Abstract
Regulated cell death by a non-apoptotic pathway known as parthanatos is increasingly recognised as a central player in pathological processes, including ischaemic tissue damage and neurodegenerative diseases. Parthanatos is activated under conditions that induce high levels of DNA damage, leading to hyperactivation of the DNA damage sensor PARP1. While this strict dependence on PARP1 activation is a defining feature of parthanatos that distinguishes it from other forms of cell death, the molecular events downstream of PARP1 activation remain poorly understood. In this mini-review, we highlight a number of important questions that remain to be answered about this enigmatic form of cell death.
Keywords: Cell death, parthanatos, PARP1, PARG, AIF
Introduction
ADP-ribosylation (ADPr) is a covalent modification of biological macromolecules catalysed by members of the ADP-ribosyltransferase family, which transfer ADP-ribose moieties from NAD+ (nicotinamide adenine dinucleotide) to target proteins or nucleic acids (Hoch and Polo 2019; Luscher et al., 2021; Suskiewicz et al., 2023). ADP-ribosyltransferases can be subdivided based on the nature of the ADPr modification they catalyse, which can be in the form of single ADP-ribose units, termed mono(ADP-ribose) (MAR) or as long and sometimes branched chains of poly(ADP-ribose) (PAR). The main human PAR-catalysing enzyme is poly(ADP-ribose) polymerase 1 (PARP1), which is a highly abundant nuclear protein that consists of three DNA-binding zinc finger domains (ZnF1, ZnF2, and ZnF3), a central BRCA1 C-terminal (BRCT) domain, a DNA-binding WGR (tryptophan, glycine, arginine) domain and a bipartite C-terminal catalytic domain composed of an auto-inhibitory helical subdomain (HD) and an ADP-ribosyl transferase (ART) subdomain. PARP1 plays central roles in the cellular response to DNA damage, due to the high affinity and specificity of its DNA-binding domains for DNA strand breaks, which lead to rapid and robust activation of PARP1 catalytic activity in response to a variety of DNA lesions (Pandey and Black 2021; Pascal 2023). Once activated, PARP1 modifies itself and a growing list of chromatin-associated proteins, including core histones, promoting the recruitment of PAR-binding DNA repair proteins to the lesion site and accelerating DNA repair (Ray Chaudhuri and Nussenzweig 2017; Hendriks et al., 2021; Caldecott, 2022). Interestingly, there is extensive literature on roles of PARP1 in many other cellular processes, such as chromatin remodelling, gene regulation and inflammation (Hottiger, 2015; Fehr et al., 2020; Kim et al., 2020), but whether PARP1 is also responding to some form of DNA damage under these conditions or if PARP1 can be catalytically activated in the absence of a DNA strand break is currently unclear.
In addition to its canonical role in accelerating DNA repair and, therefore, promoting cell survival in response to DNA lesions, PARP1 can become hyperactivated in response to high levels of acute DNA damage, triggering a regulated form of cell death termed parthanatos (Yu et al., 2002; Fatokun et al., 2014). In this setting, genetic PARP1 deletion or pharmacological PARP1 inhibition are strongly cytoprotective, and this strict PARP1 dependency is the defining feature of parthanatos that distinguishes it from other forms of cell death, such as apoptosis or necrosis (Fatokun et al., 2014).
Parthanatos can be triggered by several exogenous or endogenous sources that generate a high load of PARP1-activating DNA breaks, such as the alkylating agents MNNG (N-methyl-N’-nitro-N-nitrosoguanidine) or MMS (methyl methanesulfonate), or a variety of treatments that induce high bursts of reactive oxygen or nitrogen species, such as hydrogen peroxide (H2O2) and other oxidants. In neuronal cells, this can be achieved by overstimulation of glutamate receptors via NMDA (N-methyl-D-aspartate) or glutamate, in a process also known as glutamate excitotoxicity (Mandir et al., 2000). Several disease models that rely on DNA damage-induced tissue dysfunction, such as steptozotocin-induced diabetes and MPTP-induced Parkinsonism also rely on parthanatos for tissue demise (Yamamoto et al., 1981; Wang et al., 2003). Ischemia-reperfusion is another well-documented process that induces a burst of oxidative DNA damage, leading to PARP1-mediated cell death (Eliasson et al., 1997; Dawson and Dawson, 2018) and more recently, it has become evident that PARP1 hyperactivation and parthanatos play a pathological role in neurodegenerative disorders as well, including Parkinson’s disease and Alzheimer’s disease (Hoch et al., 2017; Kam et al., 2018; Park et al., 2020, 2022).
The variety of pathophysiological situations that lead to PARP1 hyperactivation and the likely clinical benefit of PARP inhibitors to manage these disorders have been extensively reviewed (Fatokun et al., 2014; Berger et al., 2018; Liu et al., 2022 a , b), and will only be briefly mentioned here. Likewise, other genetic or pharmacological interventions that affect the magnitude of spontaneous or induced PARP1 hyperactivation will not be discussed (Andrabi et al., 2011; Kang et al., 2011; Yang et al., 2022). Instead, this short review will focus on mechanistic considerations of the downstream events that follow excessive PARP1 catalytic activity and how they contribute to cellular demise (Figure 1). At the end of each section, we will provide a list of open questions that have not been addressed so far or that are currently unclear from the literature.
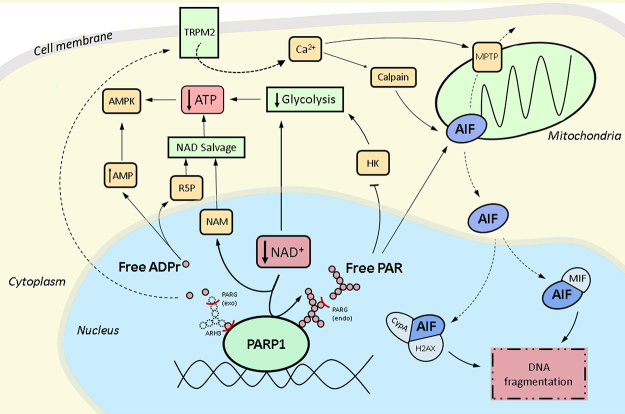
Figure 1 - . Potential pathways of cell death mediated by PARP1 hyperactivation.
PARP1 hyperactivation depletes NAD+ levels and generates protein-linked PAR chains. The hydrolases PARG and ARH3 can produce free PAR chains and/or free ADP-ribose monomers. Both NAD+ depletion and free PAR chains are thought to inhibit glycolysis, which, together with NAD+ salvage, could lead to ATP depletion. Free ADP-ribose monomers can be cleaved by Nudix hydrolases, contributing to NAD+ salvage and the generation of AMP. The ensuing changes in the AMP/ATP ratio can activate AMP-activated kinase (AMPK). Free ADPribose monomers can promote TRPM2 channel opening, increasing cytoplasmic calcium levels. This could activate calpain and/or induce opening of the mitochondrial permeability transition pore (MPTP). Free PAR chains, calpain activation or the MPTP could promote AIF release from the mitochondria, leading to AIF translocation to the nucleus. AIF/MIF or AIF/cyclophilinA/H2AX complexes could then mediate DNA fragmentation. Please refer to the text for more details. NAM: nicotinamide; R5P: ribose 5-phosphate; HK: hexokinase. Note: NAD+ salvage could, in principle, occur both in the nucleus or cytoplasm, but was drawn as shown for simplicity.
NAD+ depletion and inhibition of glycolysis
Since NAD+ is consumed in the process of ADP-ribosylation, donating the ADP-ribose moiety for target modification, and because PARP1 is a highly abundant and processive enzyme, PARP1 hyperactivation results in a rapid and profound depletion of cellular NAD+ pools (Berger 1985). Interestingly, this is accompanied by a depletion of cellular ATP, indicating that parthanatos could be a result of cellular energetic collapse (Berger 1985; Ha and Snyder 1999).
One possible explanation for PARP1-dependent ATP depletion is the unavailability of NAD+ to act as an electron acceptor for core metabolic pathways such as glycolysis and the tricarboxylic acid (TCA) cycle, with the accompanying reduction of the available NADH for oxidative phosphorylation. Early studies using astrocyte cultures indicated NAD+ depletion as the primary mediator of parthanatos, as parthanatos-associated events could be induced by other NAD+-depleting treatments and prevented by supplementation with exogenous NAD+ (Alano et al., 2004, 2010; Ying et al., 2005). Treatment with pyruvate or α-ketoglutarate, which can support TCA cycle activity but bypass glycolysis, was also sufficient to prevent cell death, indicating a glycolytic defect. As the authors pointed out, however, this scenario could be limited to situations in which glucose is the only substrate for energy metabolism in the culture medium used (artificial cerebrospinal fluid in this case), while conventional media often contain pyruvate and other carbon sources. Similarly, Zong et al. (2004) observed that cells that rely more heavily on glycolysis are more susceptible to cell death by parthanatos, which can be reversed by supplementation with pyruvate. Further evidence indicating a central role for NAD+ levels in parthanatos, is the observation that treatment with the NAD+ precursors nicotinamide riboside (NR) or nicotinamide mononucleotide (NMN) can prevent PARP1-dependent cell death in some settings (Nishida et al., 2022; Santofimia-Castaño et al., 2022). In this context, it is worth mentioning that cellular NAD+ pools are compartmentalized, and that nuclear and cytoplasmic NAD+ are in rapid equilibrium, whereas mitochondrial NAD+ pools are maintained separately (Cambronne et al., 2016; Covarrubias et al., 2021). This would indicate that nuclear PARP1 hyperactivation should impact the nuclear/cytoplasmic NAD+ pool more rapidly, which would be consistent with a larger impact of parthanatos-induced NAD+ depletion on cytoplasmic glycolysis than on mitochondrial TCA cycle. However, NAD+ can be transported across the mitochondrial membrane via the recently identified SLC25A51 transporter (Girardi et al., 2020; Kory et al., 2020; Luongo et al., 2020), and there is evidence for a mitochondrial pool of PARP1 (Szczesny et al., 2014; Herrmann et al., 2021; Lee et al., 2022), suggesting that PARP1-dependent depletion of mitochondrial NAD+ may also play a role in parthanatos execution.
Other studies in cortical neurons and glioblastoma-derived cell lines have suggested that NAD+ depletion itself is not sufficient to cause ATP depletion, glycolytic defects or cell death, only being responsible for defects in mitochondrial respiration (Andrabi et al., 2014; Fouquerel et al., 2014). In these studies, profound NAD+ depletion in the absence of PARP1 hyperactivation was insufficient to induce parthanatos and supplementation with nicotinamide riboside (NR), an NAD+ precursor, did not prevent glycolytic dysfunction (Andrabi et al., 2014; Fouquerel et al., 2014). In this scenario, PARP1 activity is thought to directly inhibit the first step of glycolysis, via the release of PAR polymers from target proteins by PAR-degrading enzymes (see below), which then bind to hexokinase-1 and inhibit its catalytic activity (Andrabi et al., 2014; Fouquerel et al., 2014). Although at odds with the NAD+-centric model that emerged from the above studies, supplying cells with pyruvate was again sufficient to overcome PARP1-mediated metabolic dysfunction, consistent with glycolysis being a core target of parthanatos (Andrabi et al., 2014) (Figure 1). As hexokinase also generates substrates for the pentose phosphate pathway (PPP), this mechanism is also consistent with the recently described depletion of reduced glutathione (GSH) and NADPH during parthanatos, which are both products of the PPP (Hossain et al., 2024).
Another model to explain the proposed uncoupling between ATP and NAD+ depletion derives from the observation that AMP, which may be generated at high levels during PAR chain degradation (see below), can inhibit the mitochondrial adenine nucleotide translocator (ANT) (Formentini et al., 2009; Buonvicino et al., 2013). This would lead to an impaired translocation of ADP into the mitochondria, inhibiting mitochondrial ATP synthesis due to the low availability of ADP for oxidative phosphorylation.
Interestingly, it has been suggested that ATP depletion is responsible for diverting cells from apoptosis to parthanatos, with PARP1 hyperactivation thus acting as a “switch” between these forms of cell death (Ha and Snyder 1999). In agreement with this, recent findings indicate that lower (but still cytotoxic) levels of DNA damage induce an intermediate level of NAD+ consumption by PARP1 that can be matched by the NAD+ salvage pathway, leading to a transient NAD+ and ATP depletion that allows cell death to proceed by apoptosis, whereas higher DNA damage loads cause a more prolonged NAD+ and ATP depletion that precludes apoptosis (Nishida et al., 2022). Conversely, PARP1 hyperactivation is also actively prevented during the apoptotic cascade via caspase-dependent cleavage of PARP1 between the DNA binding domains and the catalytic domain, which is thought to ensure that the cell can meet the energy requirements of apoptosis (D’Amours et al., 2001).
Open questions:
What factor(s) determine(s) whether NAD supplementation does or does not prevent parthanatos induction?
Is the inhibition of glycolysis necessary and/or sufficient for cell death by parthanatos?
How do free PAR chains inhibit hexokinase activity?
Are NAD+ and ATP depletion mechanistically connected?
Is there more extensive crosstalk between apoptosis and parthanatos, or is this limited to parthanatic NAD+/ATP depletion preventing apoptosis and apoptotic PARP1 cleavage preventing parthanatos?
PAR hydrolysis
The human genome encodes several hydrolases responsible for the reversal of ADP-ribosylation: those belonging to the macrodomain family - PARG, TARG1, MacroD1 and MacroD2; and those in the ADP-ribose-acceptor hydrolase family - ARH1 and ARH3 (O’Sullivan et al., 2019; Rack et al., 2020). Among these, PARG and ARH3 are crucial for the hydrolysis of PARP1-generated PAR chains, with PARG contributing the bulk of PAR hydrolysis activity, cleaving the O-glycosidic bond between ADP-ribose units, both in linear chains as well as at branching points (Rack et al., 2021). While ARH3 can also contribute to PAR hydrolysis, its main role is the release of the final serine-bound mono-ADP-ribose (Abplanalp et al., 2017; Fontana et al., 2017), serine being the main target residue for PARP1 in response to DNA damage (Palazzo et al., 2018). Similar to PARP1, PARG is a highly active enzyme, making poly-ADP-ribosylation a very transient modification that is produced and degraded within minutes of an insult (Hanzlikova et al., 2018). Interestingly, the reversal of DNA damage-induced mono-ADP-ribosylation, which can be generated either as a remnant of PARG activity or directly by PARP1, seems to be much slower, indicating a longer-lasting, and therefore different, cellular response (Longarini et al., 2023).
There is extensive but conflicting evidence as to the role of PARG in parthanatos, with several studies suggesting that PARG can either prevent or promote PARP1-dependent cell death. In favour of an inhibitory role, PARG overexpression reduced MNNG-induced cell death in mouse neuronal cultures (Andrabi et al., 2006) and reduced NMDA-induced AIF release from mitochondria (Yu et al., 2006), an important step in most parthanatos models, which is covered in more detail below. Similarly, knockdown of PARG increased cell death in mouse neurons (Andrabi et al., 2006) and PARG deletion in trophoblast stem cells increased AIF release after UV irradiation (Zhou et al., 2011). PARG +/- mice had larger infarct volumes after brain ischaemia-reperfusion injury, while mice overexpressing PARG had smaller infarct volumes (Andrabi et al., 2006). In contrast, other studies suggest that PARG is necessary for, or at least contributes to, the process of parthanatos. PARG inhibition protected mice against brain ischaemia (Lu et al., 2003), and PARG silencing rendered cells more resistant to treatment with H2O2 but not MNNG (Blenn et al., 2006). In the aforementioned studies proposing the hexokinase inhibition model, there is also conflicting evidence regarding the role of PARG. In (Fouquerel et al., 2014), PARG knockdown rescued the PARP1-dependent glycolytic defect and ATP depletion, while in (Andrabi et al., 2014) a similar rescue of glycolysis was observed after PARG overexpression. Table 1 shows a compilation of results regarding the contribution of PARG to PARP1-dependent cell death and associated effects in different models, highlighting the considerable heterogeneity currently in the literature.
Table 1 - . Contribution of PARG activity, AIF translocation and TRPM2 channel gating to cell death by parthanatos. Only studies in which the induced cell death was shown to rely on PARP1 activity have been included.
| Model | Insult | Effect of PARG on cell death | AIF translocation | TRPM2 contribution to cell death | Reference |
|---|---|---|---|---|---|
| Mouse embryos | MNNG, menadione | Protective (knockout increased cell death) | - | - | Koh et al., 2004 |
| MEFs | H2O2 | Protective (knockdown increased cell death | - | - | Blenn et al., 2006 |
| Cortical neurons | NMDA | Protective (overexpression reduced cell death | - | - | Andrabi et al., 2006 |
| Mice | middle cerebral artery occlusion (MCAO) | Protective (heterozygous KO increased tissue damage; overexpression reduced tissue damage) | - | - | Andrabi et al., 2006 |
| Cortical neurons | MNNG | Protective (overexpression prevented glycolytic defect) | - | - | Andrabi et al., 2014 |
| MEFs | MNNG | No effect | - | - | Blenn et al., 2006 |
| HK-2 cells | TGHQ | No effect | - | - | Munoz et al., 2017 |
| Mice and rats | splanchnic artery occlusion (SAO) shock | Detrimental (knockout and inhibition protected tissues) | - | - | Cuzzocrea et al., 2005 |
| Glioblastoma cells | MNNG | Detrimental (knockdown prevented ATP depletion and glycolytic defect) | - | - | Fouquerel et al., 2014 |
| Glioblastoma cells | MMS | Protective (knockdown increased cell death) | No | - | Tang et al., 2010 |
| Pancreatic cancer cells | ZZW-115 (NUPR1 inhibitor) | Protective (inhibition increased cell death) | No | - | Santofimia-Castaño et al., 2022 |
| Trophoblast stem cells | UV | Protective (knockout increased cell death) | Yes | - | Zhou et al., 2011 |
| MEFs | H2O2 | Detrimental (knockdown reduced cell death) | Yes | - | Mashimo et al., 2013 |
| Rat fibroblasts | MNNG | - | Yes | - | Yu et al., 2002 |
| Neurons | NMDA | - | Yes | - | Yu et al., 2006 |
| MEFs | H2O2 | - | Yes | - | Kolthur-Seetharam et al., 2006 |
| MEFs | MNNG | - | Yes | - | Wang et al., 2009 |
| Cortical neurons | NMDA | - | Yes | - | Wang et al., 2011 |
| Glioma cells | DPT | - | Yes | - | Ma et al., 2016 |
| SH-SY5Y cells | MNNG | - | Yes | - | Zhong et al., 2018 |
| HK-2 cells | TGHQ | - | No | - | Zhang et al., 2014 |
| Retinal cells | H2O2 | - | No | - | Jang et al., 2017 |
| Mouse bone-marrow derived macrophages | H2O2 | - | No | - | Regdon et al., 2019 |
| HEK293 expressing recombinant TRPM2 | H2O2 | - | - | Increased | Fonfria et al., 2004 |
| Rat striatal neurons | H2O2 and amyloid β-peptide(1-42) | - | - | Increased | Fonfria et al., 2005 |
| Rat cardiomyocytes | H2O2 | - | - | Increased (apoptosis markers also present) | Yang et al., 2006 |
| Mice | MCAO | - | - | Increased infarct volumes (also androgen signalling-dependent) | Shimizu et al., 2013 |
| RIN-5F (rat pancreatic β-cells) | H2O2 | - | - | Increased | Ishii et al., 2014 |
| Mouse hippocampal neurons | H2O2 | - | - | Increased (death is also partly Zn2+ dependent) | Li et al., 2017 |
| SH-SY5Y overexpressing TRPM2 | H2O2 | - | - | Increased | An et al., 2019 |
These competing roles of PARG can, at least in theory, be ascribed to two separate functions that both depend on PARG catalytic activity, but have opposing effects on parthanatos execution. One possibility is centered around the formation of free PAR chains, which are thought to inhibit hexokinase (above) and release AIF from mitochondria (below) (Figure 1). PARG activity could be required for the formation of these free PAR chains via its endoglycohydrolase activity and therefore promote parthanatos, but high PARG activity may also degrade these free PAR chains after their formation, and therefore reduce cell death by parthanatos (Mashimo et al., 2013). In this context, it is worth mentioning that, while PARG acts both as an exo- and endoglycohydrolase, its exoglycohydrolase activity is thought to be predominant (Barkauskaite et al., 2015), indicating that PAR hydrolysis generates mostly ADP-ribose monomers, not free PAR chains. Moreover, high levels of nuclear PARG catalytic activity imply that any free PAR polymers generated in the nucleus must be protected from PARG activity in order to reach the cytosol at any significant amounts. A further complication in the interpretation of the contribution of PARG to parthanatos is that long-term PARG deletion can affect the activation of PARP1, since PARP1 auto-modification inhibits its DNA binding, such that the accumulation of spontaneously auto-modified PARP1 in PARG KO cells can reduce the population of PARP1 molecules that can engage in DNA damage-induced PARylation (Gogola et al., 2018).
ARH3, on the other hand, is thought to play a protective role in parthanatos, which is ascribed to its PAR-degrading activity, which would reduce the accumulation of free PAR polymers and prevent parthanatos induction (Mashimo et al., 2013) (Figure 1). In agreement with this model, ARH3-deficient mice are more sensitive to ischaemia-reperfusion injury and ARH3-deficient human patients present neurodegenerative disorders and their fibroblasts are more sensitive to H2O2-induced parthanatos (Danhauser et al., 2018; Ghosh et al., 2018). However, an alternative explanation for neurodegeneration in these patients could be that failure to remove mono-ADP-ribosylation from core histones leads to epigenetic changes that culminate in transcription deregulation (Hanzlikova et al., 2020), which would be independent of parthanatos.
Open questions:
Do PAR hydrolases, and PARG in particular, promote or inhibit parthanatos execution?
How are free PAR chains generated at sufficiently high amounts, protected from hydrolytic enzymes and then transported out of the nucleus?
ADP-ribose monomers and Ca2+ release
While the nicotinamide moiety of NAD+ released during PAR synthesis is predominantly recycled back to NAD+ by the NAD+ salvage pathway (Covarrubias et al., 2021), the ADP-ribose moiety transferred onto target proteins and subsequently released by PAR/MAR hydrolases generates free ADP-ribose monomers (Figure 1). This free ADP-ribose can bind to the calcium channel TRPM2, which contains two ADP-ribose binding sites that regulate channel opening (Perraud et al., 2001; Huang et al., 2018; Szollosi 2021). In several cell types, increases in intracellular Ca2+ were observed upon oxidative stress, were accompanied by the accumulation of free ADP-ribose and relied on PARP1 activation and TRPM2 gating (Fonfria et al., 2004; Perraud et al., 2005; Yang et al., 2006). Although in some systems there is evidence for ADP-ribose-independent, but oxidative stress-dependent TRPM2 opening (Wehage et al., 2002), the ADPr-dependent activation requires PARG activity (Blenn et al., 2011), arguing in favour of a PARP1/PARG-dependent route of ADP-ribose generation. Consistent with this, induction of parthanatos using MNNG can also lead to calcium influx (Chiu et al., 2011). Interestingly, in a model of renal ischaemia/reperfusion injury, cell death can be prevented both by PARP1 inhibition or Ca2+ chelation, suggesting an important role of Ca2+ influx for parthanatos execution (Zhang et al., 2014). In another study, Ca2+ chelation only suppressed cell death upon H2O2 treatment, but not upon MNNG treatment, indicating that this effect could be specific to particular insults (Bentle et al., 2006). A further complication in the interpretation of this data is that Ca2+ may affect PARP1 activation by a poorly understood mechanism (Zhang et al., 2014). While TRPM2 is a cell-membrane resident channel and therefore can only cause Ca2+ influxes from the extracellular space, Ca2+ release from the endoplasmic reticulum may also contribute to parthanatos (Munoz et al., 2017; Zhong et al., 2018). Interestingly, unlike TRPM2 gating, this effect was independent of PARG, suggesting a different mechanism of channel opening (Zhang et al., 2014; Munoz et al., 2017).
Open questions:
Is ADP-ribose-induced TRPM2 gating necessary and/or sufficient for parthanatos execution?
Are there TRPM2-dependent and TRPM2-independent modes of parthanatos?
What are the downstream molecular effects of TRPM2-mediated increases in intracellular Ca2+?
ADP-ribose degradation into AMP
Free ADP-ribose can also be further degraded into AMP and ribose-5-phosphate by phosphodiesterases of the Nudix superfamily (Carreras-Puigvert et al., 2017) (Figure 1). These enzymes target the phosphodiester bond in ADP-ribose and can degrade either free ADPr or leave a phosphoribose modification on previously ADP-ribosylated proteins, although the detection of protein phosphoribose modification is currently limited to in vitro reactions (Daniels et al., 2015; Palazzo et al., 2015; O’Sullivan et al., 2019). The resultant ribose 5-phosphate could have many metabolic fates, including the formation of phosphoribosyl pyrophosphate (PRPP), which is required for the NAD+ salvage pathway (Figure 2). Interestingly, the complete cycle of NAD+ salvage, from conversion of NAD+ to an ADP-ribose unit by PARP1 back to a full NAD+ molecule using the same carbon backbones, has an energetic cost of four high-energy phosphate groups per ADP-ribose unit attached to a protein (Figure 2). Given that NAD(H) concentrations are roughly in the 0.3 mM range (Yang et al., 2007), while ATP concentrations are only around 10x higher, in the 3-4 mM range (Greiner and Glonek, 2021), the full consumption of cellular NAD+ by PARP1 and its subsequent salvage couId make a substantial contribution to ATP depletion during parthanatos. While the relative contributions of this Nudix-dependent salvage pathway as opposed to glycolysis inhibition (see above) to energetic collapse during parthanatos is unclear, the accumulation of ADP and particularly AMP may be an important signal in cell death after PARP1 hyperactivation (Formentini et al., 2009). Illustrating this, MNNG treatment of HEK-293 cells led to activation of the AMP-activated kinase (AMPK) attributed to increased AMP/ATP ratios, which then inhibited the mTORC1 signaling pathway, involved in the regulation of cell death/survival and energy metabolism (Ethier et al., 2012) (Figure 1). While this would suggest the induction of an autophagic response, as observed in some parthanatos models (Zhou et al., 2013; Jiang et al., 2018), whether AMPK activation and autophagy contribute to cell death execution by parthanatos or are protective mechanisms is currently unclear.
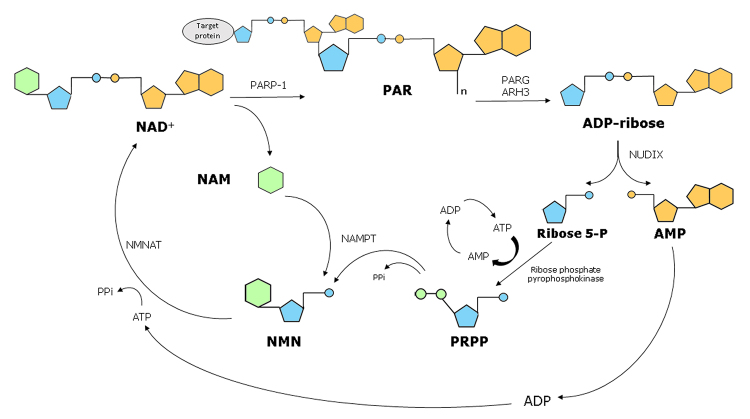
Figure 2 - . A full cycle of NAD+ salvage costs four high-energy phosphate groups per NAD+ molecule consumed by PARP1.
PARP1 activity generates PAR and releases nicotinamide (NAM). PAR is cleaved into ADP-ribose monomer by PARG and ARH3, which is further cleaved by Nudix hydrolases into ribose 5-phosphate (R5P) and AMP. While the AMP is re-phosphorylated to ATP, at the cost of two high-energy phosphate groups, the R5P is converted to phosphoribosyl pyrophosphate (PRPP), at the cost of two further high-energy phosphates. PRPP is conjugated to NAM to form nicotinamide mononucleotide (NMN) and NMN is conjugated with ATP to generate NAD+. NAMPT: nicotinamide phosphoribosyltransferase; NMNAT: Nicotinamide mononucleotide adenylyltransferase.
Open questions:
Are Nudix hydrolases required for parthanatos execution?
What is the relative contribution of AMP generated from ADP-ribose hydrolysis, as opposed to ATP depletion from glycolysis inhibition (above), for the AMPK activation/autophagy observed in parthanatos?
How do AMPK activation and autophagy affect cell death by parthanatos?
AIF translocation and DNA fragmentation
Apoptosis-Inducing Factor (AIF) is a mitochondrial flavoprotein that plays a role in the assembly of the respiratory chain complexes, but is also involved in cell death mechanisms (Susin et al., 1999; Vahsen et al., 2004). It is normally located in the inner mitochondrial membrane, facing the inter-membrane space (Otera et al., 2005), but can also be found loosely associated with the outer mitochondrial membrane (Yu et al., 2009). In response to PARP1 hyperactivation, AIF is often observed to translocate from the mitochondria to the nucleus (Yu et al., 2002), but some models of PARP1-dependent cell death do not lead to observable AIF translocation, indicating that there may be AIF-dependent and AIF-independent forms of parthanatos (Table 1). For example, retinal cells and macrophages do not seem to exhibit AIF translocation after PARP1-dependent cell death induction (Jang et al., 2017; Regdon et al., 2019). Interestingly, AIF translocation to the nucleus is also observed in response to some apoptotic stimuli (Daugas et al., 2000), which was recently suggested to also rely on PARP1 activation (Mashimo et al., 2021).
In the context of parthanatos, AIF is thought to be released from mitochondria via direct interaction with free PAR polymers (above) (Yu et al., 2006; Wang et al., 2011), but the molecular details of this process are currently unclear. Alternatively, AIF release may proceed via its proteolysis, which has been observed in some situations to rely on calpain I, which is a Ca2+ -dependent protease, and therefore could in principle respond to TRPM2-dependent Ca2+ influxes or endoplasmic reticulum calcium release (Polster et al., 2005; Norberg et al., 2008; Vosler et al., 2009; Sun et al., 2018). However, there is rather strong evidence against a central role of calpain cleavage on AIF release, at least in some parthanatos models (Wang et al., 2009). Another possible contributor to AIF release from mitochondria is the mitochondrial permeability transition pore, which is an ill-defined molecular entity that allows the non-selective diffusion of small molecules through the mitochondrial inner membrane, which can lead to mitochondrial swelling and rupture, and is associated to several cell death mechanisms (Yu et al., 2006; Bernardi et al., 2023) (Figure 1).
AIF translocation to the nucleus is associated with large scale DNA fragmentation, culminating in cell death. Two mechanisms for AIF-induced DNA cleavage have been proposed (Figure 1). One model suggests that cytoplasmic AIF interacts with macrophage migration inhibitory factor (MIF), leading to the nuclear translocation of MIF, which was identified to have a nuclease activity (Wang et al., 2016). In agreement with this model, a specific inhibitor of MIF nuclease activity was recently shown to protect cells from parthanatos in a mouse model of parkinsonism (Park et al., 2022). The second model is based on the recent identification of a nuclease activity in AIF itself, which is proposed to degrade DNA in a complex formed between AIF, cyclophilin A and histone H2AX (Artus et al., 2010; Novo et al., 2022).
Open questions:
What is the precise sequence of molecular events that promotes AIF release from mitochondria?
How does translocation of AIF promote DNA fragmentation and what protein(s) catalyse(s) DNA cleavage?
What factor(s) define(s) AIF-dependent and AIF-independent parthanatos and what process leads to DNA fragmentation in AIF-independent parthanatos?
What are the differences and similarities between apoptotic and parthanatic AIF translocation?
Conclusions
In the late 1970s, Goodwin and colleagues first showed that DNA damage can induce the depletion of NAD and ATP levels, and that PARP1 activity is central to this effect (Goodwin et al., 1978). Almost 50 years of research since then have led to the identification of a range of different stimuli that induce PARP1 hyperactivation and a variety of pathological situations in which PARP1 activation seems to contribute to cell death and tissue damage. However, several questions and inconsistencies still remain regarding the sequence of molecular events that drive cell death by parthanatos. While a number of key mechanisms have already been described, it remains unclear which events are necessary and sufficient for parthanatos execution and how each of these steps connects to the next one in the cascade. Complicating matters even further, there seem to be clear differences in how parthanatos proceeds in different cell types and at different metabolic states. With this review, we aim to highlight the urgent need for studies that determine the contribution of several steps along the cascade in single, well-defined model systems. Only by comparing all of these steps between different models in which NAD+ supplementation, PARG activity, AIF translocation or TRPM2 gating play differential roles, can we hope to shed light on whether there are multiple pathways of parthanatos or if a single pathway integrates all of these events. Although technically difficult and inherently multidisciplinary, this will be critical to better understand not only how this pathway operates, but also how other cell death mechanisms, such as apoptosis, are interconnected to parthanatos. A better definition of these mechanisms will be central to clarify the contribution of PARP1-dependent cell death to human pathology, particularly in a variety of neurodegenerative disorders, such as Parkinson’s and Alzheimer’s disease, which are of rising concern in an aging human population.
Acknowledgements
The authors wish to apologize to the colleagues whose work could not be mentioned here. Work in the NCH lab is funded by FAPESP grant 2018/18007-5.
References
- Abplanalp J, Leutert M, Frugier E, Nowak K, Feurer R, Kato J, Kistemaker HVA, Filippov DV, Moss J, Caflisch A, et al. Proteomic analyses identify ARH3 as a serine mono-ADP-ribosylhydrolase. Nat Commun. 2017;8:2055. doi: 10.1038/s41467-017-02253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci. 2010;30:2967–2978. doi: 10.1523/JNEUROSCI.5552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alano CC, Ying W, Swanson RA. Poly(ADP-ribose) polymerase-1-mediated cell death in astrocytes requires NAD+ depletion and mitochondrial permeability transition. J Biol Chem. 2004;279:18895–18902. doi: 10.1074/jbc.M313329200. [DOI] [PubMed] [Google Scholar]
- An X, Fu Z, Mai C, Wang W, Wei L, Li D, Li C, Jiang LH. Increasing the TRPM2 channel expression in human neuroblastoma SH-SY5Y cells augments the susceptibility to ROS-Induced Cell Death. Cells. 2019;8:28. doi: 10.3390/cells8010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrabi SA, Kang HC, Haince JF, Lee YI, Zhang J, Chi Z, West AB, Koehler RC, Poirier GG, Dawson TM, et al. Iduna protects the brain from glutamate excitotoxicity and stroke by interfering with poly(ADP-ribose) polymer-induced cell death. Nat Med. 2011;17:692–699. doi: 10.1038/nm.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrabi SA, Kim NS, Yu SW, Wang H, Koh DW, Sasaki M, Klaus JA, Otsuka T, Zhang Z, Koehler RC, et al. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad Sci U S A. 2006;103:18308–18313. doi: 10.1073/pnas.0606526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrabi SA, Umanah GK, Chang C, Stevens DA, Karuppagounder SS, Gagné JP, Poirier GG, Dawson VL, Dawson TM. Poly(ADP-ribose) polymerase-dependent energy depletion occurs through inhibition of glycolysis. Proc Natl Acad Sci U S A. 2014;111:10209–10214. doi: 10.1073/pnas.1405158111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artus C, Boujrad H, Bouharrour A, Brunelle M-N, Hoos S, Yuste VJ, Lenormand P, Rousselle J-C, Namane A, England P, et al. AIF promotes chromatinolysis and caspase-independent programmed necrosis by interacting with histone H2AX. EMBO J. 2010;29:1585–1599. doi: 10.1038/emboj.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkauskaite E, Jankevicius G, Ahel I. Structures and mechanisms of enzymes employed in the synthesis and degradation of PARP-dependent protein ADP-Ribosylation. Mol Cell. 2015;58:935–946. doi: 10.1016/j.molcel.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Bentle MS, Reinicke KE, Bey EA, Spitz DR, Boothman DA. Calcium-dependent modulation of poly(ADP-ribose) polymerase-1 alters cellular metabolism and DNA repair. J Biol Chem. 2006;281:33684–33696. doi: 10.1074/jbc.M603678200. [DOI] [PubMed] [Google Scholar]
- Berger NA. Poly(ADP-Ribose) in the cellular response to DNA damage. Radiat Res. 1985;101:4–15. [PubMed] [Google Scholar]
- Berger NA, Besson VC, Boulares AH, Burkle A, Chiarugi A, Clark RS, Curtin NJ, Cuzzocrea S, Dawson TM, Dawson VL, et al. Opportunities for the repurposing of PARP inhibitors for the therapy of non-oncological diseases. Br J Pharmacol. 2018;175:192–222. doi: 10.1111/bph.13748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P, Gerle C, Halestrap AP, Jonas EA, Karch J, Mnatsakanyan N, Pavlov E, Sheu S-S, Soukas AA. Identity, structure, and function of the mitochondrial permeability transition pore: Controversies, consensus, recent advances, and future directions. Cell Death Differ. 2023;30:1869–1885. doi: 10.1038/s41418-023-01187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenn C, Althaus FR, Malanga M. Poly(ADP-ribose) glycohydrolase silencing protects against H2O2-induced cell death. Biochem J. 2006;396:419–429. doi: 10.1042/BJ20051696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenn C, Wyrsch P, Bader J, Bollhalder M, Althaus FR. Poly(ADP-ribose)glycohydrolase is an upstream regulator of Ca2+ fluxes in oxidative cell death. Cell Mol Life Sci. 2011;68:1455–1466. doi: 10.1007/s00018-010-0533-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonvicino D, Formentini L, Cipriani G, Chiarugi A. Glucose deprivation converts poly(ADP-ribose) polymerase-1 hyperactivation into a transient energy-producing process. J Biol Chem. 2013;288:36530–36537. doi: 10.1074/jbc.M113.506378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldecott KW. DNA single-strand break repair and human genetic disease. Trends Cell Biol. 2022;32:733–745. doi: 10.1016/j.tcb.2022.04.010. [DOI] [PubMed] [Google Scholar]
- Cambronne XA, Stewart ML, Kim D, Jones-Brunette AM, Morgan RK, Farrens DL, Cohen MS, Goodman RH. Biosensor reveals multiple sources for mitochondrial NAD(+) Science. 2016;352:1474–1477. doi: 10.1126/science.aad5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras-Puigvert J, Zitnik M, Jemth A-S, Carter M, Unterlass JE, Hallström B, Loseva O, Karem Z, Calderón-Montaño JM, Lindskog C, et al. A comprehensive structural, biochemical and biological profiling of the human NUDIX hydrolase family. Nat Commun. 2017;8:1541. doi: 10.1038/s41467-017-01642-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu LY, Ho FM, Shiah SG, Chang Y, Lin WW. Oxidative stress initiates DNA damager MNNG-induced poly(ADP-ribose)polymerase-1-dependent parthanatos cell death. Biochem Pharmacol. 2011;81:459–470. doi: 10.1016/j.bcp.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22:119–141. doi: 10.1038/s41580-020-00313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Di Paola R, Mazzon E, Cortes U, Genovese T, Muià C, Li W, Xu W, Li JH, Zhang J, et al. PARG activity mediates intestinal injury induced by splanchnic artery occlusion and reperfusion. FASEB J. 2005;19:558–566. doi: 10.1096/fj.04-3117com. [DOI] [PubMed] [Google Scholar]
- D’Amours D, Sallmann FR, Dixit VM, Poirier GG. Gain-of-function of poly(ADP-ribose) polymerase-1 upon cleavage by apoptotic proteases: Implications for apoptosis. J Cell Sci. 2001;114:3771–3778. doi: 10.1242/jcs.114.20.3771. [DOI] [PubMed] [Google Scholar]
- Danhauser K, Alhaddad B, Makowski C, Piekutowska-Abramczuk D, Syrbe S, Gomez-Ospina N, Manning MA, Kostera-Pruszczyk A, Krahn-Peper C, Berutti R, et al. Bi-allelic ADPRHL2 mutations cause neurodegeneration with developmental delay, ataxia, and axonal neuropathy. Am J Hum Genet. 2018;103:817–825. doi: 10.1016/j.ajhg.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels CM, Thirawatananond P, Ong SE, Gabelli SB, Leung AK. Nudix hydrolases degrade protein-conjugated ADP-ribose. Sci Rep. 2015;5:18271. doi: 10.1038/srep18271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugas E, Susin SA, Zamzami N, Ferri KF, Irinopoulou T, Larochette N, Prévost M-C, Leber B, Andrews D, Penninger J, et al. Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. FASEB J. 2000;14:729–739. [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. Nitric Oxide signaling in neurodegeneration and cell death. Adv Pharmacol. 2018;82:57–83. doi: 10.1016/bs.apha.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Eliasson MJL, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang Z-Q, Dawson TM, Snyder SH, et al. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- Ethier C, Tardif M, Arul L, Poirier GG. PARP-1 modulation of mTOR signaling in response to a DNA alkylating agent. PLoS One. 2012;7:e47978. doi: 10.1371/journal.pone.0047978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatokun AA, Dawson VL, Dawson TM. Parthanatos: Mitochondrial-linked mechanisms and therapeutic opportunities. Br J Pharmacol. 2014;171:2000–2016. doi: 10.1111/bph.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr AR, Singh SA, Kerr CM, Mukai S, Higashi H, Aikawa M. The impact of PARPs and ADP-ribosylation on inflammation and host-pathogen interactions. Genes Dev. 2020;34:341–359. doi: 10.1101/gad.334425.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonfria E, Marshall ICB, Benham CD, Boyfield I, Brown JD, Hill K, Hughes JP, Skaper SD, McNulty S. TRPM2 channel opening in response to oxidative stress is dependent on activation of poly(ADP-ribose) polymerase. Br J Pharmacol. 2004;143:186–192. doi: 10.1038/sj.bjp.0705914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonfria E, Marshall IC, Boyfield I, Skaper SD, Hughes JP, Owen DE, Zhang W, Miller BA, Benham CD, McNulty S. Amyloid beta-peptide(1-42) and hydrogen peroxide-induced toxicity are mediated by TRPM2 in rat primary striatal cultures. J Neurochem. 2005;95:715–723. doi: 10.1111/j.1471-4159.2005.03396.x. [DOI] [PubMed] [Google Scholar]
- Fontana P, Bonfiglio JJ, Palazzo L, Bartlett E, Matic I, Ahel I. Serine ADP-ribosylation reversal by the hydrolase ARH3. Elife. 2017;6:e28533. doi: 10.7554/eLife.28533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formentini L, Macchiarulo A, Cipriani G, Camaioni E, Rapizzi E, Pellicciari R, Moroni F, Chiarugi A. Poly(ADP-ribose) catabolism triggers AMP-dependent mitochondrial energy failure. J Biol Chem. 2009;284:17668–17676. doi: 10.1074/jbc.M109.002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouquerel E, Goellner Eva M, Yu Z, Gagné J-P, Barbi de Moura M, Feinstein T, Wheeler D, Redpath P, Li J, Romero G, et al. ARTD1/PARP1 negatively regulates glycolysis by inhibiting hexokinase 1 independent of NAD+ Depletion. Cell Rep. 2014;8:1819–1831. doi: 10.1016/j.celrep.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh SG, Becker K, Huang H, Dixon-Salazar T, Chai G, Salpietro V, Al-Gazali L, Waisfisz Q, Wang H, Vaux KK, et al. Biallelic mutations in ADPRHL2, encoding ADP-Ribosylhydrolase 3, lead to a degenerative pediatric stress-induced epileptic ataxia syndrome. Am J Hum Genet. 2018;103:826. doi: 10.1016/j.ajhg.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi E, Agrimi G, Goldmann U, Fiume G, Lindinger S, Sedlyarov V, Srndic I, Gürtl B, Agerer B, Kartnig F, et al. Epistasis-driven identification of SLC25A51 as a regulator of human mitochondrial NAD import. Nat Commun. 2020;11:6145. doi: 10.1038/s41467-020-19871-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogola E, Duarte AA, de Ruiter JR, Wiegant WW, Schmid JA, de Bruijn R, James DI, Guerrero Llobet S, Vis DJ, Annunziato S, et al. Selective Loss of PARG Restores PARylation and Counteracts PARP Inhibitor-Mediated Synthetic Lethality. Cancer Cell. 2018;33:1078–1093.e1012. doi: 10.1016/j.ccell.2018.05.008. [DOI] [PubMed] [Google Scholar]
- Goodwin PM, Lewis PJ, Davies MI, Skidmore CJ, Shall S. The effect of gamma radiation and neocarzinostatin of NAD and ATP levels in mouse leukaemia cells. Biochim Biophys Acta Gen Subj. 1978;543:576–582. doi: 10.1016/0304-4165(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Greiner JV, Glonek T. Intracellular ATP Concentration and Implication for Cellular Evolution. Biology (Basel) 2021;10:1166. doi: 10.3390/biology10111166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci U S A. 1999;96:13978–13982. doi: 10.1073/pnas.96.24.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzlikova H, Kalasova I, Demin AA, Pennicott LE, Cihlarova Z, Caldecott KW. The Importance of Poly(ADP-Ribose) polymerase as a sensor of unligated okazaki fragments during DNA Replication. Mol Cell. 2018;71:319–331e313. doi: 10.1016/j.molcel.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzlikova H, Prokhorova E, Krejcikova K, Cihlarova Z, Kalasova I, Kubovciak J, Sachova J, Hailstone R, Brazina J, Ghosh S, et al. Pathogenic ARH3 mutations result in ADP-ribose chromatin scars during DNA strand break repair. Nat Commun. 2020;11:3391. doi: 10.1038/s41467-020-17069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks IA, Buch-Larsen SC, Prokhorova E, Elsborg JD, Rebak AKLFS, Zhu K, Ahel D, Lukas C, Ahel I, Nielsen ML. The regulatory landscape of the human HPF1- and ARH3-dependent ADP-ribosylome. Nat Commun. 2021;12:5893. doi: 10.1038/s41467-021-26172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann GK, Russell WK, Garg NJ, Yin YW. Poly(ADP-ribose) polymerase 1 regulates mitochondrial DNA repair in an NAD-dependent manner. J Biol Chem. 2021;296:100309. doi: 10.1016/j.jbc.2021.100309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch NC, Hanzlikova H, Rulten SL, Tetreault M, Komulainen E, Ju L, Hornyak P, Zeng Z, Gittens W, Rey SA, et al. XRCC1 mutation is associated with PARP1 hyperactivation and cerebellar ataxia. Nature. 2017;541:87–91. doi: 10.1038/nature20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch NC, Polo LM. ADP-ribosylation: From molecular mechanisms to human disease. Genet Mol Biol. 2019;43:e20190075. doi: 10.1590/1678-4685-GMB-2019-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MI, Lee JH, Gagné JP, Khan J, Poirier GG, King PH, Dawson VL, Dawson TM, Andrabi SA. Poly(ADP-ribose) mediates bioenergetic defects and redox imbalance in neurons following oxygen and glucose deprivation. FASEB J. 2024;38:e23556. doi: 10.1096/fj.202302559R. [DOI] [PubMed] [Google Scholar]
- Hottiger MO. Nuclear ADP-Ribosylation and its role in chromatin plasticity, cell differentiation, and epigenetics. Annu Rev Biochem. 2015;84:227–263. doi: 10.1146/annurev-biochem-060614-034506. [DOI] [PubMed] [Google Scholar]
- Huang Y, Winkler PA, Sun W, Lü W, Du J. Architecture of the TRPM2 channel and its activation mechanism by ADP-ribose and calcium. Nature. 2018;562:145–149. doi: 10.1038/s41586-018-0558-4. [DOI] [PubMed] [Google Scholar]
- Ishii M, Hagiwara T, Mori Y, Shimizu S. Involvement of TRPM2 and L-type Ca²⁺ channels in Ca²⁺ entry and cell death induced by hydrogen peroxide in rat β-cell line RIN-5F. J Toxicol Sci. 2014;39:199–209. doi: 10.2131/jts.39.199. [DOI] [PubMed] [Google Scholar]
- Jang KH, Do YJ, Son D, Son E, Choi JS, Kim E. AIF-independent parthanatos in the pathogenesis of dry age-related macular degeneration. Cell Death Dis. 2017;8:e2526. doi: 10.1038/cddis.2016.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HY, Yang Y, Zhang YY, Xie Z, Zhao XY, Sun Y, Kong WJ. The dual role of poly(ADP-ribose) polymerase-1 in modulating parthanatos and autophagy under oxidative stress in rat cochlear marginal cells of the stria vascularis. Redox Biol. 2018;14:361–370. doi: 10.1016/j.redox.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam TI, Mao X, Park H, Chou SC, Karuppagounder SS, Umanah GE, Yun SP, Brahmachari S, Panicker N, Chen R, et al. Poly(ADP-ribose) drives pathologic alpha-synuclein neurodegeneration in Parkinson’s disease. Science. 2018;362:eaat8407. doi: 10.1126/science.aat8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HC, Lee Y-I, Shin J-H, Andrabi SA, Chi Z, Gagné J-P, Lee Y, Ko HS, Lee BD, Poirier GG, et al. Iduna is a poly(ADP-ribose) (PAR)-dependent E3 ubiquitin ligase that regulates DNA damage. Proc Natl Acad Sci U S A. 2011;108:14103–14108. doi: 10.1073/pnas.1108799108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Challa S, Jones A, Kraus WL. PARPs and ADP-ribosylation in RNA biology: From RNA expression and processing to protein translation and proteostasis. Genes Dev. 2020;34:302–320. doi: 10.1101/gad.334433.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh DW, Lawler AM, Poitras MF, Sasaki M, Wattler S, Nehls MC, Stöger T, Poirier GG, Dawson VL, Dawson TM. Failure to degrade poly(ADP-ribose) causes increased sensitivity to cytotoxicity and early embryonic lethality. Proc Natl Acad Sci U S A. 2004;101:17699–17704. doi: 10.1073/pnas.0406182101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolthur-Seetharam U, Dantzer F, McBurney MW, de Murcia G, Sassone-Corsi P. Control of AIF-mediated cell death by the functional interplay of SIRT1 and PARP-1 in response to DNA damage. Cell Cycle. 2006;5:873–877. doi: 10.4161/cc.5.8.2690. [DOI] [PubMed] [Google Scholar]
- Kory N, Uit de Bos J, van der Rijt S, Jankovic N, Güra M, Arp N, Pena IA, Prakash G, Chan SH, Kunchok T, et al. MCART1/SLC25A51 is required for mitochondrial NAD transport. Sci Adv. 2020;6:eabe5310. doi: 10.1126/sciadv.abe5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Hussain M, Kim EW, Cheng SJ, Leung AKL, Fakouri NB, Croteau DL, Bohr VA. Mitochondrial PARP1 regulates NAD(+)-dependent poly ADP-ribosylation of mitochondrial nucleoids. Exp Mol Med. 2022;54:2135–2147. doi: 10.1038/s12276-022-00894-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yang W, Jiang LH. Alteration in intracellular Zn(2+) homeostasis as a result of TRPM2 channel activation contributes to ROS-induced hippocampal cell death. Front Mol Neurosci. 2017;10:414. doi: 10.3389/fnmol.2017.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Li J, Ke Y, Zeng X, Gao J, Ba X, Wang R. The key players of parthanatos: Opportunities for targeting multiple levels in the therapy of parthanatos-based pathogenesis. Cell Mol Life Sci. 2022;79:60. doi: 10.1007/s00018-021-04109-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Luo W, Wang Y. Emerging role of PARP-1 and PARthanatos in ischemic stroke. J Neurochem. 2022;160:74–87. doi: 10.1111/jnc.15464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longarini EJ, Dauben H, Locatelli C, Wondisford AR, Smith R, Muench C, Kolvenbach A, Lynskey ML, Pope A, Bonfiglio JJ, et al. Modular antibodies reveal DNA damage-induced mono-ADP-ribosylation as a second wave of PARP1 signaling. Mol Cell. 2023;83:1743–1760.e1711. doi: 10.1016/j.molcel.2023.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XC, Massuda E, Lin Q, Li W, Li JH, Zhang J. Post-treatment with a novel PARG inhibitor reduces infarct in cerebral ischemia in the rat. Brain Res. 2003;978:99–103. doi: 10.1016/s0006-8993(03)02774-4. [DOI] [PubMed] [Google Scholar]
- Luongo TS, Eller JM, Lu MJ, Niere M, Raith F, Perry C, Bornstein MR, Oliphint P, Wang L, McReynolds MR, et al. SLC25A51 is a mammalian mitochondrial NAD(+) transporter. Nature. 2020;588:174–179. doi: 10.1038/s41586-020-2741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Ahel I, Altmeyer M, Ashworth A, Bai P, Chang P, Cohen M, Corda D, Dantzer F, Daugherty MD, et al. ADP-ribosyltransferases, an update on function and nomenclature. FEBS J. 2021;289:7399–7410. doi: 10.1111/febs.16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Lu B, Feng C, Wang C, Wang Y, Luo T, Feng J, Jia H, Chi G, Luo Y, et al. Deoxypodophyllotoxin triggers parthanatos in glioma cells via induction of excessive ROS. Cancer Lett. 2016;371:194–204. doi: 10.1016/j.canlet.2015.11.044. [DOI] [PubMed] [Google Scholar]
- Mandir AS, Poitras MF, Berliner AR, Herring WJ, Guastella DB, Feldman A, Poirier GG, Wang ZQ, Dawson TM, Dawson VL. NMDA but not non-NMDA excitotoxicity is mediated by Poly(ADP-ribose) polymerase. J Neurosci. 2000;20:8005–8011. doi: 10.1523/JNEUROSCI.20-21-08005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo M, Kato J, Moss J. ADP-ribosyl-acceptor hydrolase 3 regulates poly (ADP-ribose) degradation and cell death during oxidative stress. Proc Natl Acad Sci U S A. 2013;110:18964–18969. doi: 10.1073/pnas.1312783110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo M, Onishi M, Uno A, Tanimichi A, Nobeyama A, Mori M, Yamada S, Negi S, Bu X, Kato J, et al. The 89-kDa PARP1 cleavage fragment serves as a cytoplasmic PAR carrier to induce AIF-mediated apoptosis. J Biol Chem. 2021;296:100046. doi: 10.1074/jbc.RA120.014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz FM, Zhang F, Islas-Robles A, Lau SS, Monks TJ. ROS-induced store-operated Ca2+ entry coupled to PARP-1 hyperactivation is independent of PARG activity in necrotic cell death. Toxicol Sci. 2017;158:444–453. doi: 10.1093/toxsci/kfx106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T, Naguro I, Ichijo H. NAMPT-dependent NAD(+) salvage is crucial for the decision between apoptotic and necrotic cell death under oxidative stress. Cell Death Discov. 2022;8:195. doi: 10.1038/s41420-022-01007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg E, Gogvadze V, Ott M, Horn M, Uhlén P, Orrenius S, Zhivotovsky B. An increase in intracellular Ca2+ is required for the activation of mitochondrial calpain to release AIF during cell death. Cell Death Differ. 2008;15:1857–1864. doi: 10.1038/cdd.2008.123. [DOI] [PubMed] [Google Scholar]
- Novo N, Romero-Tamayo S, Marcuello C, Boneta S, Blasco-Machin I, Velázquez-Campoy A, Villanueva R, Moreno-Loshuertos R, Lostao A, Medina M, et al. Beyond a platform protein for the degradosome assembly: The Apoptosis-Inducing Factor as an efficient nuclease involved in chromatinolysis. PNAS Nexus. 2022;2:pgac312. doi: 10.1093/pnasnexus/pgac312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan J, Tedim Ferreira M, Gagné JP, Sharma AK, Hendzel MJ, Masson JY, Poirier GG. Emerging roles of eraser enzymes in the dynamic control of protein ADP-ribosylation. Nat Commun. 2019;10:1182. doi: 10.1038/s41467-019-08859-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otera H, Ohsakaya S, Nagaura Z-I, Ishihara N, Mihara K. Export of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane space. EMBO J. 2005;24:1375–1386. doi: 10.1038/sj.emboj.7600614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo L, Leidecker O, Prokhorova E, Dauben H, Matic I, Ahel I. Serine is the major residue for ADP-ribosylation upon DNA damage. Elife. 2018;7:e34334. doi: 10.7554/eLife.34334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo L, Thomas B, Jemth A-S, Colby TD, Leidecker O, Feijs KLH, Žaja R, Loseva OI, Puigvert JC, Matic I, et al. Processing of protein ADP-ribosylation by Nudix hydrolases. Biochem J. 2015;468(2):293–301. doi: 10.1042/BJ20141554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey N, Black BE. Rapid detection and signaling of DNA damage by PARP-1. Trends Biochem Sci. 2021;46:744–757. doi: 10.1016/j.tibs.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Kam TI, Dawson TM, Dawson VL. Poly (ADP-ribose) (PAR)-dependent cell death in neurodegenerative diseases. Int Rev Cell Mol Biol. 2020;353:1–29. doi: 10.1016/bs.ircmb.2019.12.009. [DOI] [PubMed] [Google Scholar]
- Park H, Kam TI, Peng H, Chou SC, Mehrabani-Tabari AA, Song JJ, Yin X, Karuppagounder SS, Umanah GK, Rao AVS, et al. PAAN/MIF nuclease inhibition prevents neurodegeneration in Parkinson’s disease. Cell. 2022;185:1943–1959e1921. doi: 10.1016/j.cell.2022.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal JM. PARP-nucleic acid interactions: Allosteric signaling, PARP inhibitor types, DNA bridges, and viral RNA surveillance. Curr Opin Struct Biol. 2023;81:102643. doi: 10.1016/j.sbi.2023.102643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- Perraud AL, Takanishi CL, Shen B, Kang S, Smith MK, Schmitz C, Knowles HM, Ferraris D, Li W, Zhang J, et al. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J Biol Chem. 2005;280:6138–6148. doi: 10.1074/jbc.M411446200. [DOI] [PubMed] [Google Scholar]
- Polster BM, Basañez G, Etxebarria A, Hardwick JM, Nicholls DG. Calpain I induces cleavage and release of apoptosis-inducing factor from isolated mitochondria. J Biol Chem. 2005;280:6447–6454. doi: 10.1074/jbc.M413269200. [DOI] [PubMed] [Google Scholar]
- Rack JGM, Liu Q, Zorzini V, Voorneveld J, Ariza A, Honarmand Ebrahimi K, Reber JM, Krassnig SC, Ahel D, van der Marel GA, et al. Mechanistic insights into the three steps of poly(ADP-ribosylation) reversal. Nat Commun. 2021;12:4581. doi: 10.1038/s41467-021-24723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack JGM, Palazzo L, Ahel I. (ADP-ribosyl)hydrolases: Structure, function, and biology. Genes Dev. 2020;34:263–284. doi: 10.1101/gad.334631.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray Chaudhuri A, Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat Rev Mol Cell Biol. 2017;18:610–621. doi: 10.1038/nrm.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regdon Z, Robaszkiewicz A, Kovács K, Rygielska Ż, Hegedűs C, Bodoor K, Szabó É, Virág L. LPS protects macrophages from AIF-independent parthanatos by downregulation of PARP1 expression, induction of SOD2 expression, and a metabolic shift to aerobic glycolysis. Free Radic Biol Med. 2019;131:184–196. doi: 10.1016/j.freeradbiomed.2018.11.034. [DOI] [PubMed] [Google Scholar]
- Santofimia-Castaño P, Huang C, Liu X, Xia Y, Audebert S, Camoin L, Peng L, Lomberk G, Urrutia R, Soubeyran P, et al. NUPR1 protects against hyperPARylation-dependent cell death. Commun Biol. 2022;5:732. doi: 10.1038/s42003-022-03705-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Macey TA, Quillinan N, Klawitter J, Perraud AL, Traystman RJ, Herson PS. Androgen and PARP-1 regulation of TRPM2 channels after ischemic injury. J Cereb Blood Flow Metab. 2013;33:1549–1555. doi: 10.1038/jcbfm.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Sukumaran P, Selvaraj S, Cilz NI, Schaar A, Lei S, Singh BB. TRPM2 Promotes neurotoxin MPP(+)/MPTP-induced cell death. Mol Neurobiol. 2018;55:409–420. doi: 10.1007/s12035-016-0338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Suskiewicz MJ, Prokhorova E, Rack JGM, Ahel I. ADP-ribosylation from molecular mechanisms to therapeutic implications. Cell. 2023;186:4475–4495. doi: 10.1016/j.cell.2023.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesny B, Brunyanszki A, Olah G, Mitra S, Szabo C. Opposing roles of mitochondrial and nuclear PARP1 in the regulation of mitochondrial and nuclear DNA integrity: Implications for the regulation of mitochondrial function. Nucleic Acids Res. 2014;42:13161–13173. doi: 10.1093/nar/gku1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szollosi A. Two decades of evolution of our understanding of the Transient Receptor Potential Melastatin 2 (TRPM2) Cation Channel. Life. 2021;11:397. doi: 10.3390/life11050397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JB, Goellner EM, Wang XH, Trivedi RN, St Croix CM, Jelezcova E, Svilar D, Brown AR, Sobol RW. Bioenergetic metabolites regulate base excision repair-dependent cell death in response to DNA damage. Mol Cancer Res. 2010;8:67–79. doi: 10.1158/1541-7786.MCR-09-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahsen N, Candé C, Brière J-J, Bénit P, Joza N, Larochette N, Mastroberardino PG, Pequignot MO, Casares N, Lazar V, et al. AIF deficiency compromises oxidative phosphorylation. EMBO J. 2004;23:4679–4689. doi: 10.1038/sj.emboj.7600461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosler PS, Sun D, Wang S, Gao Y, Kintner DB, Signore AP, Cao G, Chen J. Calcium dysregulation induces apoptosis-inducing factor release: Cross-talk between PARP-1- and calpain-signaling pathways. Exp Neurol. 2009;218:213–220. doi: 10.1016/j.expneurol.2009.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Shimoji M, Yu S-W, Dawson TM, Dawson VL. Apoptosis inducing factor and PARP-Mediated injury in the MPTP mouse model of Parkinson’s disease. Ann N Y Acad Sci. 2003;991:132–139. doi: 10.1111/j.1749-6632.2003.tb07471.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, An R, Umanah GK, Park H, Nambiar K, Eacker SM, Kim B, Bao L, Harraz MM, Chang C, et al. A nuclease that mediates cell death induced by DNA damage and poly(ADP-ribose) polymerase-1. Science. 2016;354:aad6872. doi: 10.1126/science.aad6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kim NS, Haince JF, Kang HC, David KK, Andrabi SA, Poirier GG, Dawson VL, Dawson TM. Poly(ADP-ribose) (PAR) binding to apoptosis-inducing factor is critical for PAR polymerase-1-dependent cell death (parthanatos) Sci Signal. 2011;4:ra20. doi: 10.1126/scisignal.2000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kim NS, Li X, Greer PA, Koehler RC, Dawson VL, Dawson TM. Calpain activation is not required for AIF translocation in PARP-1-dependent cell death (parthanatos) J Neurochem. 2009;110:687–696. doi: 10.1111/j.1471-4159.2009.06167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehage E, Eisfeld J, Heiner I, Jüngling E, Zitt C, Lückhoff A. Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem. 2002;277:23150–23156. doi: 10.1074/jbc.M112096200. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Uchigata Y, Okamoto H. Streptozotocin and alloxan induce DNA strand breaks and poly(ADP-ribose) synthetase in pancreatic islets. Nature. 1981;294:284–286. doi: 10.1038/294284a0. [DOI] [PubMed] [Google Scholar]
- Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang KT, Chang WL, Yang PC, Chien CL, Lai MS, Su MJ, Wu ML. Activation of the transient receptor potential M2 channel and poly(ADP-ribose) polymerase is involved in oxidative stress-induced cardiomyocyte death. Cell Death Differ. 2006;13:1815–1826. doi: 10.1038/sj.cdd.4401813. [DOI] [PubMed] [Google Scholar]
- Yang M, Wang C, Zhou M, Bao L, Wang Y, Kumar A, Xing C, Luo W, Wang Y. KDM6B promotes PARthanatos via suppression of O6-methylguanine DNA methyltransferase repair and sustained checkpoint response. Nucleic Acids Res. 2022;50:6313–6331. doi: 10.1093/nar/gkac471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W, Alano CC, Garnier P, Swanson RA. NAD+ as a metabolic link between DNA damage and cell death. J Neurosci Res. 2005;79:216–223. doi: 10.1002/jnr.20289. [DOI] [PubMed] [Google Scholar]
- Yu S-W, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, Dawson VL. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad Sci U S A. 2006;103:18314–18319. doi: 10.1073/pnas.0606528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- Yu SW, Wang Y, Frydenlund DS, Ottersen OP, Dawson VL, Dawson TM. Outer mitochondrial membrane localization of apoptosis-inducing factor: Mechanistic implications for release. ASN Neuro. 2009;1:e00021. doi: 10.1042/AN20090046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Xie R, Munoz FM, Lau SS, Monks TJ. PARP-1 hyperactivation and reciprocal elevations in intracellular Ca2+ during ROS-induced nonapoptotic cell death. Toxicol Sci. 2014;140:118–134. doi: 10.1093/toxsci/kfu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Song R, Pang Q, Liu Y, Zhuang J, Chen Y, Hu J, Hu J, Liu Y, Liu Z, et al. Propofol inhibits parthanatos via ROS-ER-calcium-mitochondria signal pathway in vivo and vitro. Cell Death Dis. 2018;9:932. doi: 10.1038/s41419-018-0996-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Ng S, Huang Q, Wu Y-T, Li Z, Yao SQ, Shen H-M. AMPK mediates a pro-survival autophagy downstream of PARP-1 activation in response to DNA alkylating agents. FEBS Letters. 2013;587:170–177. doi: 10.1016/j.febslet.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Feng X, Koh DW. Activation of cell death mediated by apoptosis-inducing factor due to the absence of poly(ADP-ribose) glycohydrolase. Biochemistry. 2011;50:2850–2859. doi: 10.1021/bi101829r. [DOI] [PubMed] [Google Scholar]
- Zong W-X, Ditsworth D, Bauer DE, Wang Z-Q, Thompson CB. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004;18:1272–1282. doi: 10.1101/gad.1199904. [DOI] [PMC free article] [PubMed] [Google Scholar]