Abstract
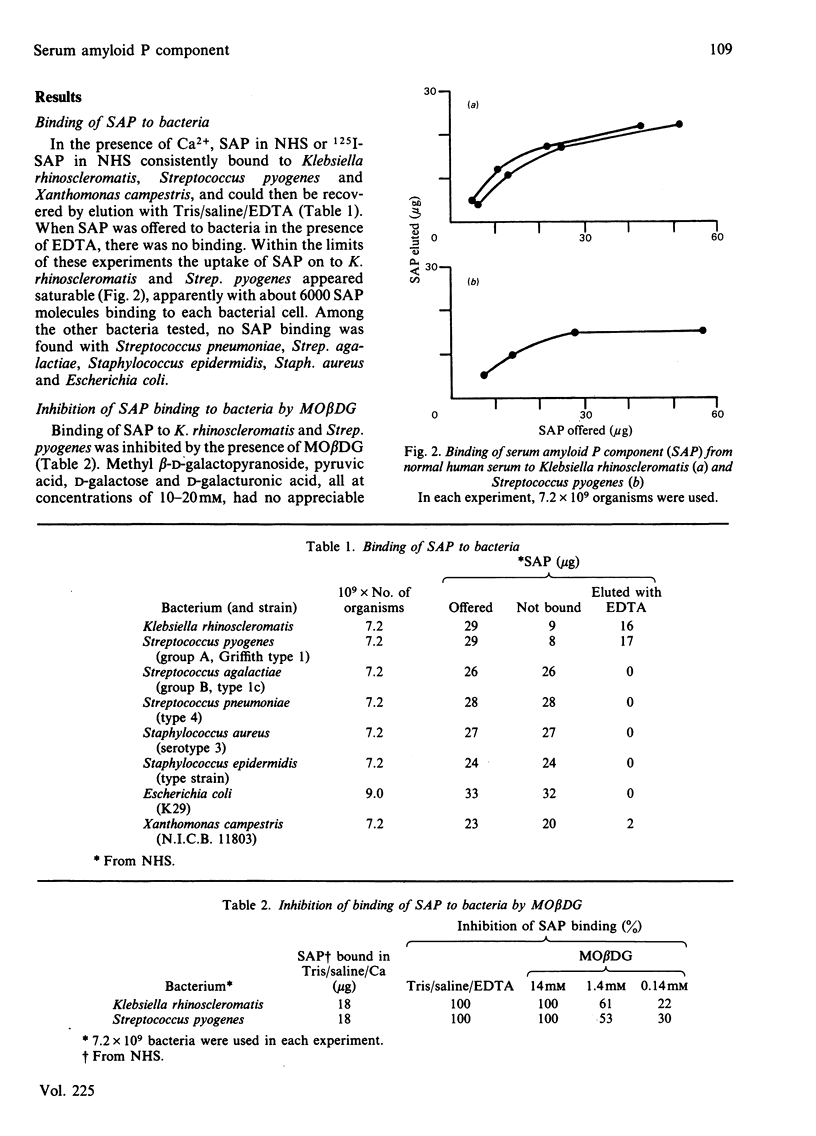
Serum amyloid P component (SAP), a normal plasma glycoprotein, has recently been shown to have Ca2+-dependent binding specificity for methyl 4,6-O-(1-carboxyethylidene)-beta-D-galactopyranoside (MO beta DG) [Hind, Collins, Renn, Cook, Caspi, Baltz & Pepys (1984) J. Exp. Med. 159, 1058-1069]. SAP was found to bind in vitro to Klebsiella rhinoscleromatis, the cell wall of which is known to contain this particular cyclic pyruvate acetal of galactose. SAP also bound in similar amounts (approx. 6000 molecules per organism) to group A Streptococcus pyogenes, but very much less was taken up on Xanthomonas campestris, which contains the 4,6-cyclic pyruvate acetal of mannose. No SAP bound to Escherichia coli, which contains the 4,6-cyclic pyruvate acetal of glucose, or to Streptococcus pneumoniae type 4, which contains the 2,3-cyclic pyruvate acetal of alpha- rather than beta-galactopyranoside, or to other organisms (Streptococcus agalactiae, Staphylococcus aureus and Staphylococcus epidermidis), the carbohydrate structures of which are less well characterized. Binding of SAP to those organisms which it did recognize was completely inhibited or reversed by millimolar concentrations of free MO beta DG. SAP, a human plasma protein, thus behaves as a lectin and may be a useful probe for its particular specific ligand in the cell walls of bacteria and other organisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltz M. L., De Beer F. C., Feinstein A., Pepys M. B. Calcium-dependent aggregation of human serum amyloid P component. Biochim Biophys Acta. 1982 Feb 18;701(2):229–236. doi: 10.1016/0167-4838(82)90118-2. [DOI] [PubMed] [Google Scholar]
- Baltz M. L., de Beer F. C., Feinstein A., Munn E. A., Milstein C. P., Fletcher T. C., March J. F., Taylor J., Bruton C., Clamp J. R. Phylogenetic aspects of C-reactive protein and related proteins. Ann N Y Acad Sci. 1982;389:49–75. doi: 10.1111/j.1749-6632.1982.tb22125.x. [DOI] [PubMed] [Google Scholar]
- De Beer F. C., Pepys M. B. Isolation of human C-reactive protein and serum amyloid P component. J Immunol Methods. 1982;50(1):17–31. doi: 10.1016/0022-1759(82)90300-3. [DOI] [PubMed] [Google Scholar]
- Hind C. R., Collins P. M., Renn D., Cook R. B., Caspi D., Baltz M. L., Pepys M. B. Binding specificity of serum amyloid P component for the pyruvate acetal of galactose. J Exp Med. 1984 Apr 1;159(4):1058–1069. doi: 10.1084/jem.159.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchcraft C. L., Gewurz H., Hansen B., Dyck R. F., Pepys M. B. Agglutination of complement-coated erythrocytes by serum amyloid P-component. J Immunol. 1981 Mar;126(3):1217–1219. [PubMed] [Google Scholar]
- Jansson P. E., Kenne L., Lindberg B. Structure of extracellular polysaccharide from Xanthomonas campestris. Carbohydr Res. 1975 Dec;45:275–282. doi: 10.1016/s0008-6215(00)85885-1. [DOI] [PubMed] [Google Scholar]
- Kamei A., Nakazawa K., Takeuchi N., Akashi S., Kagabe K. Isolation and characterization of a pyruvic acid-carrying sugar from extracellular polysaccharide of Escherichia coli 36M. J Biochem. 1977 Aug;82(2):599–602. [PubMed] [Google Scholar]
- Mold C., Nakayama S., Holzer T. J., Gewurz H., Du Clos T. W. C-reactive protein is protective against Streptococcus pneumoniae infection in mice. J Exp Med. 1981 Nov 1;154(5):1703–1708. doi: 10.1084/jem.154.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B., Baltz M. L. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Dash A. C., Fletcher T. C., Richardson N., Munn E. A., Feinstein A. Analogues in other mammals and in fish of human plasma proteins, C-reactive protein and amyloid P component. Nature. 1978 May 11;273(5658):168–170. doi: 10.1038/273168a0. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Dash A. C. Isolation of amyloid P component (protein AP) from normal serum as a calcium-dependent binding protein. Lancet. 1977 May 14;1(8020):1029–1031. doi: 10.1016/s0140-6736(77)91260-0. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Dyck R. F., de Beer F. C., Skinner M., Cohen A. S. Binding of serum amyloid P-component (SAP) by amyloid fibrils. Clin Exp Immunol. 1979 Nov;38(2):284–293. [PMC free article] [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- WHEAT R. W., DORSCH C., GODOY G. OCCURRENCE OF PYRUVIC ACID IN THE CAPSULAR POLYSACCHARIDE OF KLEBSIELLA RHINOSCLEROMATIS. J Bacteriol. 1965 Feb;89:539–539. doi: 10.1128/jb.89.2.539-539.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yother J., Volanakis J. E., Briles D. E. Human C-reactive protein is protective against fatal Streptococcus pneumoniae infection in mice. J Immunol. 1982 May;128(5):2374–2376. [PubMed] [Google Scholar]
- de Beer F. C., Baltz M. L., Holford S., Feinstein A., Pepys M. B. Fibronectin and C4-binding protein are selectively bound by aggregated amyloid P component. J Exp Med. 1981 Oct 1;154(4):1134–1139. doi: 10.1084/jem.154.4.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]


