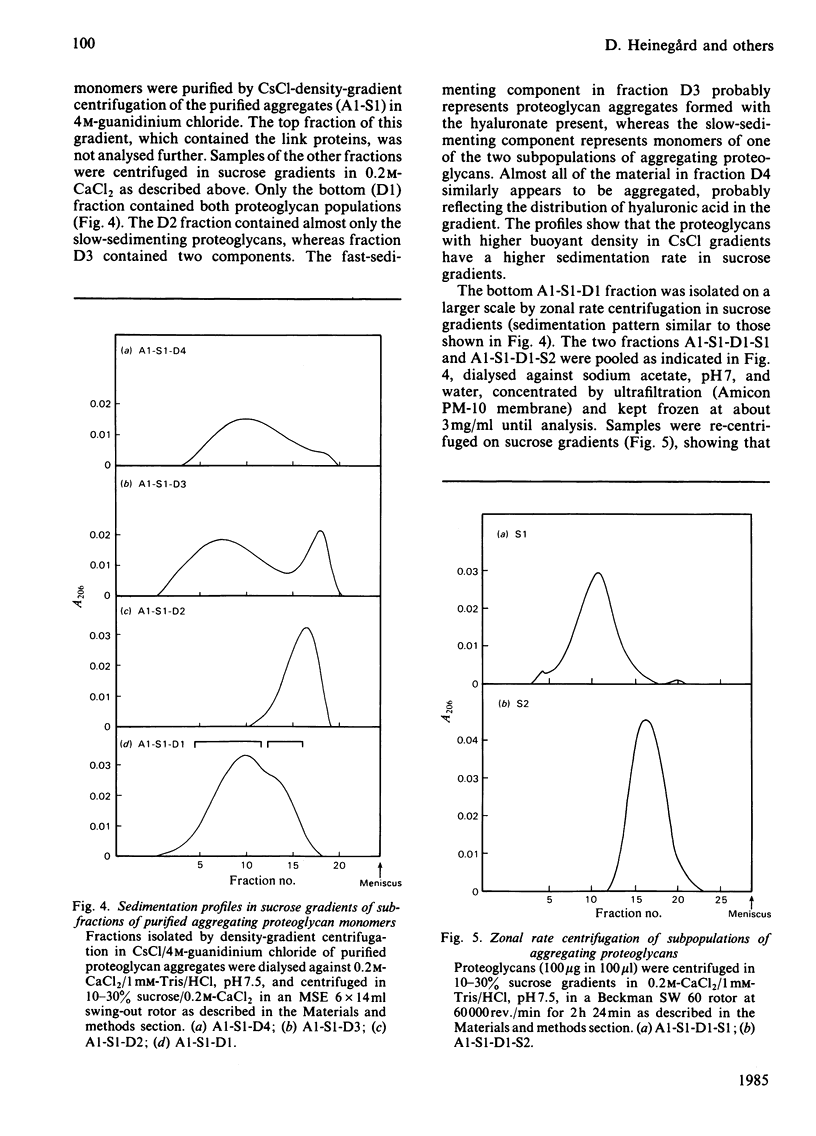
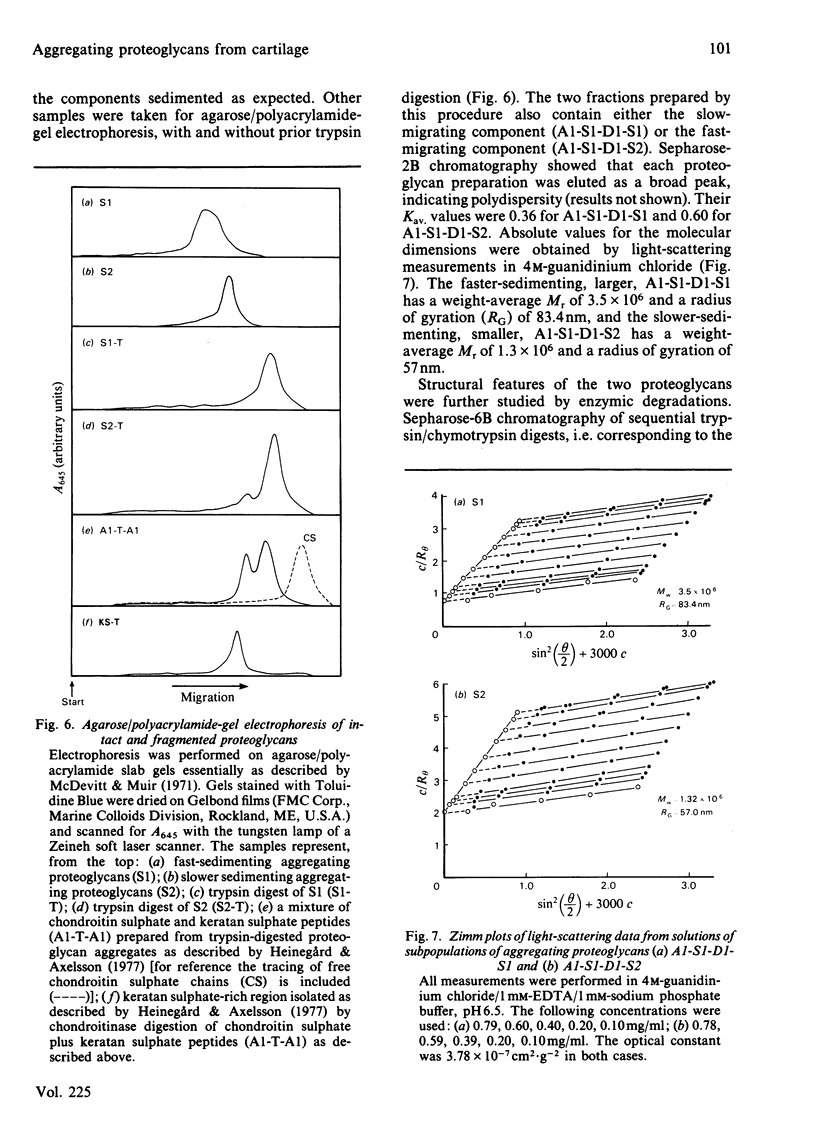
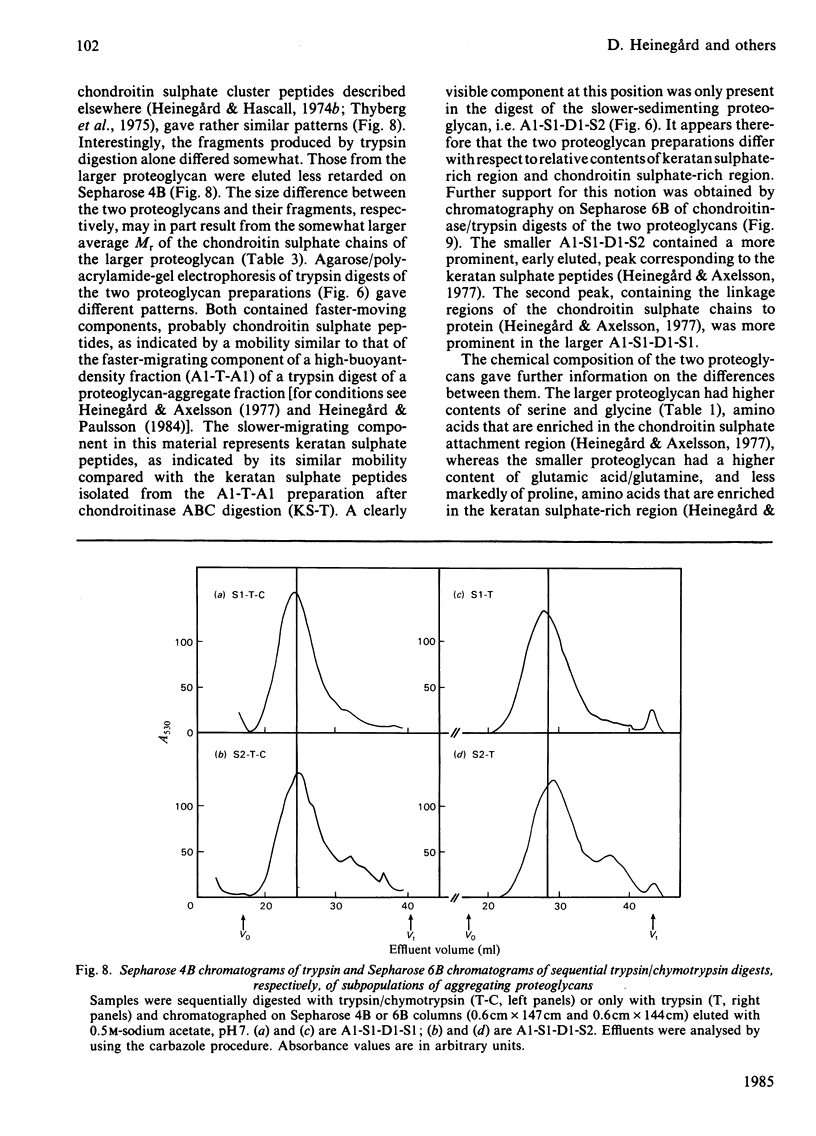
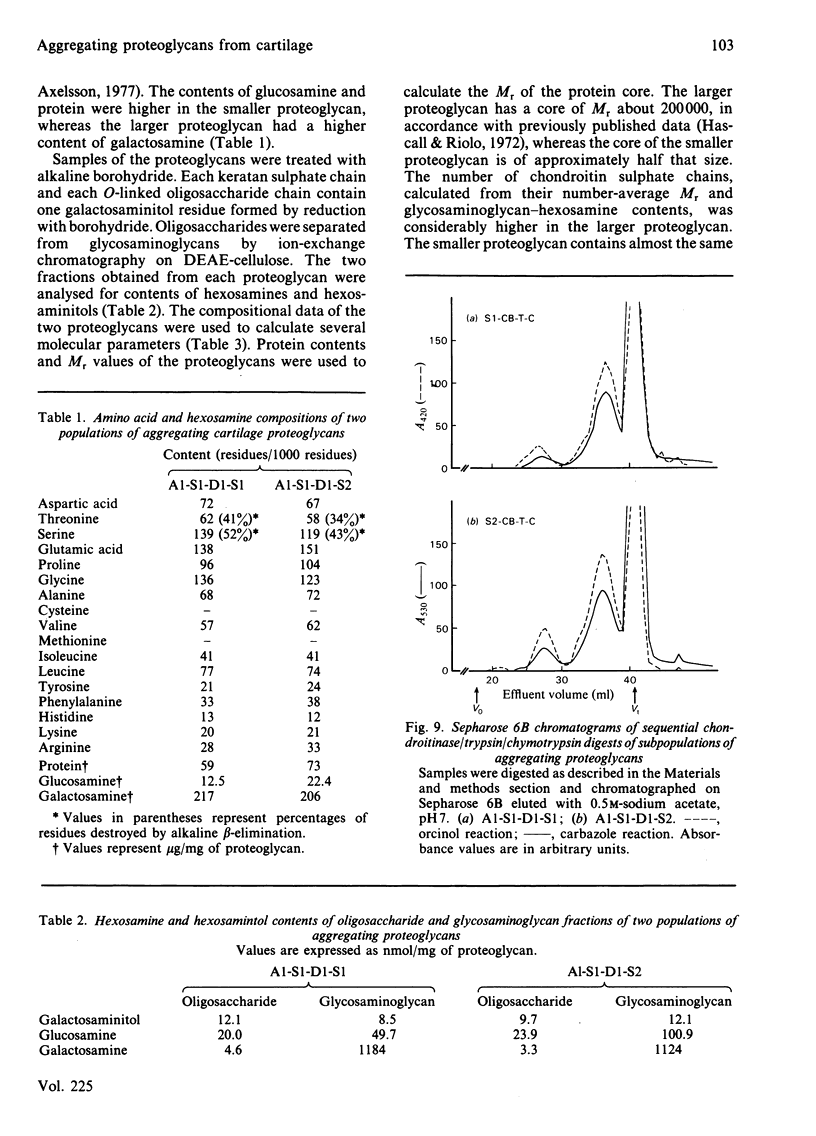
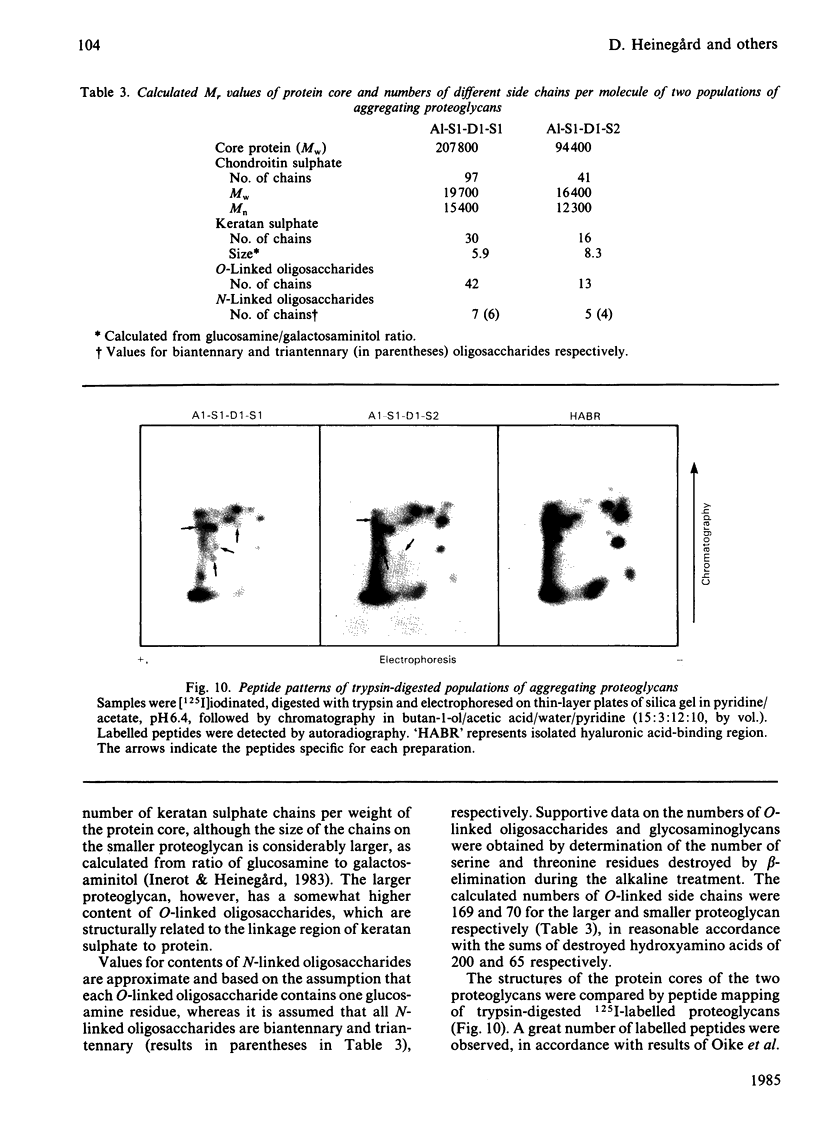
Intermediary gel immunoelectrophoresis was used to show that purified aggregating cartilage proteoglycans from 2-year-old steers contain two distinct populations of molecules and that only one of these is immunologically related to non-aggregating cartilage proteoglycans. The two types of aggregating proteoglycans were purified by density-gradient centrifugation in 3.5M-CsCl/4M-guanidinium chloride and separated by zonal rate centrifugation in sucrose gradients. The higher-buoyant-density faster-sedimenting proteoglycan represented 43% of the proteoglycans in the extract. It had a weight-average Mr of 3.5 X 10(6), did not contain a well-defined keratan sulphate-rich region, had a quantitatively dominant chondroitin sulphate-rich region and contained 5.9% protein and 23% hexosamine. The lower-buoyant-density, more slowly sedimenting, proteoglycan represented 15% of the proteoglycans in the extract. It had a weight-average Mr of 1.3 X 10(6), contained both the keratan sulphate-rich and the chondroitin sulphate-rich regions and contained 7.3% protein and 23% hexosamine. Each of the proteoglycan preparations showed only one band on agarose/polyacrylamide-gel electrophoresis. The larger proteoglycan had a lower mobility than the smaller. The distribution of chondroitin sulphate chains along the chondroitin sulphate-rich region was similar for the two types of proteoglycans. The somewhat larger chondroitin sulphate chains of the larger proteoglycan could not alone account for the larger size of the proteoglycan. Peptide patterns after trypsin digestion of the proteoglycans showed great similarities, although the presence of a few peptides not shared by both populations indicates that the core proteins are partially different.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Franzén A., Björnsson S., Heinegård D. Zonal rate centrifugation of proteoglycans in sucrose gradients. Anal Biochem. 1982 Feb;120(1):38–45. doi: 10.1016/0003-2697(82)90314-1. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Binding of oligosaccharides of hyaluronic acid to proteoglycans. Biochem J. 1973 Dec;135(4):905–908. doi: 10.1042/bj1350905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. II. Oligosaccharide competitors of the proteoglycan-hyaluronic acid interaction. J Biol Chem. 1974 Jul 10;249(13):4242–4249. [PubMed] [Google Scholar]
- Hascall V. C., Riolo R. L. Characteristics of the protein-keratan sulfate core and of keratan sulfate prepared from bovine nasal cartilage proteoglycan. J Biol Chem. 1972 Jul 25;247(14):4529–4538. [PubMed] [Google Scholar]
- Heinegård D. K., Hascall V. C. Characteristics of the nonaggregating proteoglycans isolated from bovine nasal cartilage. J Biol Chem. 1979 Feb 10;254(3):927–934. [PubMed] [Google Scholar]
- Heinegård D., Axelsson I. Distribution of keratan sulfate in cartilage proteoglycans. J Biol Chem. 1977 Mar 25;252(6):1971–1979. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Characterization of chondroitin sulfate isolated from trypsin-chymotrypsin digests of cartilage proteoglycans. Arch Biochem Biophys. 1974 Nov;165(1):427–441. doi: 10.1016/0003-9861(74)90182-9. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Paulsson M., Inerot S., Carlström C. A novel low-molecular weight chondroitin sulphate proteoglycan isolated from cartilage. Biochem J. 1981 Aug 1;197(2):355–366. doi: 10.1042/bj1970355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D. Polydispersity of cartilage proteoglycans. Structural variations with size and buoyant density of the molecules. J Biol Chem. 1977 Mar 25;252(6):1980–1989. [PubMed] [Google Scholar]
- Hoffman P., Mashburn T. A., Jr, Hsu D., Trivedi D., Diep J. Variable nature of cartilage proteoglycans. J Biol Chem. 1975 Sep 25;250(18):7251–7256. [PubMed] [Google Scholar]
- Hoffman P. Selective aggregation of proteoglycans with hyaluronic acid. J Biol Chem. 1979 Dec 10;254(23):11854–11860. [PubMed] [Google Scholar]
- Inerot S., Heinegård D. Bovine tracheal cartilage proteoglycans. Variations in structure and composition with age. Coll Relat Res. 1983 May;3(3):245–262. doi: 10.1016/s0174-173x(83)80007-7. [DOI] [PubMed] [Google Scholar]
- Kitchen R. G., Cleland R. L. Dilute solution properties of proteoglycan fractions from bovine nasal cartilage. Biopolymers. 1978 Mar;17(3):759–783. doi: 10.1002/bip.1978.360170316. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., De Luca S., Nilsson B., Hascall V. C., Caputo C. B., Kimura J. H., Heinegard D. Oligosaccharides on proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1980 Jul 10;255(13):6084–6091. [PubMed] [Google Scholar]
- McDevitt C. A., Muir H. Gel electrophoresis of proteoglycans and glycosaminoglycans on large-pore composite polyacrylamide-agarose gels. Anal Biochem. 1971 Dec;44(2):612–622. doi: 10.1016/0003-2697(71)90250-8. [DOI] [PubMed] [Google Scholar]
- Nilsson B., De Luca S., Lohmander S., Hascall V. C. Structures of N-linked and O-linked oligosaccharides on proteoglycan monomer isolated from the Swarm rat chondrosarcoma. J Biol Chem. 1982 Sep 25;257(18):10920–10927. [PubMed] [Google Scholar]
- Oike Y., Kimata K., Shinomura T., Suzuki S., Takahashi N., Tanabe K. A mapping technique for probing the structure of proteoglycan core molecules. J Biol Chem. 1982 Aug 25;257(16):9751–9758. [PubMed] [Google Scholar]
- Pearson J. P., Mason R. M. The stability of bovine nasal cartilage proteoglycans during isolation and storage. Biochim Biophys Acta. 1977 Jun 23;498(1):176–188. doi: 10.1016/0304-4165(77)90098-8. [DOI] [PubMed] [Google Scholar]
- Sheehan J. K., Nieduszynski I. A., Phelps C. F. Self-association of proteoglycan subunits from pig laryngeal cartilage. Biochem J. 1978 Apr 1;171(1):109–114. doi: 10.1042/bj1710109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanescu V., Maroteaux P., Sobczak E. Proteoglycan populations of baboon (Papio papio) articular cartilage. Biochem J. 1977 Apr 1;163(1):103–109. doi: 10.1042/bj1630103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanescu V., Sweet M. B. Characterization of a proteoglycan of high electrophoretic mobility. Biochim Biophys Acta. 1981 Feb 18;673(1):101–113. [PubMed] [Google Scholar]
- Swann D. A., Powell S., Sotman S. The heterogeneity of cartilage proteoglycans. Isolation of different types of proteoglycans from bovine articular cartilage. J Biol Chem. 1979 Feb 10;254(3):945–954. [PubMed] [Google Scholar]
- Thyberg J., Lohmander S., Heinegård D. Proteoglycans of hyaline cartilage: Electron-microscopic studies on isolated molecules. Biochem J. 1975 Oct;151(1):157–166. doi: 10.1042/bj1510157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triphaus G. F., Schmidt A., Buddecke E. Age-related changes in the incorporation of [35S]sulfate into two proteoglycan populations from human cartilage. Hoppe Seylers Z Physiol Chem. 1980 Dec;361(12):1773–1779. doi: 10.1515/bchm2.1980.361.2.1773. [DOI] [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of the molecular weight and molecular-weight distribution of chondroitin sulphate. J Chromatogr. 1971 Jul 8;59(1):87–97. doi: 10.1016/s0021-9673(01)80009-1. [DOI] [PubMed] [Google Scholar]
- Wieslander J., Heinegárd D. Immunochemical analysis of cartilage proteoglycans. Radioimmunoassay of the molecules and the substructures. Biochem J. 1980 Jun 1;187(3):687–694. doi: 10.1042/bj1870687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieslander J., Heinegård D. Immunochemical analysis of cartilage proteoglycans. Antigenic determinants of substructures. Biochem J. 1979 Apr 1;179(1):35–45. doi: 10.1042/bj1790035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieslander J., Heinegård D. Immunochemical analysis of cartilage proteoglycans. Cross-reactivity of molecules isolated from different species. Biochem J. 1981 Oct 1;199(1):81–87. doi: 10.1042/bj1990081. [DOI] [PMC free article] [PubMed] [Google Scholar]