Abstract
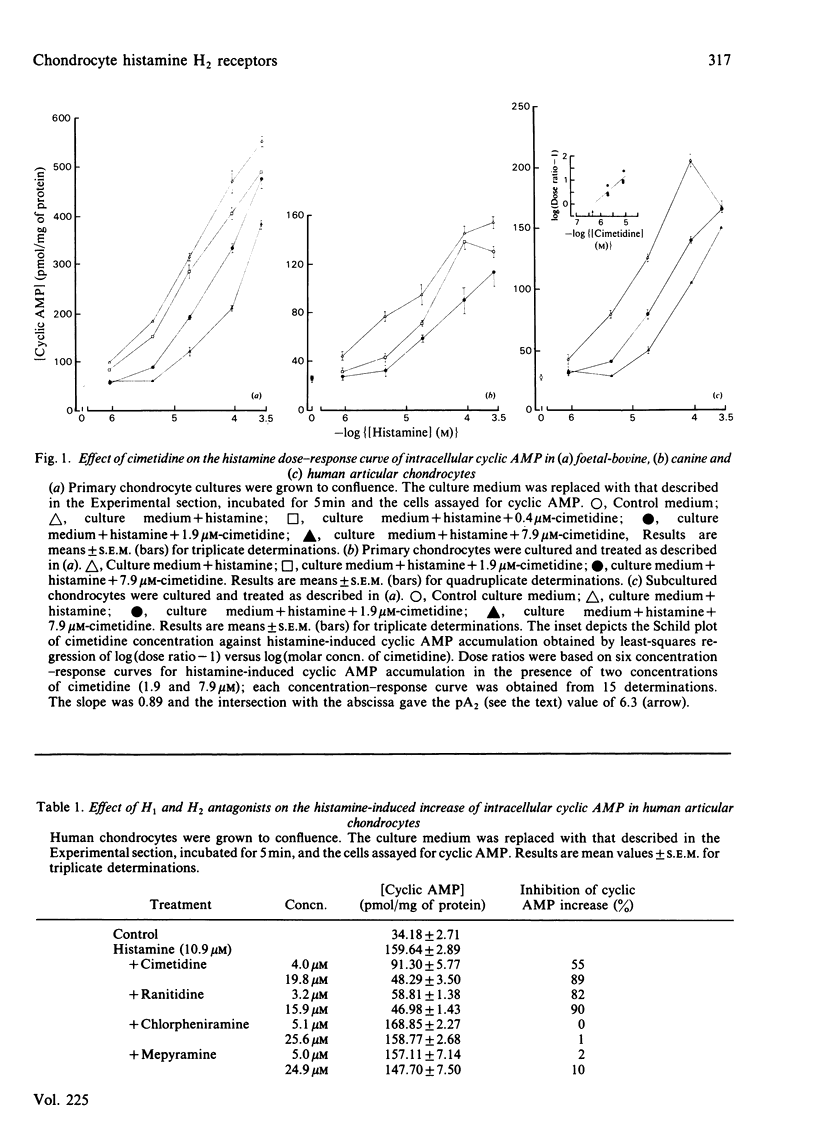
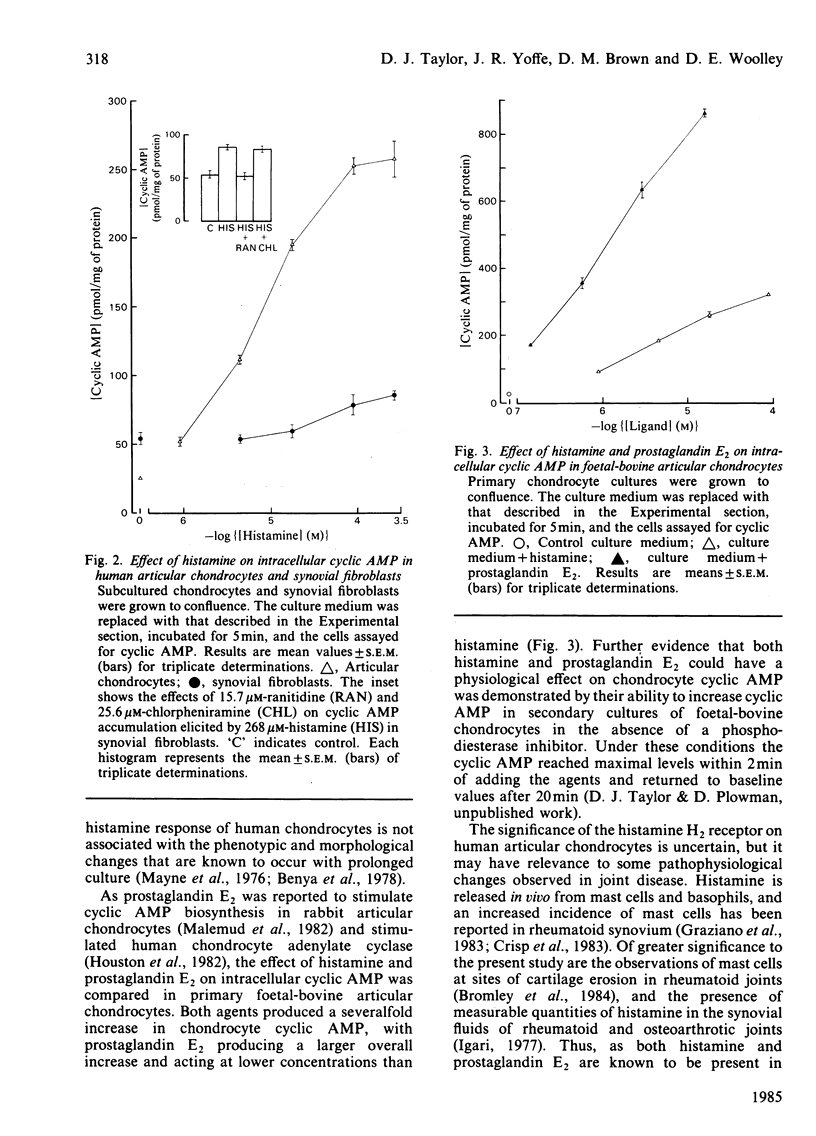
Histamine (1-100 microM) induced a concentration-dependent increase in intracellular cyclic AMP in monolayer cultures of human, canine and foetal-bovine articular chondrocytes. The dose-response curve for histamine in each culture was progressively displaced to the right with increasing concentrations of cimetidine, an H2-receptor antagonist. The histamine-induced cyclic AMP elevation in human articular chondrocytes was also significantly decreased by ranitidine, another H2 antagonist, but not by the H1 antagonists mepyramine and chlorpheniramine. These findings indicate that histamine activates chondrocyte adenylate cyclase through an H2 receptor. The cyclic AMP response of human chondrocytes to histamine was many times greater than that measured for synovial fibroblasts under similar conditions. Such findings suggest that mast-cell-chondrocyte interactions in vivo may contribute to changed chondrocyte metabolism in joint disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Haboubi H. A., Zeitlin I. J. The actions of cimetidine hydrochloride and mepyramine maleate in rat adjuvant arthritis. Eur J Pharmacol. 1982 Feb 26;78(2):175–185. doi: 10.1016/0014-2999(82)90234-5. [DOI] [PubMed] [Google Scholar]
- Benya P. D., Padilla S. R., Nimni M. E. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell. 1978 Dec;15(4):1313–1321. doi: 10.1016/0092-8674(78)90056-9. [DOI] [PubMed] [Google Scholar]
- Black J. W., Duncan W. A., Durant C. J., Ganellin C. R., Parsons E. M. Definition and antagonism of histamine H 2 -receptors. Nature. 1972 Apr 21;236(5347):385–390. doi: 10.1038/236385a0. [DOI] [PubMed] [Google Scholar]
- Bromley M., Fisher W. D., Woolley D. E. Mast cells at sites of cartilage erosion in the rheumatoid joint. Ann Rheum Dis. 1984 Feb;43(1):76–79. doi: 10.1136/ard.43.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Krane S. M., Russell R. G., Robinson D. R. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):945–949. doi: 10.1073/pnas.73.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. J., Schor S. L., Grant M. E. Effects of matrix macromolecules on chondrocyte gene expression: synthesis of a low molecular weight collagen species by cells cultured within collagen gels. J Cell Biol. 1982 Jun;93(3):767–774. doi: 10.1083/jcb.93.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston J. P., McGuire M. K., Meats J. E., Ebsworth N. M., Russell R. G., Crawford A., Mac Neil S. Adenylate cyclase of human articular chondrocytes. Responsiveness to prostaglandins and other hormones. Biochem J. 1982 Oct 15;208(1):35–42. doi: 10.1042/bj2080035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F. Mediation of local homeostasis and inflammation by leukotrienes and other mast cell-dependent compounds. Nature. 1981 Sep 10;293(5828):103–108. doi: 10.1038/293103a0. [DOI] [PubMed] [Google Scholar]
- Malemud C. J., Moskowitz R. W., Papay R. S. Correlation of the biosynthesis of prostaglandin and cyclic AMP in monolayer cultures of rabbit articular chondrocytes. Biochim Biophys Acta. 1982 Mar 15;715(1):70–79. doi: 10.1016/0304-4165(82)90051-4. [DOI] [PubMed] [Google Scholar]
- Mayne R., Vail M. S., Mayne P. M., Miller E. J. Changes in type of collagen synthesized as clones of chick chondrocytes grow and eventually lose division capacity. Proc Natl Acad Sci U S A. 1976 May;73(5):1674–1678. doi: 10.1073/pnas.73.5.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meats J. E., McGuire M. B., Russell R. G. Human synovium releases a factor which stimulates chondrocyte production of PGE and plasminogen activator. Nature. 1980 Aug 28;286(5776):891–892. doi: 10.1038/286891a0. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Yoffe J. R., Woolley D. E. Histamine H2 receptors on foetal-bovine articular chondrocytes. Biochem J. 1983 May 15;212(2):517–520. doi: 10.1042/bj2120517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang P. W., Lehotay D. C., Murphy B. E. Competitive binding assay for adenosine 3',5'-monophosphate employing a bovine adrenal protein: application to urine, plasma and tissues. J Clin Endocrinol Metab. 1972 Dec;35(6):809–817. doi: 10.1210/jcem-35-6-809. [DOI] [PubMed] [Google Scholar]
- von der Mark K., Gauss V., von der Mark H., Müller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977 Jun 9;267(5611):531–532. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]


