Abstract
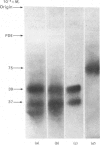
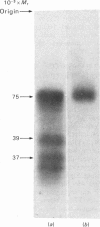
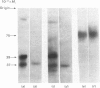
Light-activated hydrolysis of cyclic GMP is achieved through the photoexcitation of rhodopsin, a process which then triggers the replacement of GDP for GTP by a retinal guanosine 5'-triphosphatase referred to as 'transducin'. The transducin-GTP complex then switches on the phosphodiesterase [Fung, Hurley & Stryer (1981) Proc. Natl. Acad. Sci. U.S.A. 78, 152-156]. The bovine transducin consists of an alpha-subunit (39000 Mr), which is a GTP-binding component, together with a beta-(37000 Mr) and a gamma-subunit (10000 Mr). We have purified retinal transducin from cow, pig, chick and frog. The enzyme specific activities and sodium dodecyl sulphate/polyacrylamide-gel-electrophoretic profiles indicate that this enzyme is similar in all species except the frog. Whereas the bovine, pig and chick transducins consist of major 37000- and 39000-Mr components, that of the frog consists of a single 75000-Mr component. Labelling of the GTP-binding components with the photoaffinity label 8-azidoguanosine [gamma-32P]triphosphate demonstrated that the 37000-Mr components of the cow, pig and chick and the 75000-Mr component of the frog were major GTP-binding components. In addition, peptide maps of radioiodinated tryptic peptides indicate that the frog 75000-Mr protein is highly related to the pig transducin. These results demonstrate evolutionary conservation of retinal transducin and the presence of a higher-Mr, but nonetheless highly conserved form, of transducin in the frog. The relationship of this component to the recently reported rod-outer-segment inhibitor protein [Yamazaki, Stein, Chernoff & Bitensky (1983) J. Biol. Chem. 258, 8188-8194] is discussed.
Full text
PDF

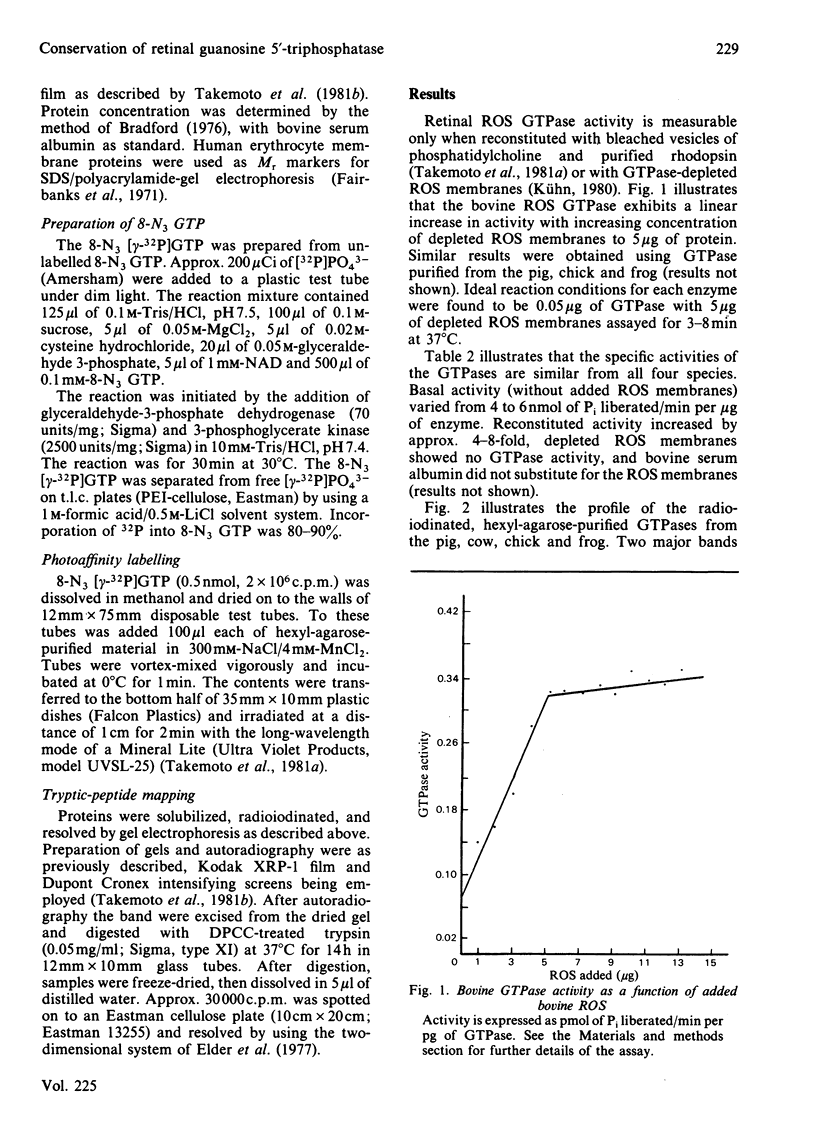
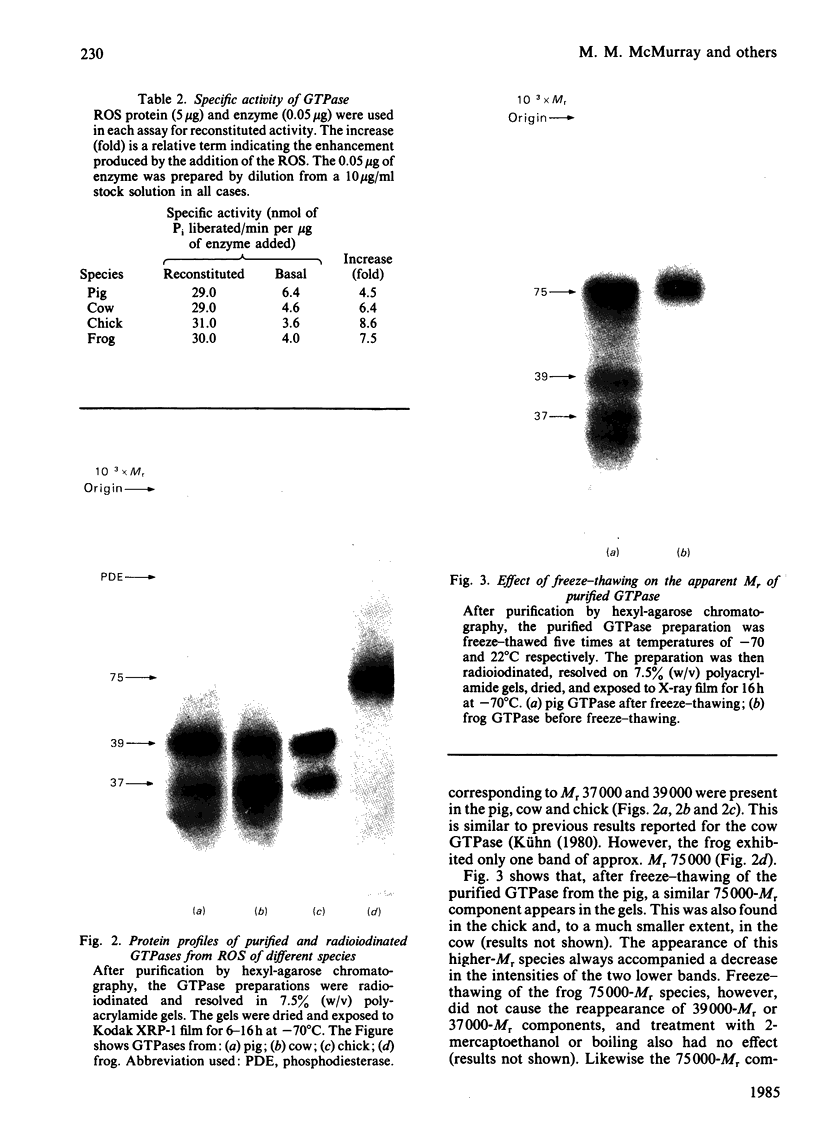
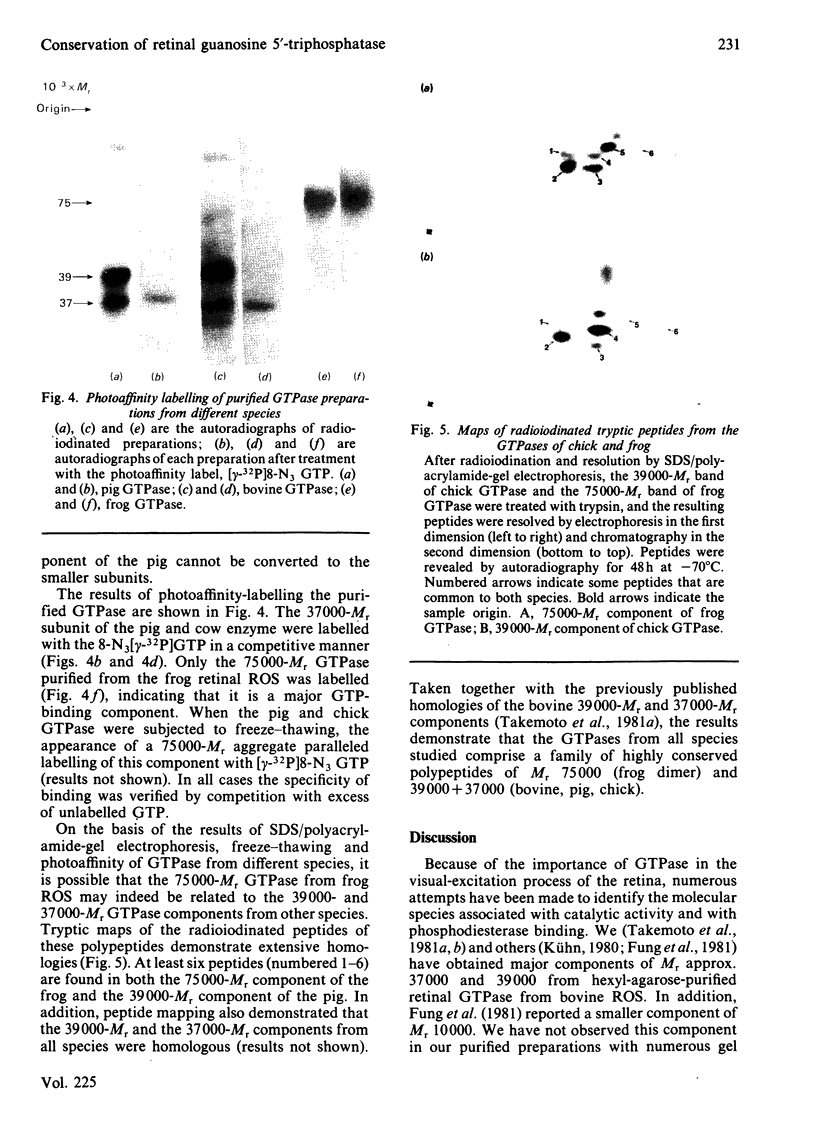

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abood M. E., Hurley J. B., Pappone M. C., Bourne H. R., Stryer L. Functional homology between signal-coupling proteins. Cholera toxin inactivates the GTPase activity of transducin. J Biol Chem. 1982 Sep 25;257(18):10540–10543. [PubMed] [Google Scholar]
- Baehr W., Devlin M. J., Applebury M. L. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979 Nov 25;254(22):11669–11677. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fung B. K. Characterization of transducin from bovine retinal rod outer segments. I. Separation and reconstitution of the subunits. J Biol Chem. 1983 Sep 10;258(17):10495–10502. [PubMed] [Google Scholar]
- Fung B. K., Hurley J. B., Stryer L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci U S A. 1981 Jan;78(1):152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. B. Isolation and recombination of bovine rod outer segment cGMP phosphodiesterase and its regulators. Biochem Biophys Res Commun. 1980 Jan 29;92(2):505–510. doi: 10.1016/0006-291x(80)90362-9. [DOI] [PubMed] [Google Scholar]
- Kühn H. Light- and GTP-regulated interaction of GTPase and other proteins with bovine photoreceptor membranes. Nature. 1980 Feb 7;283(5747):587–589. doi: 10.1038/283587a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manning D. R., Gilman A. G. The regulatory components of adenylate cyclase and transducin. A family of structurally homologous guanine nucleotide-binding proteins. J Biol Chem. 1983 Jun 10;258(11):7059–7063. [PubMed] [Google Scholar]
- Takemoto D. J., Haley B. E., Hansen J., Pinkett O., Takemoto L. J. GTPase from rod outer segments: characterization by photoaffinity labeling and tryptic peptide mapping. Biochem Biophys Res Commun. 1981 Sep 16;102(1):341–347. doi: 10.1016/0006-291x(81)91527-8. [DOI] [PubMed] [Google Scholar]
- Wheeler G. L., Bitensky M. W. A light-activated GTPase in vertebrate photoreceptors: regulation of light-activated cyclic GMP phosphodiesterase. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4238–4242. doi: 10.1073/pnas.74.10.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki A., Stein P. J., Chernoff N., Bitensky M. W. Activation mechanism of rod outer segment cyclic GMP phosphodiesterase. Release of inhibitor by the GTP/GTP-binding protein. J Biol Chem. 1983 Jul 10;258(13):8188–8194. [PubMed] [Google Scholar]