Abstract
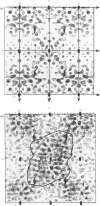
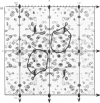
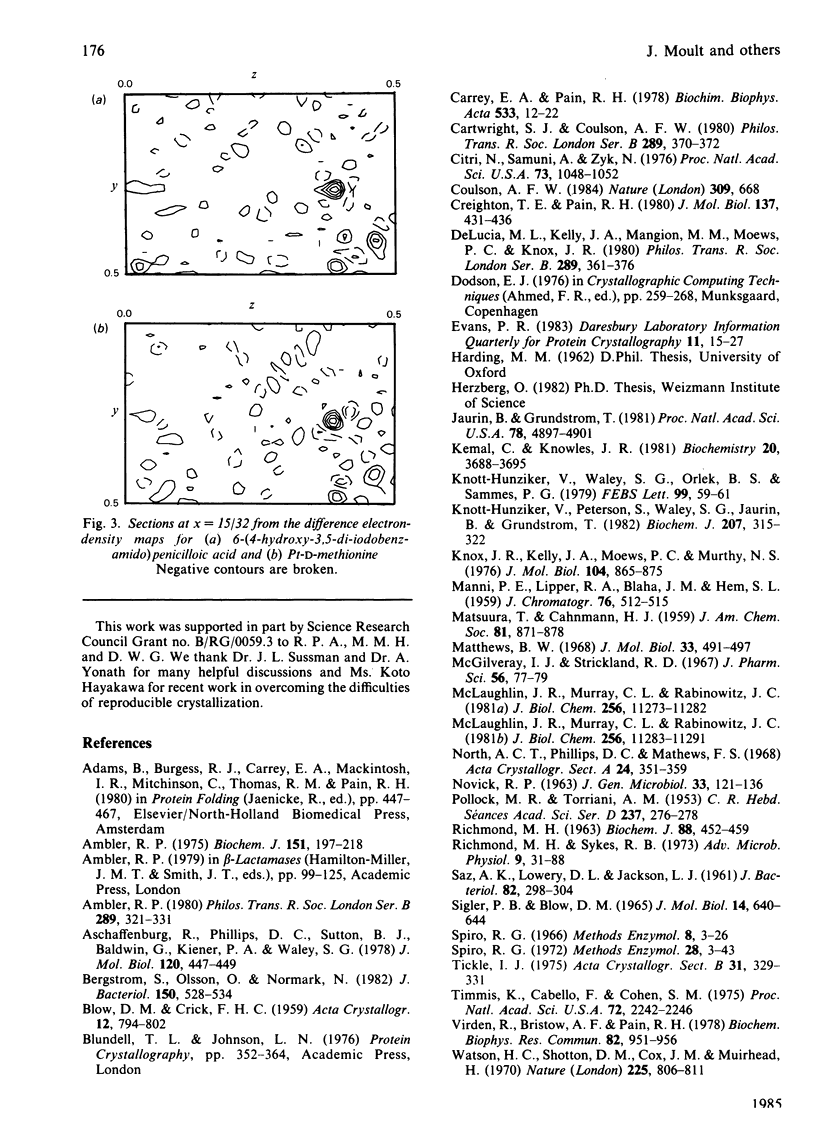
The preparation, crystallization and low-resolution structure determination of beta-lactamase (EC 3.5.2.6, 'penicillinase') from Staphylococcus aureus is described. The enzyme crystallizes in space group I222 with 1 molecule per asymmetric unit and cell dimensions a = 5.45(1), b = 9.39(1) and c = 13.87(2) nm. The structure was determined at 0.5 nm resolution by using phases calculated from (NH4)2Pt(CN)4 and KAu(CN)2 derivatives. The mean figure of merit mean value of m, for the 1106 reflexions used was 0.70. Difference Fourier syntheses for data collected from crystals soaked in platinum D-methionine and in 6-(4-hydroxy-3,5-di-iodobenzamido)penicilloic acid revealed the likely position of the active site of the enzyme.
Full text
PDF






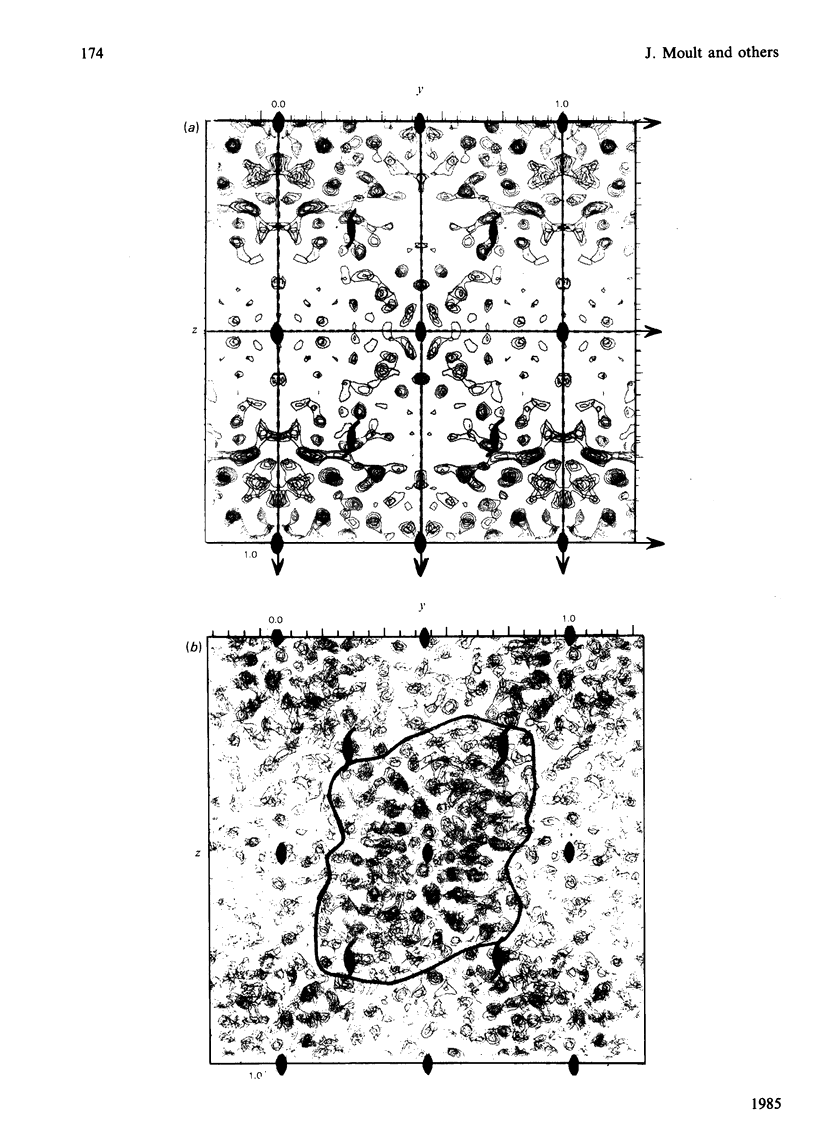
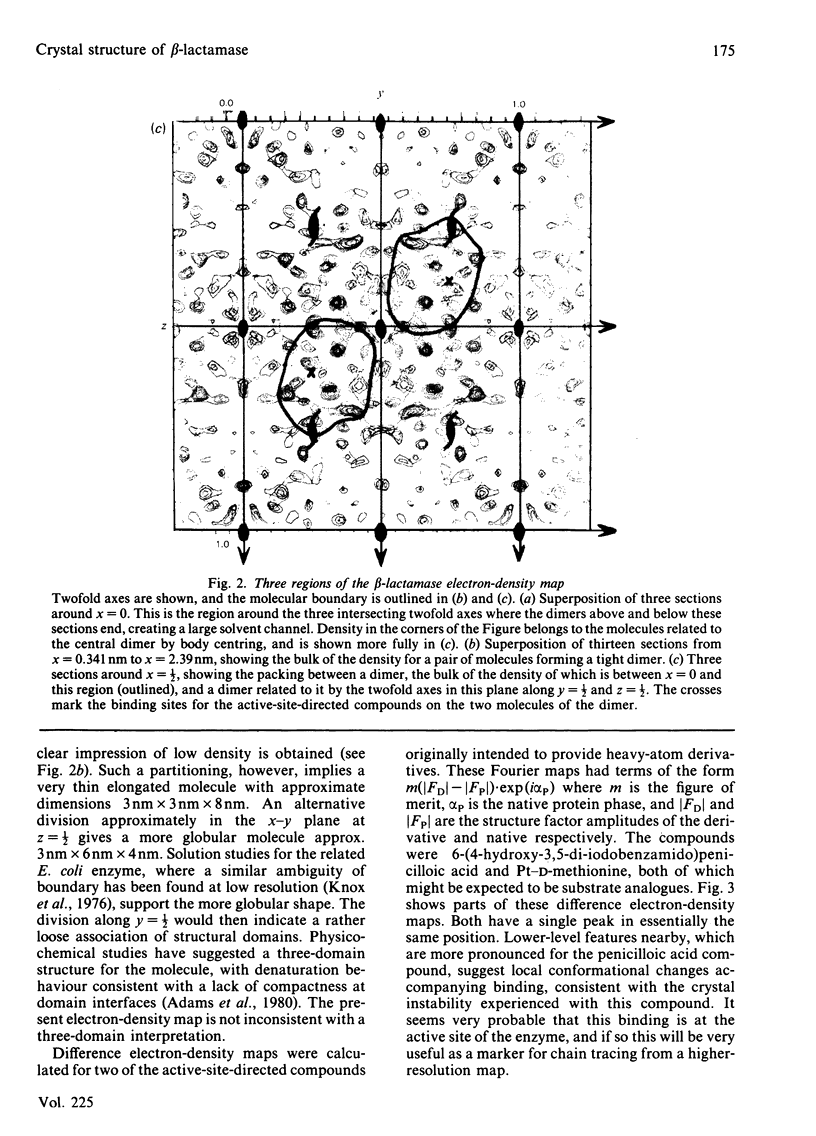
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P. The amino acid sequence of Staphylococcus aureus penicillinase. Biochem J. 1975 Nov;151(2):197–218. doi: 10.1042/bj1510197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- Aschaffenburg R., Phillips D. C., Sutton B. J., Baldwin G., Kiener P. A., Waley S. G. Preliminary crystallographic data for beta-lactamase I from Bacillus cereus 569. J Mol Biol. 1978 Apr 15;120(3):447–449. doi: 10.1016/0022-2836(78)90430-8. [DOI] [PubMed] [Google Scholar]
- Bergström S., Olsson O., Normark S. Common evolutionary origin of chromosomal beta-lactamase genes in enterobacteria. J Bacteriol. 1982 May;150(2):528–534. doi: 10.1128/jb.150.2.528-534.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrey E. A., Pain R. H. Conformation of a stable intermediate on the folding pathway of Staphylococcus aureus penicillinase. Biochim Biophys Acta. 1978 Mar 28;533(1):12–22. doi: 10.1016/0005-2795(78)90542-1. [DOI] [PubMed] [Google Scholar]
- Cartwright S. J., Coulson A. F. Active site of staphylococcal beta-lactamase. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):370–372. [PubMed] [Google Scholar]
- Citri N., Samuni A., Zyk N. Acquisition of substrate-specific parameters during the catalytic reaction of penicillinase. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1048–1052. doi: 10.1073/pnas.73.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson A. F. Microbiology: proteins that bind the beta-lactam antibiotics. Nature. 1984 Jun 21;309(5970):668–668. doi: 10.1038/309668a0. [DOI] [PubMed] [Google Scholar]
- Creighton T. E., Pain R. H. Unfolding and refolding of Staphylococcus aureus penicillinase by urea-gradient electrophoresis. J Mol Biol. 1980 Mar 15;137(4):431–436. doi: 10.1016/0022-2836(80)90167-9. [DOI] [PubMed] [Google Scholar]
- DeLucia M. L., Kelly J. A., Mangion M. M., Moews P. C., Knox J. R. Tertiary and secondary structure analysis of penicillin-binding proteins. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):374–376. [PubMed] [Google Scholar]
- Jaurin B., Grundström T. ampC cephalosporinase of Escherichia coli K-12 has a different evolutionary origin from that of beta-lactamases of the penicillinase type. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4897–4901. doi: 10.1073/pnas.78.8.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemal C., Knowles J. R. Penicillanic acid sulfone: interaction with RTEM beta-lactamase from Escherichia coli at different pH values. Biochemistry. 1981 Jun 23;20(13):3688–3695. doi: 10.1021/bi00516a004. [DOI] [PubMed] [Google Scholar]
- Knott-Hunziker V., Petursson S., Waley S. G., Jaurin B., Grundström T. The acyl-enzyme mechanism of beta-lactamase action. The evidence for class C Beta-lactamases. Biochem J. 1982 Nov 1;207(2):315–322. doi: 10.1042/bj2070315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott-Hunziker V., Waley S. G., Orlek B. S., Sammes P. G. Penicillinase active sites: labelling of serine-44 in beta-lactamase I by 6beta-bromopenicillanic acid. FEBS Lett. 1979 Mar 1;99(1):59–61. doi: 10.1016/0014-5793(79)80248-3. [DOI] [PubMed] [Google Scholar]
- Knox J. R., Kelly J. A., Moews P. C., Murthy N. S. 5-5A crystallographic structure of penicillin beta-lactamase and radius of gyration in solution. J Mol Biol. 1976 Jul 15;104(4):865–875. doi: 10.1016/0022-2836(76)90187-x. [DOI] [PubMed] [Google Scholar]
- Manni P. E., Lipper R. A., Blaha J. M., Hem S. L. Analysis of potassium penicillin G and its degradation products by thin-layer chromatography. J Chromatogr. 1973 Feb 28;76(2):512–515. doi: 10.1016/s0021-9673(01)96943-2. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- McGilveray I. J., Strickland R. D. Detection and separation of penicillins by thin-layer chromatography. J Pharm Sci. 1967 Jan;56(1):77–79. doi: 10.1002/jps.2600560116. [DOI] [PubMed] [Google Scholar]
- McLaughlin J. R., Murray C. L., Rabinowitz J. C. Unique features in the ribosome binding site sequence of the gram-positive Staphylococcus aureus beta-lactamase gene. J Biol Chem. 1981 Nov 10;256(21):11283–11291. [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R., TORRIANI A. M. Purification et caractéristiques physicochimiques de la pénicillinase de Bacillus cereus. C R Hebd Seances Acad Sci. 1953 Jul 20;237(3):276–278. [PubMed] [Google Scholar]
- RICHMOND M. H. PURIFICATION AND PROPERTIES OF THE EXOPENICILLINASE FROM STAPHYLOCOCCUS AUREUS. Biochem J. 1963 Sep;88:452–459. doi: 10.1042/bj0880452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- SAZ A. K., LOWERY D. L., JACKSON L. J. Staphylococcal penicillinase. I. Inhibition and stimulation of activity. J Bacteriol. 1961 Aug;82:298–304. doi: 10.1128/jb.82.2.298-304.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigler P. B., Blow D. M. A means of promoting heavy-atom binding in protein crystals. J Mol Biol. 1965 Dec;14(2):640–644. doi: 10.1016/s0022-2836(65)80220-0. [DOI] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Cloning, isolation, and characterization of replication regions of complex plasmid genomes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2242–2246. doi: 10.1073/pnas.72.6.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virden R., Bristow A. F., Pain R. H. Reversible inhibition of penicillinase by quinacillin: evaluation of mechanisms involving two conformational states of the enzyme. Biochem Biophys Res Commun. 1978 Jun 14;82(3):951–956. doi: 10.1016/0006-291x(78)90875-6. [DOI] [PubMed] [Google Scholar]
- Watson H. C., Shotton D. M., Cox J. M., Muirhead H. Three-dimensional Fourier synthesis of tosyl-elastase at 3.5 å resolution. Nature. 1970 Feb 28;225(5235):806–811. doi: 10.1038/225806a0. [DOI] [PubMed] [Google Scholar]