Abstract
Background:
Isocitrate dehydrogenase (IDH)-mutant grade 2 gliomas are malignant brain tumors causing considerable morbidity and premature death. Vorasidenib, an oral brain-penetrant inhibitor of mutant IDH1 and IDH2 enzymes, showed preliminary activity in IDH-mutant gliomas.
Methods:
In this double-blind phase 3 trial, patients with residual or recurrent grade 2 IDH-mutant glioma and no prior treatment other than surgery were randomized to vorasidenib (40 mg oral once daily) or matched placebo in 28-day cycles. The primary endpoint was imaging-based progression-free survival per blinded independent review committee. The key secondary endpoint was time to next intervention. Crossover to vorasidenib from placebo was permitted upon confirmed imaging-based disease progression.
Results:
A total of 331 patients were randomized to vorasidenib (n=168) or placebo (n=163). At a median follow-up of 14.2 months, 226 (68.3%) patients remained on treatment. Progression-free survival was significantly improved in the vorasidenib group versus the placebo group (hazard ratio, 0.39; 95% confidence interval, 0.27 to 0.56; two-sided P=0.000000135; median 27.7 vs 11.1 months, respectively). Time to next intervention was significantly improved in the vorasidenib group versus the placebo group (hazard ratio, 0.26; 95% confidence interval, 0.15 to 0.43; two-sided P=0.000000037). Adverse events of grade 3 or higher occurring in ≥10% of patients were increased alanine aminotransferase (10% with vorasidenib and 0% with placebo).
Conclusion:
In patients with grade 2 IDH-mutant glioma, vorasidenib significantly prolonged progression-free survival and delayed time to next intervention with a predominantly low-grade safety profile. (Funded by Servier Pharmaceuticals; INDIGO ClinicalTrials.gov number, NCT04164901)
Gliomas are the most common malignant primary brain tumor in adults and are further divided by the World Health Organization (WHO) Classification into distinct tumor subtypes and tumor grades based on a combination of histological and molecular features.1 Mutations in the genes encoding the metabolic enzymes isocitrate dehydrogenase 1 (IDH1) or 2 (IDH2) are present in nearly all grade 2 diffuse gliomas in adults.2–4 The mutant enzyme produces the metabolite 2-hydroxyglutarate, which accumulates in glioma tissue and competitively inhibits various α-ketoglutarate-dependent enzymes, resulting in a broad range of changes in DNA hydroxymethylation, gene expression, cellular differentiation, and the tumor microenvironment.5,6 Given their unique molecular pathogenesis, gliomas with IDH mutations are classified as distinct disease entities in the most recent update to the WHO Classification.1 Gliomas that harbor a mutation in IDH1/IDH2 and an unbalanced translocation between chromosomes 1 and 19 (“1p/19q-codel”) are defined as oligodendrogliomas, while IDH-mutant gliomas without 1p/19q codeletion (“1p/19q-non-codel”) are defined as astrocytomas.7,8 IDH-mutant grade 2 oligodendrogliomas and astrocytomas grow continuously, albeit slowly, infiltrate the normal brain, and eventually become aggressive tumors with accelerated tumor growth and neovascularization, reflected by appearance of contrast enhancement on magnetic resonance imaging (MRI).9,10
The combination of radiation and chemotherapy has become standard-of-care for the postoperative treatment of patients with IDH-mutant grade 3 gliomas11,12 and for patients with IDH-mutant grade 2 gliomas who are considered high risk for early disease progression.13 While adjuvant chemoradiation can result in long-lasting disease remissions, treatment is not curative and is associated with radiation-induced neurocognitive dysfunction, chemotherapy-associated DNA hypermutation, and other toxicities.14–16 To delay these potential long-term toxicities, many patients with IDH-mutant grade 2 gliomas do not receive immediate adjuvant chemoradiation following their initial diagnosis, and are instead monitored with serial brain MRI scans.17–19 This “watch-and-wait” period provides an opportunity for the evaluation of novel therapies with the potential to postpone the need for radiation and chemotherapy, preserve quality of life, and alter the natural history of diffuse glioma.
Vorasidenib is a dual inhibitor of the mutant IDH1 and IDH2 enzymes that was developed for penetration across the blood–brain barrier.20 During initial clinical evaluation, vorasidenib showed a predominantly low-grade safety profile and preliminary antitumor activity in patients with non-enhancing glioma.21 In a peri-operative trial, vorasidenib showed >90% reduction in the concentration of the oncometabolite, 2-hydroxyglutarate, in resected tumor, which was associated with reversed gene expression and epigenetic changes typically associated with IDH mutation in glioma.22 The current phase 3 trial was conducted to determine whether vorasidenib, when given at 40 mg daily oral dose, could prolong progression-free survival and delay the initiation of further anticancer therapy in patients with residual or recurrent IDH-mutant grade 2 gliomas who had undergone surgery as their only treatment and were not in need of immediate chemotherapy or radiotherapy in the opinion of the treating physician.
METHODS
TRIAL DESIGN AND RANDOMIZATION
This global, double-blind, randomized, placebo-controlled phase 3 study (NCT04164901) assessed the efficacy and safety of vorasidenib, as compared with placebo, in patients with residual or recurrent grade 2 IDH-mutant glioma. Patients received 40 mg vorasidenib or placebo orally, once-daily in continuous 28-day cycles. A central interactive web response system was used to randomly assign patients in a 1:1 ratio to receive vorasidenib or placebo in identical labeled study drug containers to ensure patients, investigators, study site staff and the sponsor were blinded to treatment assignment. Randomization was stratified by locally determined chromosome 1p19q status (codeleted or non-codeleted) and baseline tumor size (longest diameter of ≥2 cm or <2 cm).23–27 Imaging was done using a standardized imaging protocol.28 Treatment continued until imaging-based disease progression was confirmed by blinded independent review committee, unacceptable toxicity, the need for other anticancer therapy as determined by the investigator, or pregnancy. Patients randomized to the placebo arm were eligible to cross over to vorasidenib treatment upon blinded review-confirmed imaging-based disease progression.
TRIAL OVERSIGHT
Written informed consent was provided by all patients or their legal guardians before participating in the trial, and approval from the institutional review board or independent ethics committee was obtained at each trial site. An independent data monitoring committee regularly reviewed safety and other clinical data, as well as the efficacy data following prespecified interim analyses 1 and 2. The study was unblinded following the recommendation of the data monitoring committee based on early demonstration of efficacy following the planned second interim analysis (data cut-off date: September 6, 2022). This trial was conducted according to International Conference on Harmonisation of Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. This trial was designed by the former sponsor, Agios Pharmaceuticals, in collaboration with the investigators. After the start of the trial, Servier Pharmaceuticals acquired the Agios’ oncology business. Data were collected by the investigators and their research staff. The authors analyzed the data in collaboration with the sponsor. Drafts of the manuscript were written by the first author and revised in collaboration with all the authors and the sponsor, all of whom vouch for the completeness and accuracy of the data and for the adherence of the trial to the protocol. Assistance in manuscript preparation was provided by a professional medical writer funded by the sponsor.
PATIENTS
Patients aged 12 years and older with residual or recurrent histologically confirmed grade 2 oligodendroglioma or astrocytoma (per WHO 2016 criteria)29 with centrally confirmed IDH1 and IDH2 mutation status were eligible. An investigational clinical trial assay, based on the Oncomine™ Dx Target Test and developed in partnership with Thermo Fisher Scientific Inc. (Life Technologies Corporation, Carlsbad, CA, U.S.A.), was used to centrally confirm the detection of IDH1 R132H/C/G/S/L mutation variants or IDH2 R172K/M/W/S/G mutation variants. Other key eligibility criteria included a Karnofsky performance scale score of at least 80, at least one prior surgery (with the most recent surgery occurring between 1 and 5 years before randomization), no other anticancer treatment for glioma, no need for glucocorticoids for signs/symptoms from glioma, not being in immediate need of chemotherapy or radiation, and adequate hepatic and renal function. Patients had measurable non-enhancing disease (≥1 target lesion measuring ≥1 cm × ≥1 cm) that was centrally assessed based on, at minimum, 2D T1-weighted MRI pre- and postcontrast enhancement, 2D T2-weighted MRI, and 2D fluid-attenuated inversion recovery scans, confirmed by blinded review before enrollment. Any enhancement was minimal, non-nodular, and non-measurable. Other major exclusion criteria included the presence of any features assessed as high risk by the investigator (including uncontrolled seizures, brain stem involvement and clinically relevant functional or neurocognitive deficits caused by the tumor) and a heart-rate corrected QT interval of ≥450 msec using Fridericia’s formula.
ENDPOINTS AND ASSESSMENTS
The primary endpoint of the study was progression-free survival, defined as the time from randomization to the first documented imaging-based progressive disease (as assessed by blinded independent assessment per modified Response Assessment for Neuro-oncology for Low-Grade Gliomas [RANO-LGG])30 or death from any cause, whichever occurred earlier. The key secondary endpoint was time to next intervention, defined as the time from randomization to the initiation of the first subsequent anticancer therapy (including vorasidenib, for patients randomized to placebo who subsequently crossed over) or death from any cause. Secondary endpoints included objective response and safety, as well as tumor growth rate by volume (determined by blinded independent review), health-related quality of life, and overall survival, which will be reported elsewhere. Objective response was determined by blinded independent review per modified RANO-LGG. Safety and adverse-event profiles were assessed through physical examination, including neurological status, Karnofsky performance scale scores, vital signs, 12-lead electrocardiograms, clinical laboratory evaluations (hematologic, chemical, and coagulation studies), and adverse events (according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0).31
STATISTICAL ANALYSES
The full analysis set, which included all patients who underwent randomization (according to the intention-to-treat principle), was used for all efficacy analyses, unless otherwise specified. The safety analysis set, which included all patients who received at least one dose of vorasidenib or placebo, was used for all safety analyses, unless otherwise specified. Categorical data were summarized by frequency distributions. Continuous data were summarized by descriptive statistics. Time-to-event endpoints were estimated using the Kaplan–Meier method, with point estimates and 95% confidence intervals (CIs) provided where appropriate. All reported P values are two-sided.
With a sample size of ~340 patients, 164 progression-free survival events would provide ≥90% power to detect a hazard ratio (HR) of 0.6 using log-rank test at a one-sided significance level of 0.025. The study followed a group-sequential design with three prespecified analyses (interim analysis 1, futility at ~55 progression-free survival events; interim analysis 2, superiority/futility at ~123 progression-free survival events; final analysis at ~164 progression-free survival events), with predefined Gamma family (–24) alpha-spending function to determine the efficacy boundaries. To control the overall type 1 error in the study, the fixed sequence testing32 was used to adjust for the multiple statistical testing of the primary and key secondary efficacy end points; time to next intervention was tested only if progression-free survival reached statistical significance. All stratified analyses were conducted based on randomization stratification factors using interactive web response system data: chromosome 1p19q codeletion status (codeleted or non-codeleted) and baseline tumor size per local assessment (longest diameter of ≥2 cm or <2 cm). The primary efficacy analysis compared the progression-free survival between the two treatment arms using a stratified log-rank test. A stratified Cox proportional hazards model was used to estimate the HR of progression-free survival, along with its 95% CI. The key secondary efficacy analysis compared the time to next intervention between the two treatment arms using a stratified log-rank test. A stratified Cox proportional hazards model was used to estimate the HR of time to next intervention, along with its 95% CI. Prespecified subgroup analyses were performed for both progression-free survival and time to next intervention.
RESULTS
CHARACTERISTICS OF THE PATIENTS
From January 2020 to February 2022, a total of 331 patients were enrolled at 77 centers across 10 countries (58.3% of patients were from North America, 29.3% from Western Europe, and 12.4% from Israel). Overall, 168 patients were randomly assigned to the vorasidenib group, and 163 patients to the placebo group (Fig. 1). The two groups were generally balanced with respect to baseline characteristics (Table 1). The median age was 40.5 years in the vorasidenib group and 39.0 years in the placebo group. Over 50% of patients in both groups had a Karnofsky performance status score of 100. All patients had undergone prior brain tumor surgery, of whom 21.5% had undergone two or more tumor surgeries before enrollment. The median interval between the last glioma surgery and randomization was 2.4 years. Both groups included similar numbers of astrocytomas and oligodendrogliomas. The tumor size at baseline was at least 2 cm for most patients (>80%) in both groups.
Figure 1. Enrollment and Randomization of Patients.
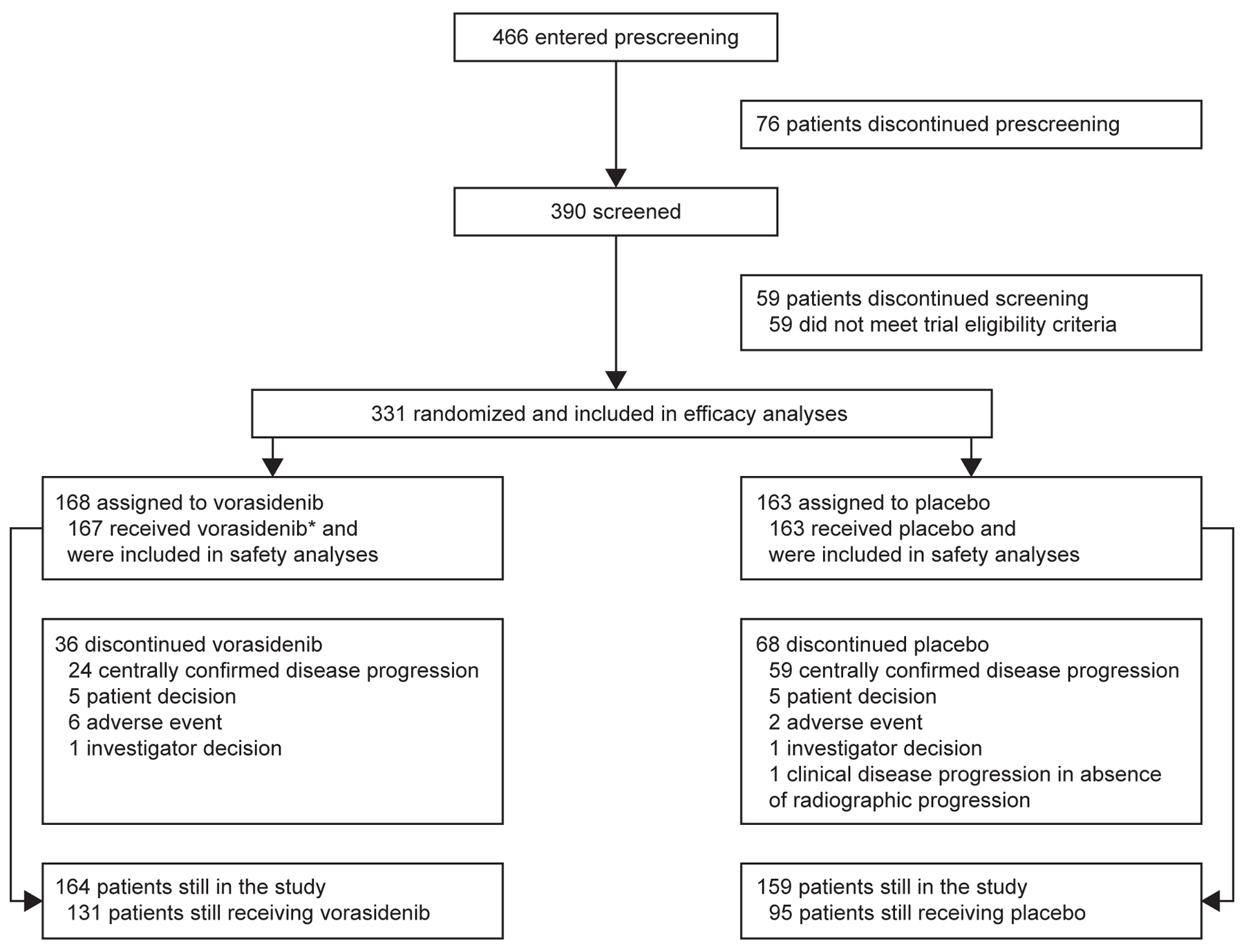
*One patient withdrew consent from study treatment and later withdrew consent from the study overall.
Table 1. Baseline Patient and Tumor Characteristics (Full Analysis Set).
| Vorasidenib (N=168) | Placebo (N=163) | |
|---|---|---|
| Median age (range) – year | 40.5 (21–71) | 39.0 (16–65) |
| Age category – year, no. (%) 16–<18 18–<40 40–<65 ≥65 |
0 76 (45.2) 90 (53.6) 2 (1.2) |
1 (0.6) 87 (53.4) 74 (45.4) 1 (0.6) |
| Sex – no. (%) Male/female |
101/67 (60.1/39.9) |
86/77 (52.8/47.2) |
| Geographic region – no. (%) North America Western Europe Rest of the World |
86 (51.2) 57 (33.9) 25 (14.9) |
107 (65.6) 40 (24.5) 16 (9.8) |
| Karnofsky performance score – no. (%) 100 90–80 |
90 (53.6) 77 (45.8) |
87 (53.4) 76 (46.6) |
| Location of tumor at initial diagnosis – no. (%) Frontal lobe Frontoparietal Frontotemporal Parietal lobe Temporal lobe Other |
93 (55.4) 4 (2.4) 9 (5.4) 19 (11.3) 21 (12.5) 22 (13.5) |
95 (58.3) 6 (3.7) 12 (7.4) 11 (6.7) 20 (12.3) 19 (11.7) |
| Time from initial diagnosis to randomization – month Mean (SD) Median (range) |
39.6 (28.9) 35.4 (12–234) |
37.5 (29.4) 29.6 (11–230) |
| Prior surgeries for glioma – no. (%) 1/≥2 |
126/42 (75.0/25.0) |
134/29 (82.2/17.8) |
| Time from last surgery for glioma to randomization – year Mean (SD) Median (range) |
2.7 (1.1) 2.5 (0.2–5.2)† |
2.6 (1.3) 2.2 (0.9–5.0) |
| Histological subtype – no. (%) Oligodendroglioma/astrocytoma |
88/80 (52.4/47.6) |
84/79 (51.5/48.5) |
|
IDH1 mutation status – no. (%)‡ IDH1 positive R132C R132G R132H R132L R132S |
163 (97.0) 8 (4.8) 5 (3.0) 146 (86.9) 2 (1.2) 2 (1.2) |
152 (93.3) 7 (4.3) 1 (0.6) 138 (84.7) 4 (2.5) 2 (1.2) |
|
IDH2 mutation status – no. (%) IDH2 positive R172K R172W R172G |
5 (3.0) 3 (1.8) 0 2 (1.2) |
11 (6.7) 10 (6.1) 1 (0.6) 0 |
| Chromosome 1p19q codeletion status – no. (%)║ Codeleted/non-codeleted |
88/80 (52.4/47.6) |
84/79 (51.5/48.5) |
| Tumor size at baseline – no. (%)║ Longest diameter of ≥2 cm/<2 cm |
139/29 (82.7/17.3) |
137/26 (84.0/16.0) |
IDH isocitrate dehydrogenase; SD standard deviation.
One patient had a biopsy during prescreening to obtain tumor tissue for IDH mutation status testing, which was allowed per protocol;
Two patients had CDKN2A homozygous deletion. See the Supplementary appendix for more detail
Data are reported as collected by electronic case report forms.
FOLLOW-UP AND OUTCOMES
As of September 6, 2022, the median follow-up period was 14.0 months in the vorasidenib group (interquartile range, 10.1 to 17.9) and 14.3 months in the placebo group (interquartile range, 10.0 to 18.1). No patients were lost to follow-up for the primary outcome and no deaths were noted in either treatment group.
Imaging-based progression per blinded independent review occurred in 135 out of 331 randomized patients: 88 out of 163 patients (54.0%) in the placebo group and 47 out of 168 patients (28.0%) in the vorasidenib group. The primary endpoint, imaging-based progression-free survival per blinded independent review, was significantly improved in the vorasidenib group compared with the placebo group. Median imaging-based progression-free survival measured from randomization to first documentation of progressive disease per blinded independent review or death was 27.7 months (95% CI, 17.0 to not estimable) in the vorasidenib group compared with 11.1 months (95% CI, 11.0 to 13.7) in the placebo group (Fig. 2A) (P=0.000000135). The HR comparing the vorasidenib group with the placebo group was 0.39 (95% CI, 0.27 to 0.56). A prespecified analysis of imaging-based progression-free survival based on investigator assessment yielded similar results to the primary analysis (HR, 0.35; 95% CI, 0.23 to 0.54). See Table S1 for a summary of progression-free survival.
Figure 2. Progression-free Survival and Time to Next Intervention in the Full Analysis Set.
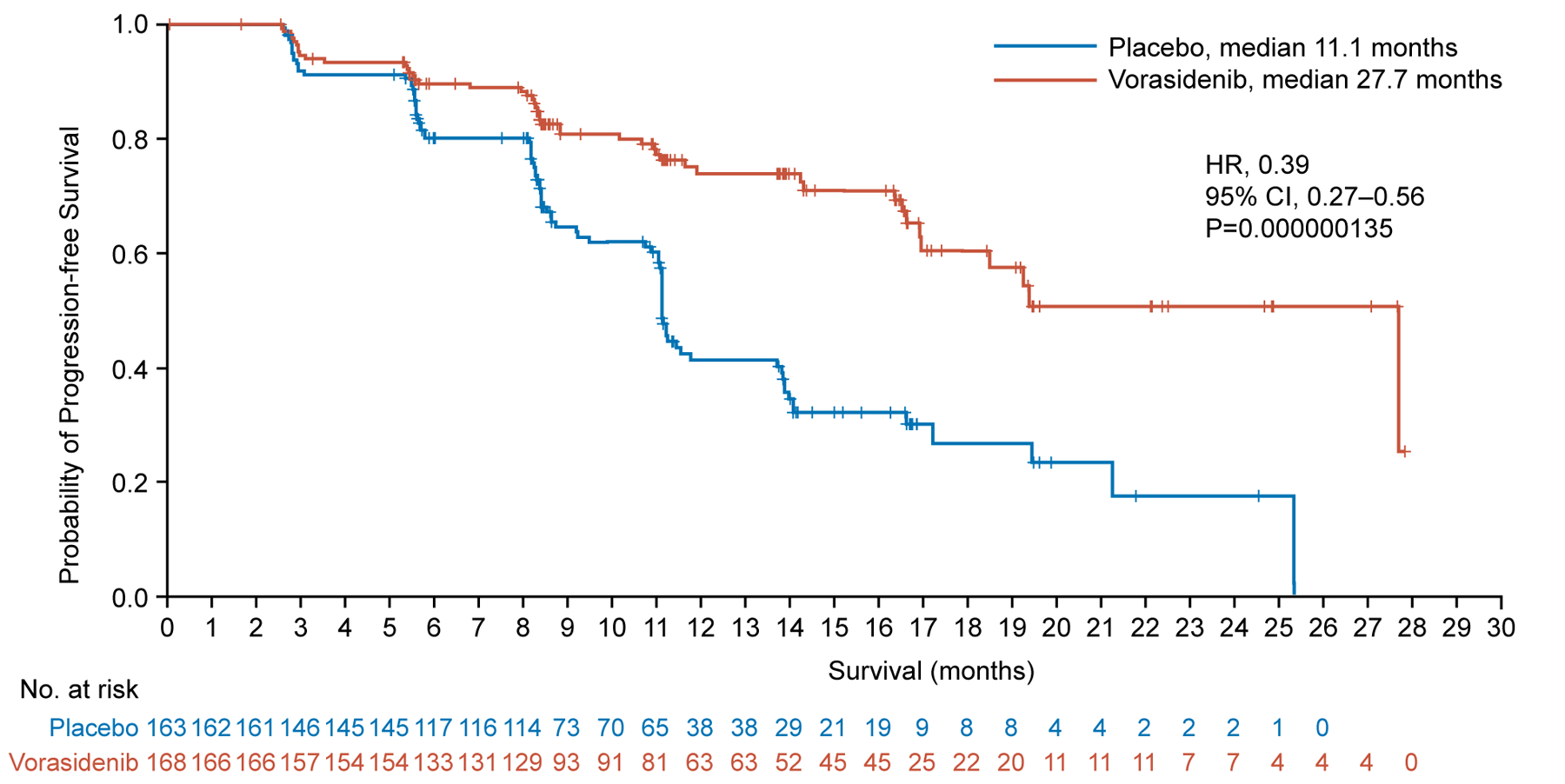
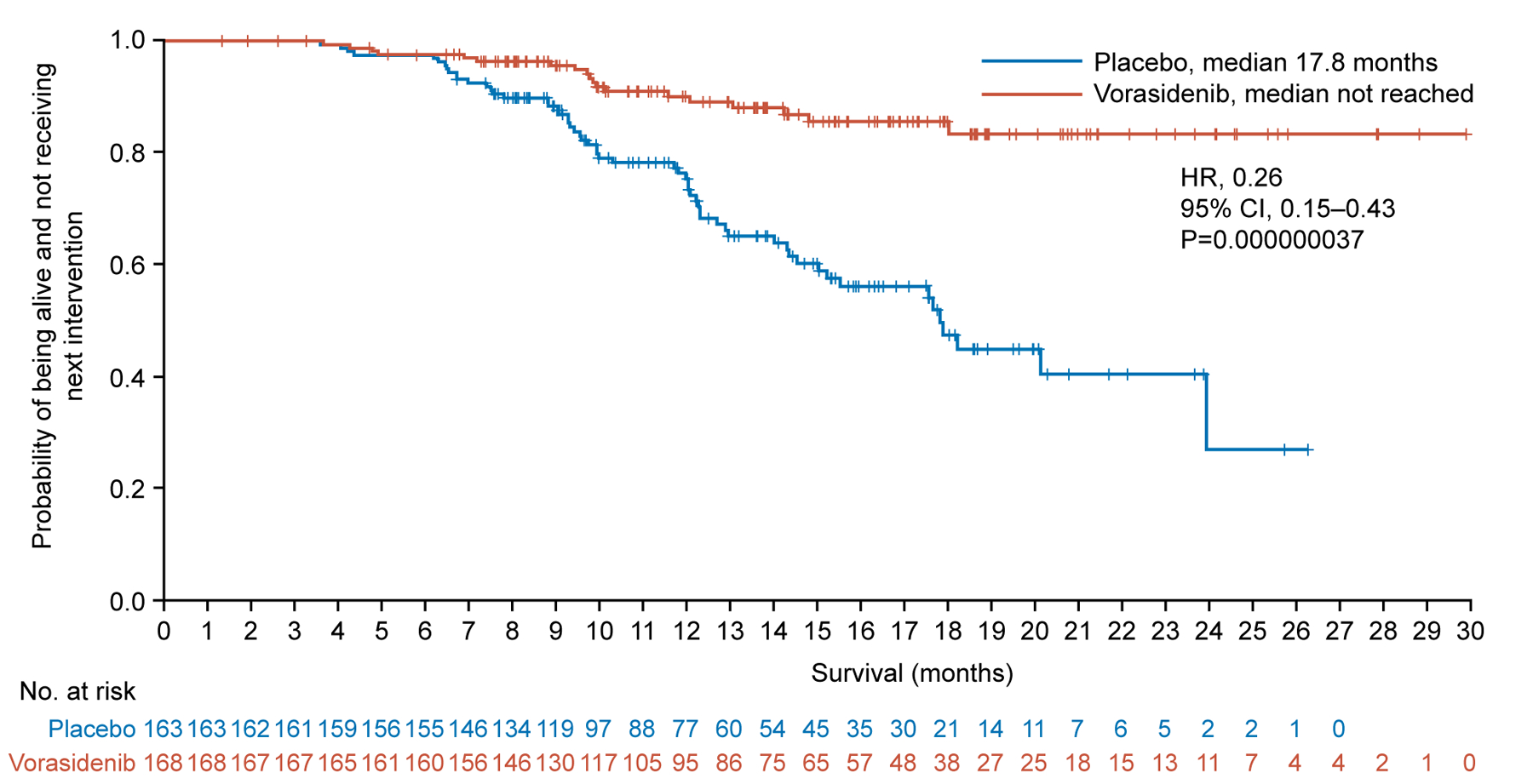
Panel A shows the Kaplan–Meier plot of the probability of imaging-based progression-free survival per blinded independent review among patients assigned to the vorasidenib group as compared with those assigned to the placebo group. Panel B shows the Kaplan–Meier plot of the probability of time to next anticancer treatment intervention among patients assigned to the vorasidenib group as compared with those assigned to the placebo group.
+Censored
CI confidence interval; HR hazard ratio.
Time to next intervention was significantly improved in the vorasidenib group compared with the placebo group (HR, 0.26; 95% CI, 0.15 to 0.43; P=0.000000037). Median time to next intervention was 17.8 months (95% CI, 15.0 to not estimable) for patients in the placebo group and was not reached for patients in the vorasidenib group (Fig. 2B). The probability of not receiving a next treatment intervention by 18 months was 85.6% (95% CI, 77.8% to 90.8%) in the vorasidenib group versus 47.4% (95% CI, 35.8% to 58.2%) in the placebo group; by 24 months the probability was 83.4% (95% CI, 74.0% to 89.6%) versus 27.0% (95% CI, 7.9% to 50.8%), respectively. Overall, 77 randomized patients received another anticancer intervention following discontinuation of blinded treatment. In the placebo group, 58 patients (35.6%) received another anticancer intervention, including crossover to vorasidenib (52 patients, 31.9%), surgery, chemotherapy, and/or radiation. In the vorasidenib group, 19 patients (11.3%) received another anticancer therapy including surgery, chemotherapy, and/or radiation) (Table S2).
The results of subgroup analyses for progression-free survival (Fig. 3A) and time to next intervention (Fig. 3B) favored vorasidenib across all subgroups, including 1p19q codeletion status (see Fig. S1 and Table S3), which reflects the histopathological subtype.
Figure 3. Subgroup Analyses of Progression-free Survival and Time to Next Intervention in the Full Analysis Set.
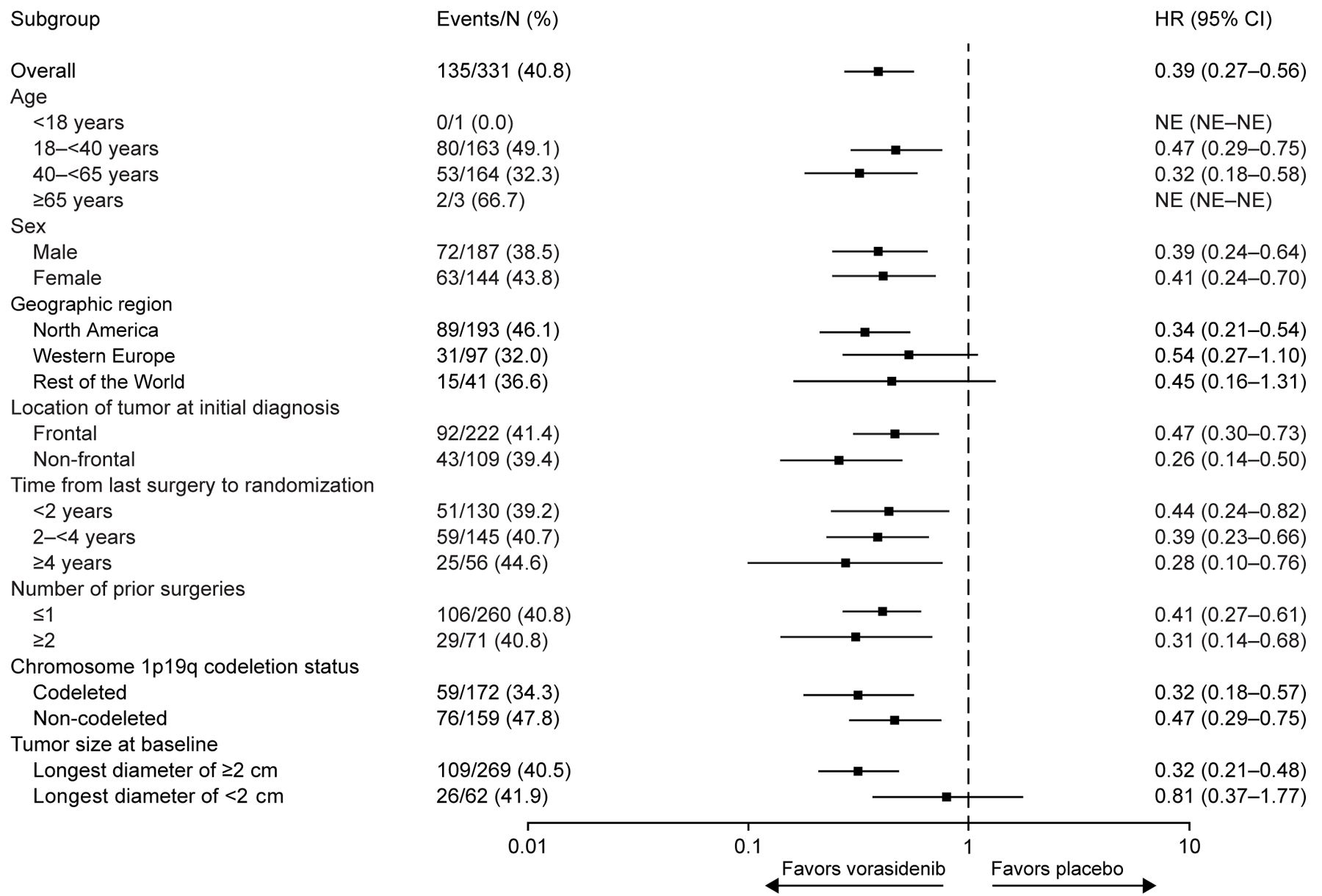
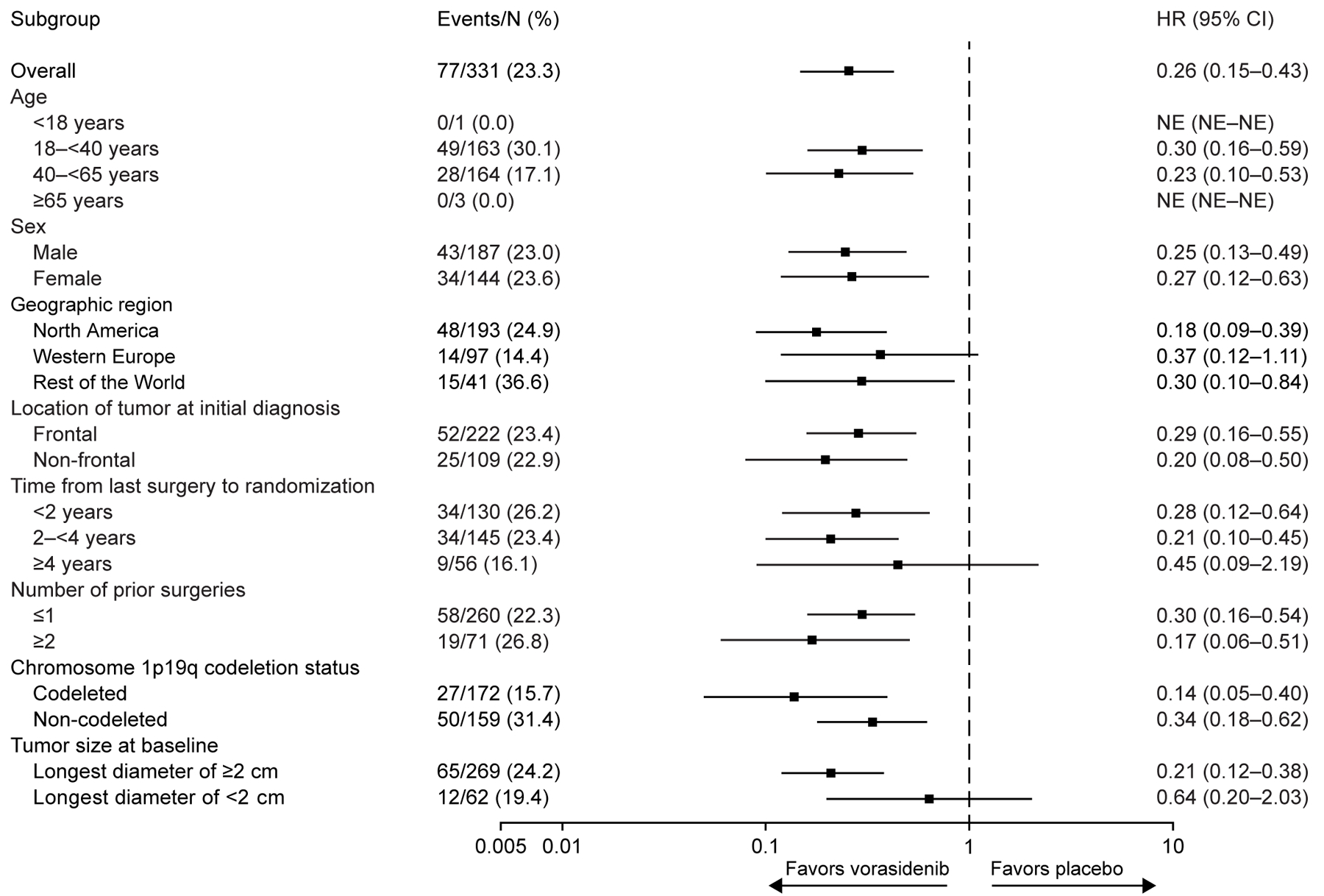
Panel A shows a forest plot of HRs for imaging-based progression-free survival per blinded independent review in key subgroups. Panel B shows a forest plot of HRs for time to next intervention in key subgroups. Subgroup analyses are based on stratification factor data as entered in the interactive web response system. For data shown in both panels, the widths of the CIs have not been adjusted for multiplicity. Thus, the CIs should not be used to reject (or not reject) the trial agent effects.
CI confidence interval; HR hazard ratio; NE not estimable.
Best overall responses by blinded independent review are shown in Table S4.
SAFETY
Overall, vorasidenib was associated with mainly low grade toxicity; treatment-emergent adverse events of any grade (≥10%) are presented in Table 2. Treatment-emergent adverse events of grade 3 or higher were observed among 27 patients (16.2%) in the vorasidenib group and nine patients (5.5%) in the placebo group. The most common treatment-emergent adverse event of grade 3 or higher was increased alanine aminotransferase (vorasidenib 9.6%, placebo 0%). Other grade 3 or higher events more common with vorasidenib were increased aspartate aminotransferase (vorasidenib 4.2%, placebo 0%) and increased gamma-glutamyltransferase (vorasidenib 3.0%, placebo 1.2%). Serious treatment-related adverse events occurred in 1.8% of patients in the vorasidenib group and in no patients in the placebo group (see Supplementary appendix for more information). Treatment-emergent adverse events led to treatment discontinuation in six patients (3.6%) in the vorasidenib group and in two patients (1.2%) in the placebo group. Treatment-emergent adverse events led to dose reduction in 18 patients (10.8%) in the vorasidenib group and five patients (3.1%) in the placebo group. Treatment interruption due to treatment-emergent adverse events occurred in 50 patients (29.9%) in the vorasidenib group and 37 patients (22.7%) in the placebo group.
Table 2. Most Common Treatment-emergent Adverse Events (Safety Analysis Set)*.
| Vorasidenib (N=167) | Placebo (N=163) | |||
|---|---|---|---|---|
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| Any adverse event – no. (%) | 141 (84.4) | 27 (16.2) | 128 (78.5) | 9 (5.5) |
| Increased alanine aminotransferase | 65 (38.9) | 16 (9.6) | 24 (14.7) | 0 |
| Increased aspartate aminotransferase | 48 (28.7) | 7 (4.2) | 13 (8.0) | 0 |
| Increased gamma-glutamyltransferase | 26 (15.6) | 5 (3.0) | 8 (4.9) | 2 (1.2) |
| COVID-19 | 55 (32.9) | 0 | 47 (28.8) | 0 |
| Fatigue | 54 (32.3) | 1 (0.6) | 52 (31.9) | 2 (1.2) |
| Headache | 45 (26.9) | 0 | 44 (27.0) | 1 (0.6) |
| Diarrhea | 41 (24.6) | 1 (0.6) | 27 (16.6) | 1 (0.6) |
| Nausea | 36 (21.6) | 0 | 37 (22.7) | 0 |
| Dizziness | 25 (15.0) | 0 | 26 (16.0) | 0 |
| Seizure | 23 (13.8) | 7 (4.2) | 19 (11.7) | 4 (2.5) |
| Constipation | 21 (12.6) | 0 | 20 (12.3) | 0 |
The safety analysis set included all the patients who received at least one dose of study treatment. Events listed are those of any grade that occurred in at least 10% of the patients in the vorasidenib group.
DISCUSSION
Diffuse gliomas with IDH mutation represent the most common malignant primary brain tumors diagnosed in adults aged <50, are not curable with current therapies, and continuously grow and infiltrate the normal brain in the absence of treatment.9,10,17 Treatment with vorasidenib significantly prolonged imaging-based progression-free survival per blinded independent review and time to next intervention in patients who were not in need of immediate chemotherapy or radiotherapy. The study met the primary and key secondary endpoints in the preplanned second interim analysis, and after unblinding, all patients in the placebo group were subsequently offered crossover to the vorasidenib arm. Though no formal statistical testing was planned for subgroup analyses, results were generally consistent, favoring vorasidenib across nearly all subgroups assessed. In some subgroups, such as those with tumors <2 cm, results should be interpreted with caution due to the small number of events. Vorasidenib had a safety profile of mainly low grade toxicities; grade ≥3 adverse events were more common in the vorasidenib group than in the placebo group, though the rates of serious adverse events and treatment discontinuations were low. Additional endpoints, including the impact of treatment on seizures, health-related quality of life and neurocognition, are planned to be reported at a later time. Follow-up for overall survival remains ongoing.
The INDIGO trial is a phase 3 clinical trial with a molecularly targeted therapy for IDH-mutant glioma. Molecularly targeted therapies have the greatest potential for long-term disease-modifying impact when deployed at the earliest disease stage.33 IDH mutations occur early in the disease course.34 The patient population in the current study represents the earliest clinical phase in tumorigenesis of IDH-mutant WHO grade 2 glioma, within 1 to 5 years of surgery, before any other cancer therapy, and before any measurable contrast-enhancement of the tumor on MRI. The “watch-and-wait” period for these patients represents an opportunity to detect a clear signal of antitumor activity for novel therapies using a placebo-controlled study design, and our study establishes a foundation for future trials with a similar design. Current treatment recommendations for IDH-mutant glioma define “risk” based on age, extent of resection, and grade of disease; however, limited data justify categorizing risk based on these factors alone.35 The INDIGO trial allowed for investigator discretion when determining risk while still requiring exclusion of high-risk features (such as enhancing disease or brainstem involvement) and uncontrolled disease-related symptoms. As such, findings could be generalized to the real-world setting in how these patients are managed.
Ivosidenib and enasidenib, inhibitors of mutant IDH1 and IDH2, respectively, have shown single-agent activity for the treatment of IDH1- or IDH2-mutant acute myeloid leukemia36,37 and IDH1-mutant cholangiocarcinoma.38 Both agents have also shown activity in combination therapy regimens.39–41 While the current study documents single-agent activity of vorasidenib in patients with previously untreated WHO grade 2 glioma, additional trials will be required to define the role of vorasidenib, alone or as part of combination therapy regimens, for patients with glioma who have already received prior cancer therapy or who present with WHO grade 3 or 4 disease. The ongoing molecular examination of pretreatment tumor biopsies and the determination of tumor volume growth rates before and after study enrollment, an approach that has been useful in our earlier clinical trials,21,42,43 will help determine opportunities for mechanism-based combinations. Such data on patients on this study are not yet available.
Supplementary Material
ACKNOWLEDGMENTS
We thank the patients who participated in the INDIGO trial, their families / caregivers, and our co-investigators. This study was sponsored by Servier Pharmaceuticals LLC. We are grateful for baseline comutation analysis provided by Adriana E. Tron, PhD, and Sung Choe, PhD, from Servier Pharmaceuticals LLC. Medical editorial assistance was provided by Debbi Gorman, PhD, from Cogent (an AMICULUM agency), funded by Servier Pharmaceuticals LLC.
Footnotes
A complete list of investigators in the phase 3, multicenter, randomized, double-blind, placebo-controlled study of vorasidenib in patients with residual or recurrent grade 2 glioma with an IDH1 or IDH2 mutation (INDIGO) is provided in the Supplementary appendix, available at NEJM.org.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 2021;23(8):1231–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008;321(5897):1807–12. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med 2009;360(8):765–73. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol 2009;118(4):469–74. [DOI] [PubMed] [Google Scholar]
- 5.Lu C, Ward PS, Kapoor GS, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 2012;483(7390):474–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 2011;19(1):17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Research N, Brat DJ, Verhaak RG, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 2015;372(26):2481–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 2015;372(26):2499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandonnet E, Delattre JY, Tanguy ML, et al. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol 2003;53(4):524–8. [DOI] [PubMed] [Google Scholar]
- 10.Rees J, Watt H, Jager HR, et al. Volumes and growth rates of untreated adult low-grade gliomas indicate risk of early malignant transformation. Eur J Radiol 2009;72(1):54–64. [DOI] [PubMed] [Google Scholar]
- 11.Lassman AB, Hoang-Xuan K, Polley MC, et al. Joint final report of EORTC 26951 and RTOG 9402: phase III trials with procarbazine, lomustine, and vincristine chemotherapy for anaplastic oligodendroglial tumors. J Clin Oncol 2022;40(23):2539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Bent MJ, Tesileanu CMS, Wick W, et al. Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053–22054): second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol 2021;22(6):813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med 2016;374(14):1344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein M, Heimans JJ, Aaronson NK, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet 2002;360(9343):1361–8. [DOI] [PubMed] [Google Scholar]
- 15.Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol 2009;8(9):810–8. [DOI] [PubMed] [Google Scholar]
- 16.Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science 2014;343(6167):189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller JJ, Gonzalez Castro LN, McBrayer S, et al. Isocitrate dehydrogenase (IDH) mutant gliomas: a Society for Neuro-Oncology (SNO) consensus review on diagnosis, management, and future directions. Neuro Oncol 2023;25(1):4–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohile NA, Messersmith H, Gatson NT, et al. Therapy for diffuse astrocytic and oligodendroglial tumors in adults: ASCO-SNO guideline. J Clin Oncol 2022;40(4):403–26. [DOI] [PubMed] [Google Scholar]
- 19.Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 2021;18(3):170–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konteatis Z, Artin E, Nicolay B, et al. Vorasidenib (AG-881): a first-in-class, brain-penetrant dual inhibitor of mutant IDH1 and 2 for treatment of glioma. ACS Med Chem Lett 2020;11(2):101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mellinghoff IK, Penas-Prado M, Peters KB, et al. Vorasidenib, a dual inhibitor of mutant IDH1/2, in recurrent or progressive glioma; results of a first-in-human phase I trial. Clin Cancer Res 2021;27(16):4491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mellinghoff IK, Lu M, Wen PY, et al. Vorasidenib and ivosidenib in IDH1-mutant low-grade glioma: a randomized, perioperative phase 1 trial. Nat Med 2023;29(3):615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw EG, Berkey B, Coons SW, et al. Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial. J Neurosurg 2008;109(5):835–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Bent MJ, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet 2005;366(9490):985–90. [DOI] [PubMed] [Google Scholar]
- 25.Weller M, van den Bent M, Tonn JC, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol 2017;18(6):e315–e29. [DOI] [PubMed] [Google Scholar]
- 26.Wijnenga MMJ, French PJ, Dubbink HJ, et al. The impact of surgery in molecularly defined low-grade glioma: an integrated clinical, radiological, and molecular analysis. Neuro Oncol 2018;20(1):103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.U.S. National Library of Medicine. Trial in low grade glioma patients: wait or treat (IWOT). NCT03763422. February 1, 2022. Available at: https://www.clinicaltrials.gov/ct2/show/NCT03763422 (accessed April 2023). [Google Scholar]
- 28.Ellingson BM, Bendszus M, Boxerman J, et al. Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro Oncol 2015;17(9):1188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wesseling P, Capper D. WHO 2016 Classification of gliomas. Neuropathol Appl Neurobiol 2018;44(2):139–50. [DOI] [PubMed] [Google Scholar]
- 30.van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol 2011;12(6):583–93. [DOI] [PubMed] [Google Scholar]
- 31.Institute NC. Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. November 27, 2017. Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5×7.pdf (accessed April 2023). [Google Scholar]
- 32.Westfall PH, Krishen K. Optimally weighted, fixed sequence and gatekeeper multiple testing procedures. J Stat Plan Inference 2001;99(1):25–40. [Google Scholar]
- 33.Thompson CB. Attacking cancer at its root. Cell 2009;138(6):1051–4. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol 2009;174(4):1149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geurts M, van den Bent MJ. On high-risk, low-grade glioma: What distinguishes high from low? Cancer 2019;125(2):174–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017;130(6):722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med 2018;378(25):2386–98. [DOI] [PubMed] [Google Scholar]
- 38.Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2020;21(6):796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DiNardo CD, Schuh AC, Stein EM, et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): a single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol 2021;22(11):1597–608. [DOI] [PubMed] [Google Scholar]
- 40.DiNardo CD, Stein AS, Stein EM, et al. Mutant isocitrate dehydrogenase 1 inhibitor ivosidenib in combination with azacitidine for newly diagnosed acute myeloid leukemia. J Clin Oncol 2021;39(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stein EM, DiNardo CD, Fathi AT, et al. Ivosidenib or enasidenib combined with intensive chemotherapy in patients with newly diagnosed AML: a phase 1 study. Blood 2021;137(13):1792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mellinghoff IK, Ellingson BM, Touat M, et al. Ivosidenib in isocitrate dehydrogenase 1-mutated advanced glioma. J Clin Oncol 2020;38(29):3398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellingson BM, Kim GHJ, Brown M, et al. Volumetric measurements are preferred in the evaluation of mutant IDH inhibition in non-enhancing diffuse gliomas: evidence from a phase I trial of ivosidenib. Neuro Oncol 2022;24(5):770–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


