Abstract
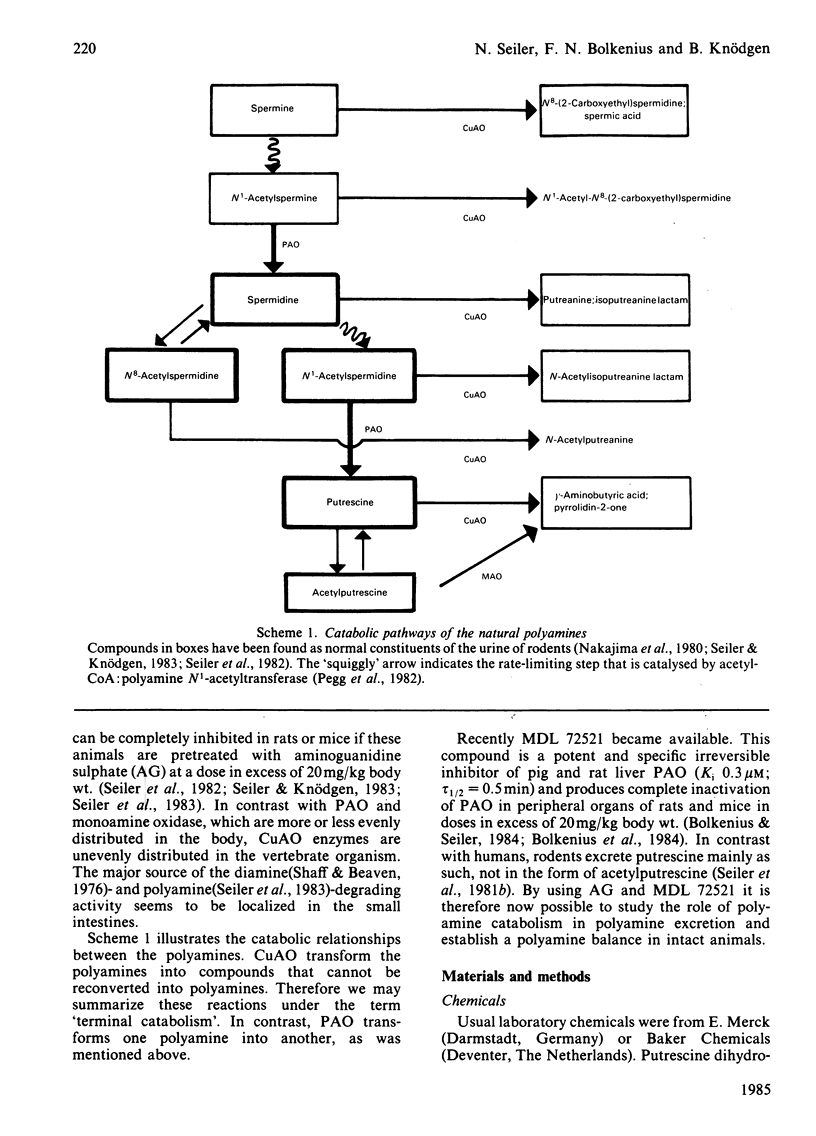
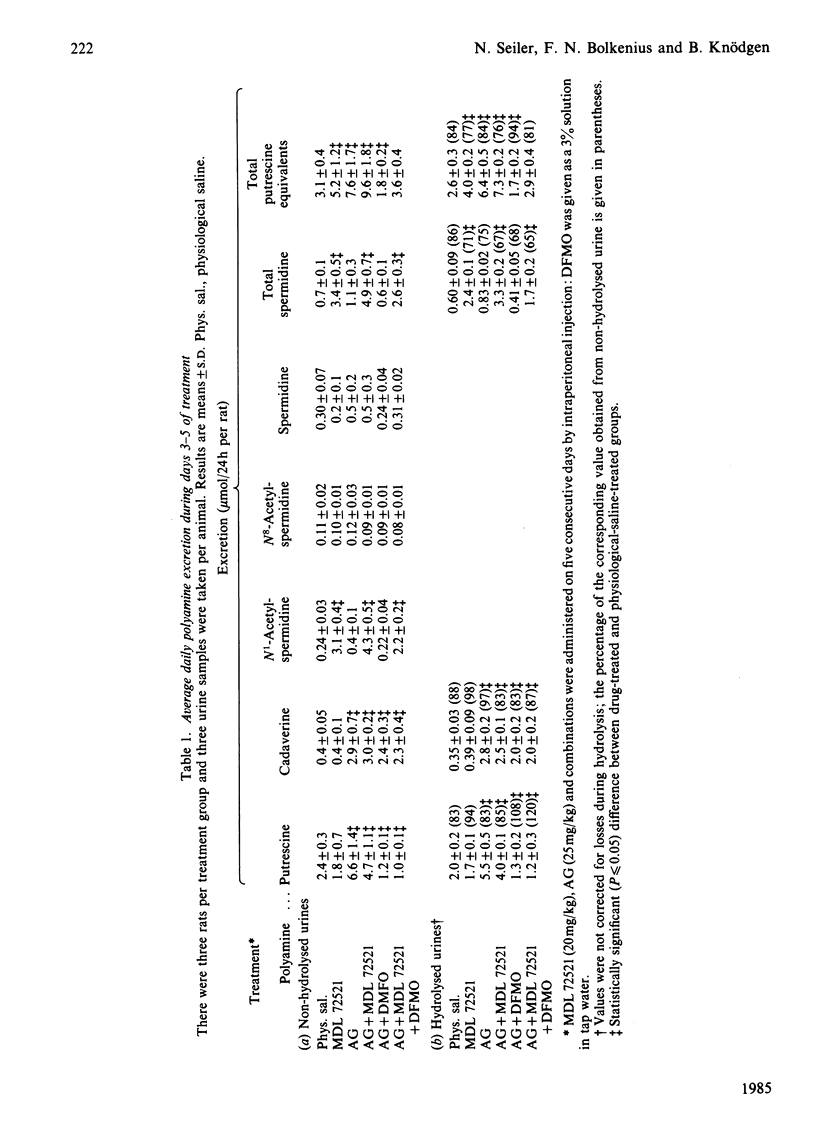
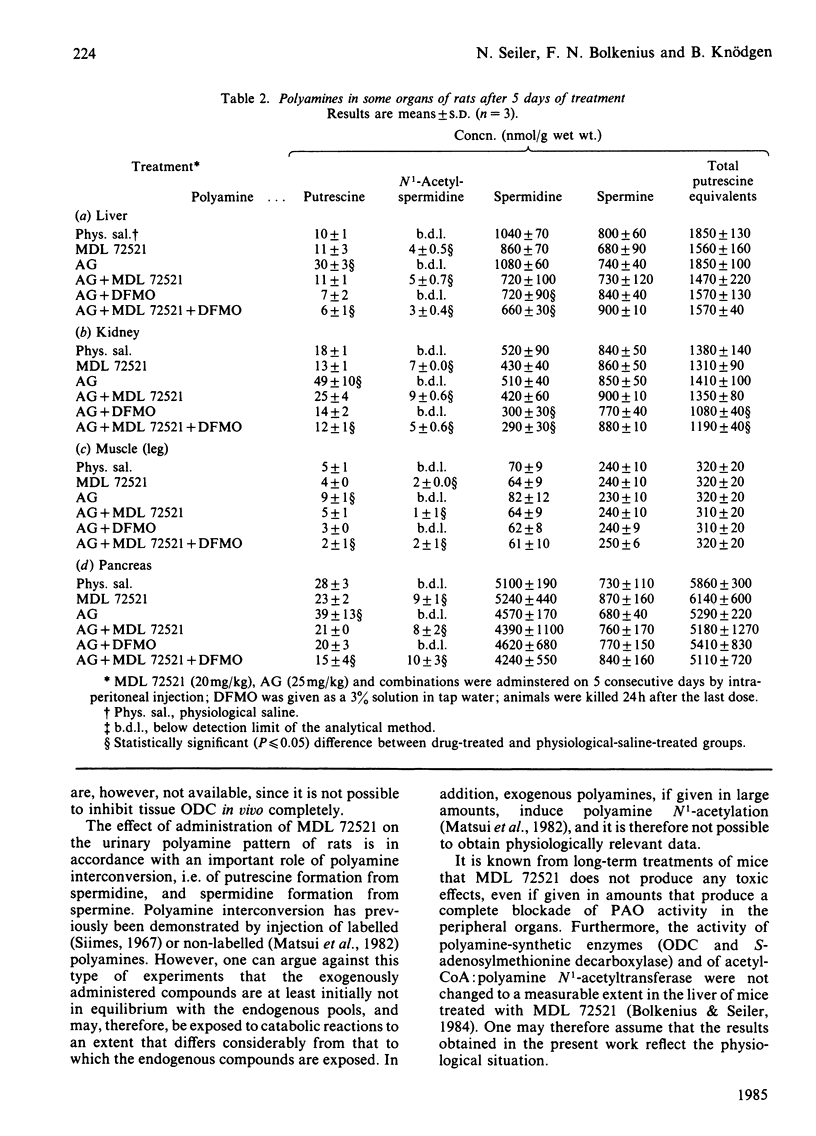
Complete inhibition of polyamine catabolism is possible by combined administration of two compounds. Aminoguanidine (25 mg/kg body wt., intraperitoneally) inhibits all reactions that are catalysed by copper-containing amine oxidases (CuAO). The products of the CuAO-catalysed reactions cannot be reconverted into polyamines (terminal catabolism) and therefore usually escape observation. N1-Methyl-N2-(buta-2,3-dienyl)butane-1,4-diamine (MDL 72521) is a new inhibitor of polyamine oxidase. It inhibits completely the degradation of N1-acetylspermidine and N1-acetylspermine. The enhanced excretion of N1-acetylspermidine in urine after administration of 20 mg of MDL 72521/day per kg body wt. is a measure of the rate of spermidine degradation in vivo to putrescine, and thus of the quantitative significance of the interconversion pathway. From the enhancement of total polyamine excretion by aminoguanidine-treated rats, one can calculate that only about 40% of the polyamines that are destined for elimination are usually observed in the urine, the other 60% being catabolized along the CuAO-catalysed pathways. The normally observed urinary polyamine pattern gives, therefore, an unsatisfactory picture of the actual polyamine elimination. Although aminoguanidine alone is sufficient to block terminal polyamine catabolism, rats that were treated with a combination of aminoguanidine and MDL 72521 excrete more polyamines than those that received aminoguanidine alone. The reason is that a certain proportion of putrescine, which is formed by degradation of spermidine, is normally reutilized for polyamine biosynthesis. In MDL 72521-treated animals this proportion appears in the urine in the form of N1-acetylspermidine. Thus it is possible to determine polyamine interconversion and re-utilization in vivo and to establish a polyamine balance in intact rats by using specific inhibitors of the CuAO and of polyamine oxidase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antrup H., Seiler N. On the turnover of polyamines spermidine and spermine in mouse brain and other organs. Neurochem Res. 1980 Feb;5(2):123–143. doi: 10.1007/BF00964327. [DOI] [PubMed] [Google Scholar]
- Bolkenius F. N., Seiler N. Acetylderivatives as intermediates in polyamine catabolism. Int J Biochem. 1981;13(3):287–292. doi: 10.1016/0020-711x(81)90080-x. [DOI] [PubMed] [Google Scholar]
- Gahl W. A., Pitot H. C. Acetylated polyamines as substrates for human pregnancy serum diamine oxidase. Life Sci. 1981 Nov 23;29(21):2177–2179. doi: 10.1016/0024-3205(81)90488-4. [DOI] [PubMed] [Google Scholar]
- Gahl W. A., Pitot H. C. Polyamine degradation in foetal and adult bovine serum. Biochem J. 1982 Mar 15;202(3):603–611. doi: 10.1042/bj2020603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G. Spermine oxidase: an amine oxidase with specificity for spermine and spermidine. J Exp Med. 1953 Mar;97(3):345–355. doi: 10.1084/jem.97.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä E. Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase. Biochemistry. 1977 Jan 11;16(1):91–100. doi: 10.1021/bi00620a015. [DOI] [PubMed] [Google Scholar]
- Matsui I., Pösö H., Pegg A. E. Conversion of exogenous spermidine into putrescine after administration to rats. Biochim Biophys Acta. 1982 Nov 24;719(2):199–207. doi: 10.1016/0304-4165(82)90089-7. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Noto T., Kato N. Isolation and identification of polyamine metabolites in urine of animals. Physiol Chem Phys. 1980;12(5):401–410. [PubMed] [Google Scholar]
- Pegg A. E., McGill S. Decarboxylation of ornithine and lysine in rat tissues. Biochim Biophys Acta. 1979 Jun 6;568(2):416–427. doi: 10.1016/0005-2744(79)90310-3. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Seely J. E., Pösö H., della Ragione F., Zagon I. A. Polyamine biosynthesis and interconversion in rodent tissues. Fed Proc. 1982 Dec;41(14):3065–3072. [PubMed] [Google Scholar]
- Russell D. H. Clinical relevance of polyamines. Crit Rev Clin Lab Sci. 1983;18(3):261–311. doi: 10.3109/10408368209085073. [DOI] [PubMed] [Google Scholar]
- Scalabrino G., Ferioli M. E. Polyamines in mammalian tumors. Part II. Adv Cancer Res. 1982;36:1–102. doi: 10.1016/s0065-230x(08)60422-4. [DOI] [PubMed] [Google Scholar]
- Seiler N., Bolkenius F. N., Knödgen B., Haegele K. The determination of N1-acetylspermine in mouse liver. Biochim Biophys Acta. 1981 Aug 5;676(1):1–7. doi: 10.1016/0304-4165(81)90002-7. [DOI] [PubMed] [Google Scholar]
- Seiler N., Bolkenius F. N., Knödgen B., Mamont P. Polyamine oxidase in rat tissues. Biochim Biophys Acta. 1980 Oct;615(2):480–488. doi: 10.1016/0005-2744(80)90514-8. [DOI] [PubMed] [Google Scholar]
- Seiler N., Bolkenius F. N., Rennert O. M. Interconversion, catabolism and elimination of the polyamines. Med Biol. 1981 Dec;59(5-6):334–346. [PubMed] [Google Scholar]
- Seiler N., Knödgen B. Determination of the naturally occurring monoacetyl derivatives of di- and polyamines. J Chromatogr. 1979 Oct 11;164(2):155–168. doi: 10.1016/s0378-4347(00)81184-6. [DOI] [PubMed] [Google Scholar]
- Seiler N., Knödgen B., Haegele K. N-(3-aminopropyl)pyrrolidin-2-one, a product of spermidine catabolism in vivo. Biochem J. 1982 Oct 15;208(1):189–197. doi: 10.1042/bj2080189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N., Knödgen B. High-performance liquid chromatographic procedure for the simultaneous determination of the natural polyamines and their monoacetyl derivatives. J Chromatogr. 1980 Dec 12;221(2):227–235. doi: 10.1016/s0378-4347(00)84307-8. [DOI] [PubMed] [Google Scholar]
- Seiler N., Knödgen B. N-(3-aminopropyl)pyrrolidin-2-one: a physiological excretory product deriving from spermidine. Int J Biochem. 1983;15(7):907–915. doi: 10.1016/0020-711x(83)90166-0. [DOI] [PubMed] [Google Scholar]
- Shaff R. E., Beaven M. A. Turnover and synthesis of diamine oxidase (DAO) in rat tissues. Studies with heparin and cycloheximide. Biochem Pharmacol. 1976 May 1;25(9):1057–1062. doi: 10.1016/0006-2952(76)90496-2. [DOI] [PubMed] [Google Scholar]
- Siimes M. Studies on the metabolism of 1,4-14C-spermidine and 1,4-14C-spermine in the rat. Acta Physiol Scand Suppl. 1967;298:1–66. [PubMed] [Google Scholar]
- Suzuki O., Matsumoto T., Oya M., Katsumata Y. Metabolism of acetylpolyamines by monoamine oxidase, diamine oxidase and polyamine oxidase. Biochim Biophys Acta. 1981 Oct 12;677(2):190–193. doi: 10.1016/0304-4165(81)90084-2. [DOI] [PubMed] [Google Scholar]


