Abstract
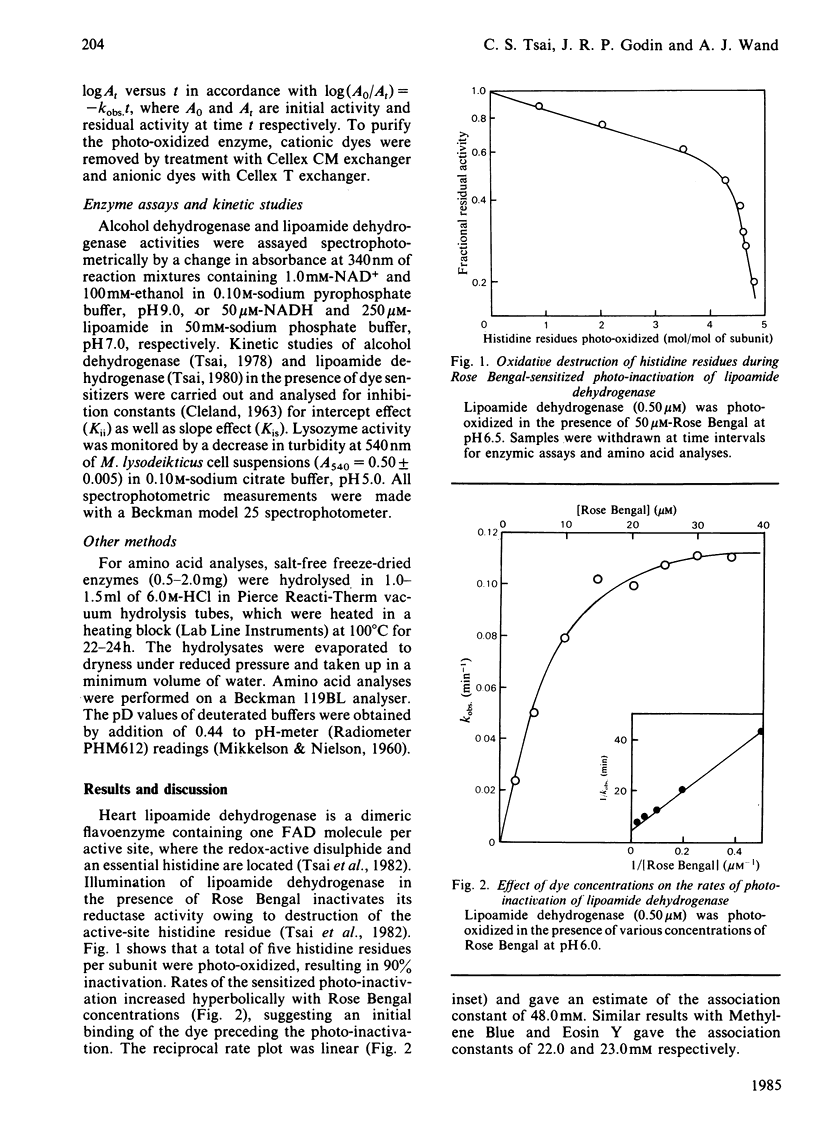
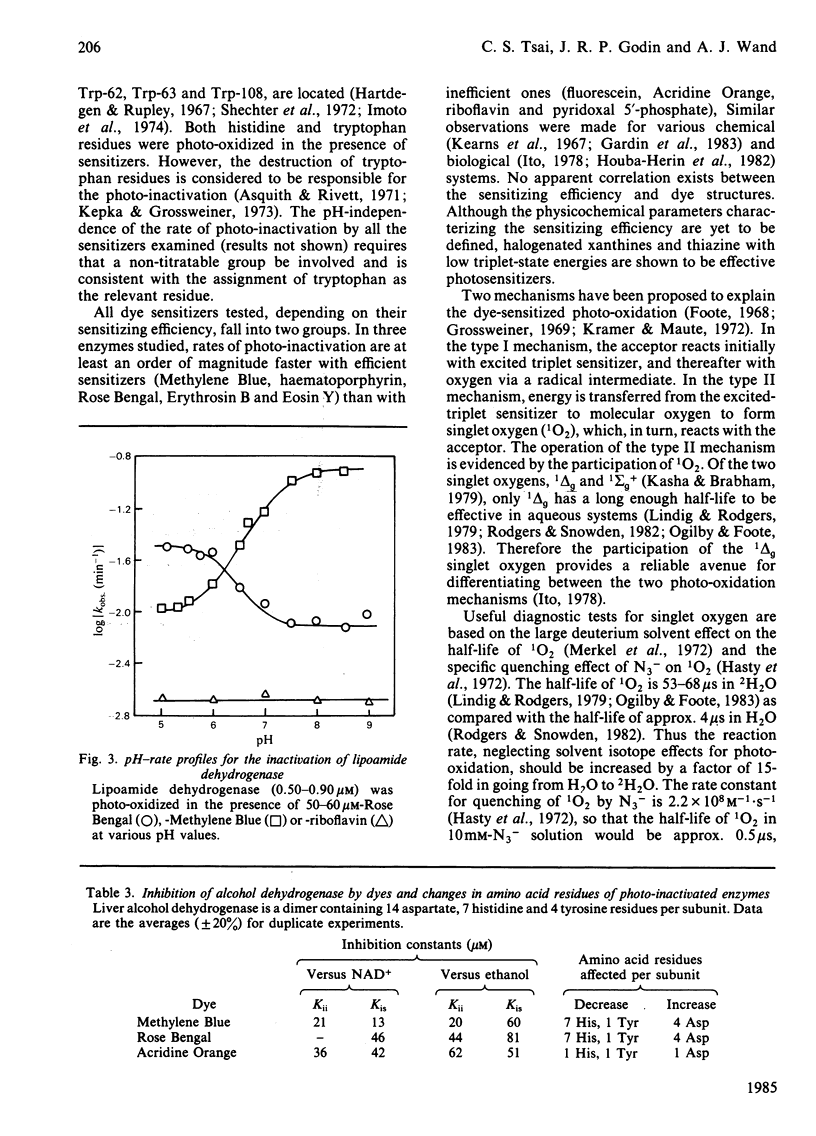
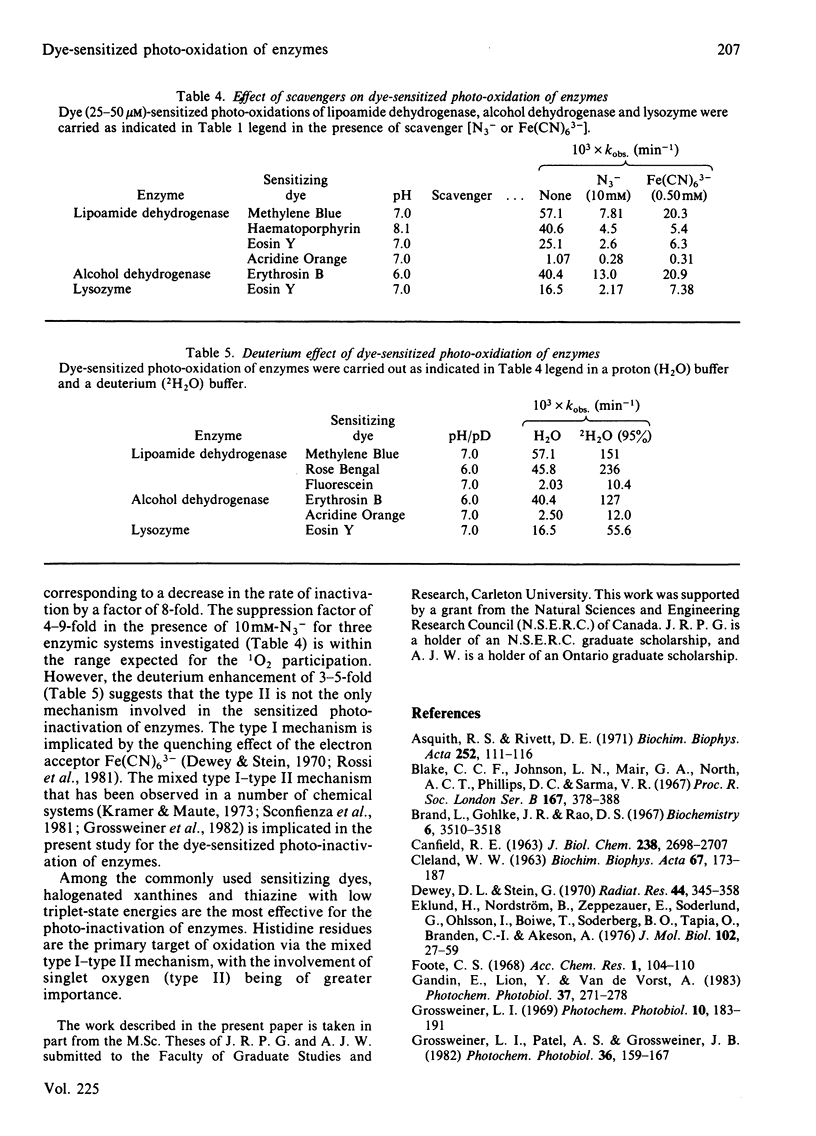
Heart lipoamide dehydrogenase, liver alcohol dehydrogenase and egg-white lysozyme are photo-oxidized in the presence of various dye sensitizers. The photodynamic process is preceded by the binding between the enzyme and the sensitizers. Among the commonly used dyes, halogenated xanthines and thiazine are effective sensitizers for the photo-inactivation of these three enzymes. Histidine residues are the primary target for the sensitized photo-oxidation that inactivates lipoamide dehydrogenase and alcohol dehydrogenase. However, the destruction of tryptophan residues is responsible for the photo-inactivation of lysozyme. The deuterium medium effect and the quenching effect by various scavengers of the potential photo-oxidative intermediates implicate the participation of the mixed type I-type II mechanism, with the involvement of singlet oxygen being of greater importance, in the photo-inactivation of the enzymes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asquith R. S., Rivett D. E. Studies on the photooxidation of tryptophan. Biochim Biophys Acta. 1971 Oct;252(1):111–116. doi: 10.1016/0304-4165(71)90098-5. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Johnson L. N., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Crystallographic studies of the activity of hen egg-white lysozyme. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):378–388. doi: 10.1098/rspb.1967.0035. [DOI] [PubMed] [Google Scholar]
- Brand L., Gohlke J. R., Rao D. S. Evidence for binding of rose bengal and anilinonaphthalenesulfonates at the active site regions of liver alcohol dehydrogenase. Biochemistry. 1967 Nov;6(11):3510–3518. doi: 10.1021/bi00863a024. [DOI] [PubMed] [Google Scholar]
- CANFIELD R. E. THE AMINO ACID SEQUENCE OF EGG WHITE LYSOZYME. J Biol Chem. 1963 Aug;238:2698–2707. [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- Dewey D. L., Stein G. The action of atomic hydrogen, hydrated electrons, and ionizing radiation on bacteriophage T7 in aqueous solution. Radiat Res. 1970 Nov;44(2):345–358. [PubMed] [Google Scholar]
- Eklund H., Nordström B., Zeppezauer E., Söderlund G., Ohlsson I., Boiwe T., Söderberg B. O., Tapia O., Brändén C. I., Akeson A. Three-dimensional structure of horse liver alcohol dehydrogenase at 2-4 A resolution. J Mol Biol. 1976 Mar 25;102(1):27–59. doi: 10.1016/0022-2836(76)90072-3. [DOI] [PubMed] [Google Scholar]
- Grossweiner L. I. Molecular mechanisms in photodynamic action. Photochem Photobiol. 1969 Sep;10(3):183–191. doi: 10.1111/j.1751-1097.1969.tb05678.x. [DOI] [PubMed] [Google Scholar]
- Grossweiner L. I., Patel A. S., Grossweiner J. B. Type I and type II mechanisms in the photosensitized lysis of phosphatidylcholine liposomes by hematoporphyrin. Photochem Photobiol. 1982 Aug;36(2):159–167. doi: 10.1111/j.1751-1097.1982.tb04358.x. [DOI] [PubMed] [Google Scholar]
- Hartdegen F. J., Rupley J. A. The oxidation by iodine of tryptophan 108 in lysozyme. J Am Chem Soc. 1967 Mar 29;89(7):1743–1745. doi: 10.1021/ja00983a044. [DOI] [PubMed] [Google Scholar]
- Houba-Herin N., Calberg-Bacq C. M., Piette J., Van de Vorst A. Mechanisms for dye-mediated photodynamic action: singlet oxygen production, deoxyguanosine oxidation and phage inactivating efficiencies. Photochem Photobiol. 1982 Sep;36(3):297–306. doi: 10.1111/j.1751-1097.1982.tb04378.x. [DOI] [PubMed] [Google Scholar]
- Imoto T., Fujimoto M., Yagishita K. The role of tryptophan-62 in the enzymic reaction of lysozyme. J Biochem. 1974 Oct;76(4):745–753. [PubMed] [Google Scholar]
- Ito T. Cellular and subcellular mechanisms of photodynamic action: the 1O2 hypothesis as a driving force in recent research. Photochem Photobiol. 1978 Oct-Nov;28(4-5):493–508. doi: 10.1111/j.1751-1097.1978.tb06957.x. [DOI] [PubMed] [Google Scholar]
- Kepka A. G., Grossweiner L. I. Photodynamic inactivation of lysozyme by eosin. Photochem Photobiol. 1973 Jul;18(1):49–61. doi: 10.1111/j.1751-1097.1973.tb06392.x. [DOI] [PubMed] [Google Scholar]
- Nihira T., Toraya T., Fukui S. Pyridoxal-5'-phosphate-sensitized photoinactivation of tryptophanase and evidence for essential histidyl residues in the active sites. Eur J Biochem. 1979 Nov;101(2):341–347. doi: 10.1111/j.1432-1033.1979.tb19726.x. [DOI] [PubMed] [Google Scholar]
- REED L. J., KOIKE M., LEVITCH M. E., LEACH F. R. Studies on the nature and reactions of protein-bound lipoic acid. J Biol Chem. 1958 May;232(1):143–158. [PubMed] [Google Scholar]
- Schnuriger B., Bourdon J., Bedu J. Photosensitized oxidation through stearate monomolecular films. Photochem Photobiol. 1968 Nov;8(5):361–368. doi: 10.1111/j.1751-1097.1968.tb05881.x. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Burstein Y., Patchornik A. Sulfenylation of tryptophan-62 in hen egg-white lysozyme. Biochemistry. 1972 Feb 15;11(4):653–660. doi: 10.1021/bi00754a030. [DOI] [PubMed] [Google Scholar]
- Tomita M., Irie M., Ukita T. Sensitized photooxidation of histidine and its derivatives. Products and mechanism of the reaction. Biochemistry. 1969 Dec;8(12):5149–5160. doi: 10.1021/bi00840a069. [DOI] [PubMed] [Google Scholar]
- Tsai C. S. Kinetic and mechanistic studies of methylated liver alcohol dehydrogenase. Biochem J. 1978 Aug 1;173(2):483–496. doi: 10.1042/bj1730483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. S. Kinetic studies of multifunctional reactions catalysed by lipoamide dehydrogenase. Int J Biochem. 1980;11(5):407–413. doi: 10.1016/0020-711x(80)90311-0. [DOI] [PubMed] [Google Scholar]
- Tsai C. S., Wand A. J., Godin J. R., Buchanan G. W. Multifunctionality of lipoamide dehydrogenase: role of histidine residues in reductase reaction. Arch Biochem Biophys. 1982 Sep;217(2):721–729. doi: 10.1016/0003-9861(82)90553-7. [DOI] [PubMed] [Google Scholar]
- WEIL L., SEIBLES T. S. Photooxidation of crystalline ribonuclease in the presence of methylene blue. Arch Biochem Biophys. 1955 Feb;54(2):368–377. doi: 10.1016/0003-9861(55)90049-7. [DOI] [PubMed] [Google Scholar]


