Abstract
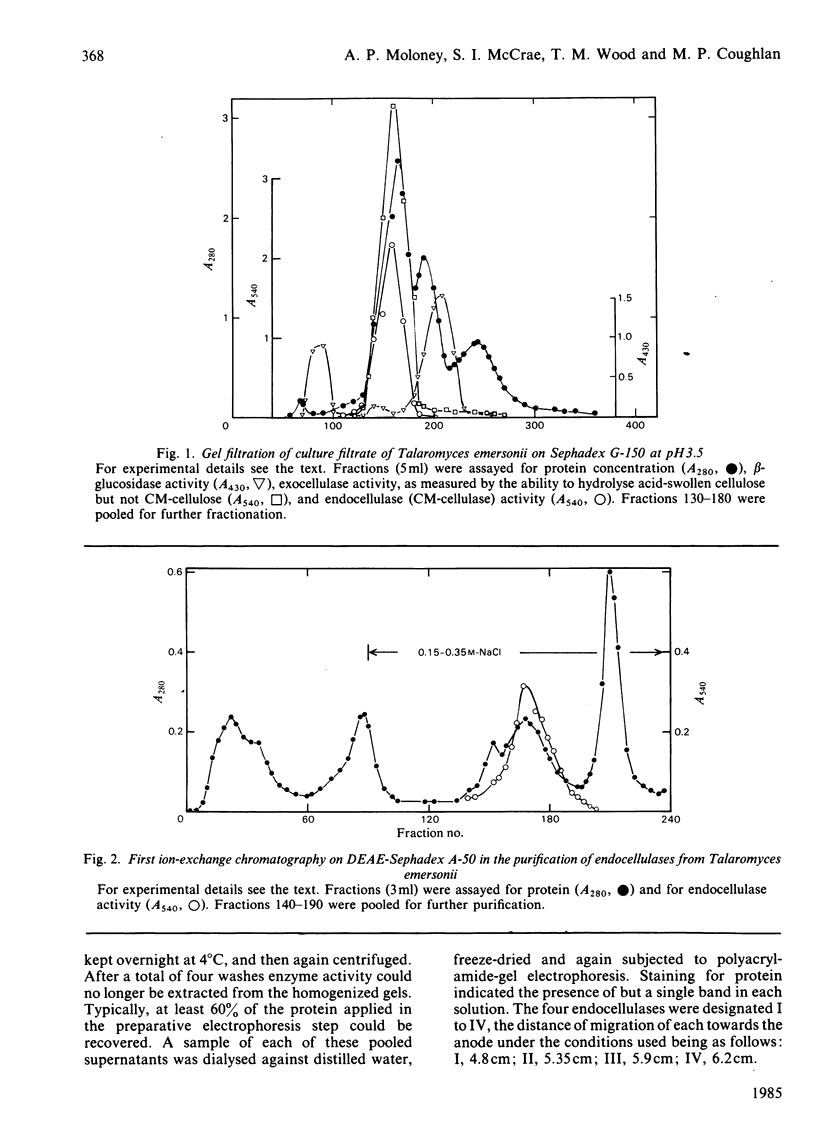
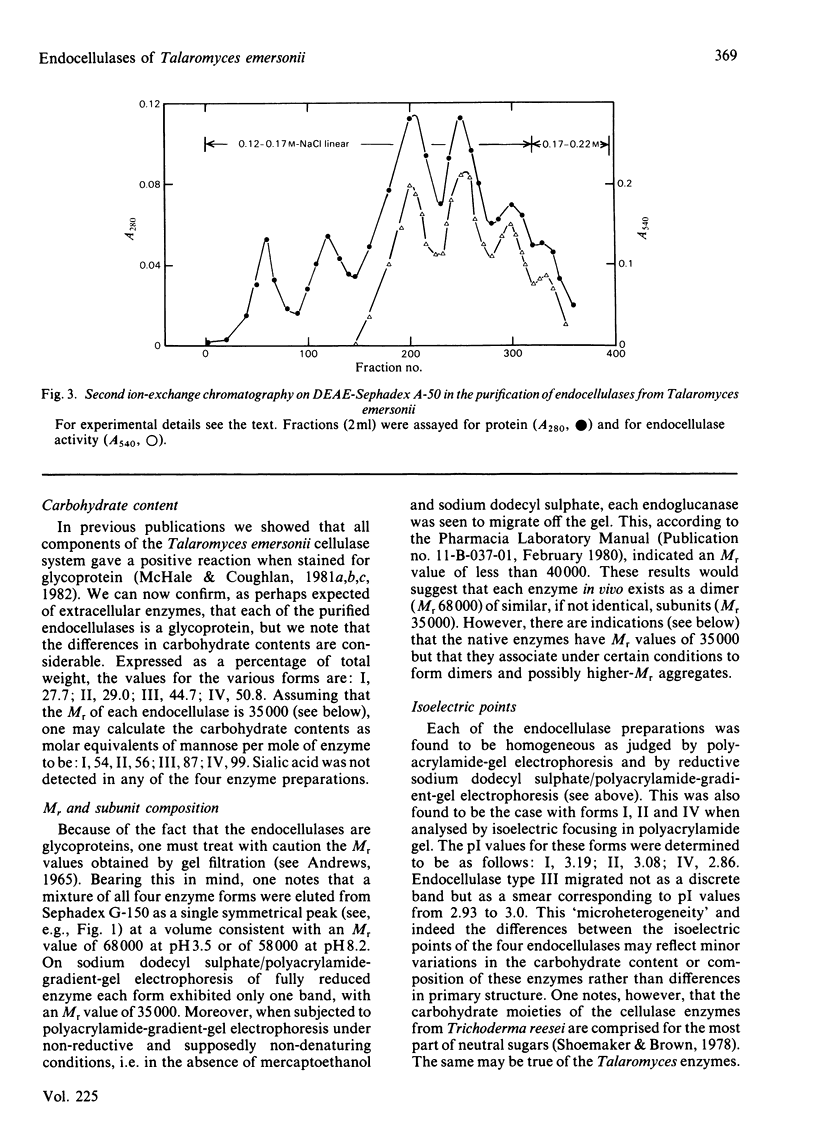
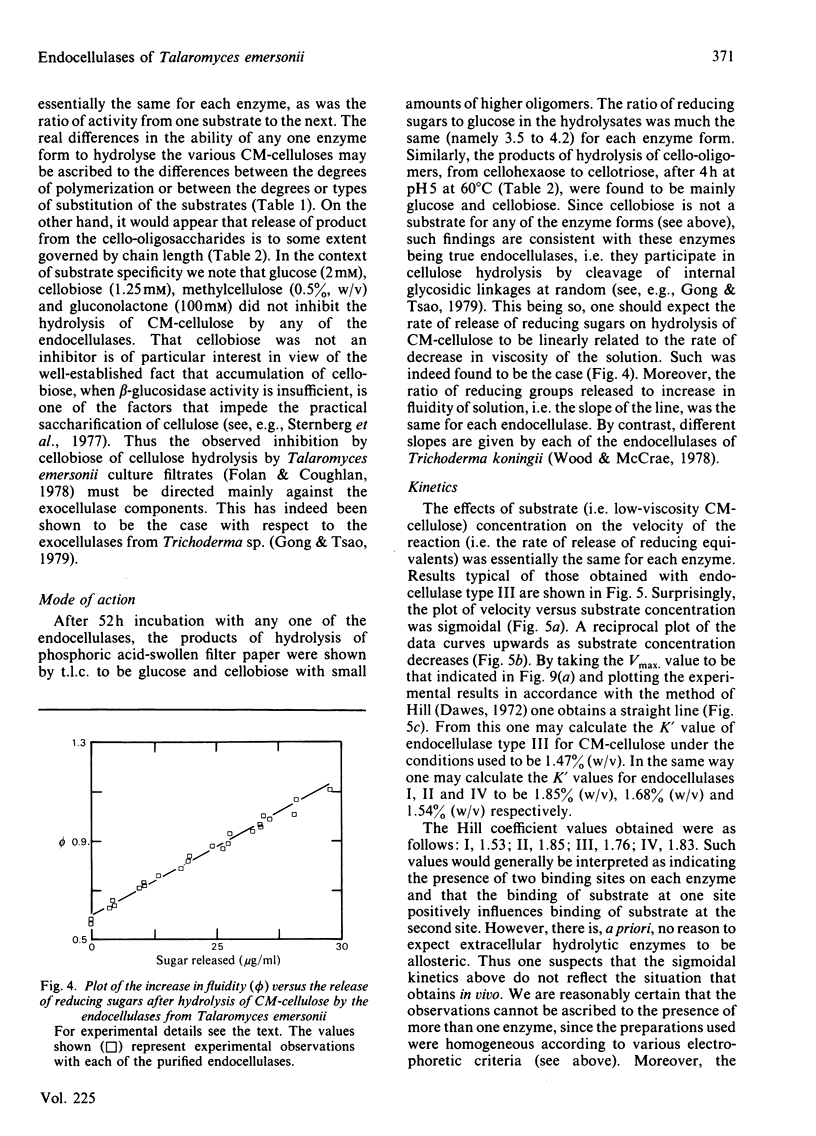
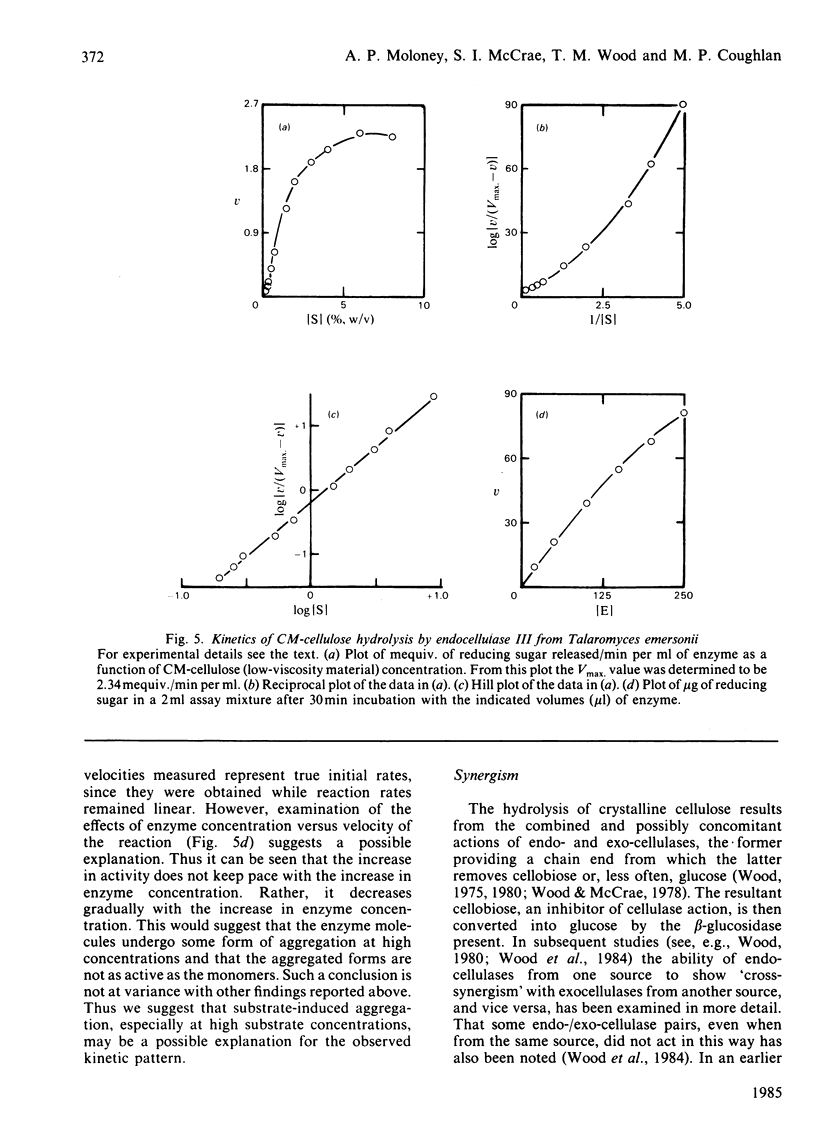
Culture filtrates of Talaromyces emersonii were found to contain four endocellulases termed I, II, III and IV, the last having the greatest electrophoretic mobility towards the anode in homogeneous 5%-(w/v)-polyacrylamide gels at pH 4.5. All four are glycoproteins, the carbohydrate contents being: I, 27.7%; II, 29.0%; III, 44.7%; IV, 50.8. Each form is eluted as a single peak corresponding to an Mr value of 68000 on gel filtration at pH 3.5 and as a single band corresponding to an Mr value of 35000 on reductive sodium dodecyl sulphate/polyacrylamide-gradient-gel electrophoresis. However, we believe that the latter represents the native Mr value. The pI values for each lie between pH 2.8 and 3.2. Activity in each case is optimal at pH 5.5-5.8 and at 75-80 degrees C. Half-life values at pH5 and 75 degrees C were from 2 to 4h. The specific activity with any individual substrate was much the same for each enzyme, as was the ratio of activity from one substrate to the next. Possible reasons for the observation that plots of velocity versus substrate concentration are sigmoidal are discussed. We believe that the finding of four endocellulases reflects differential glycosylation of a single enzyme form rather than genetically determined differences in primary structure.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan M. P., Folan M. A. Cellulose and cellulases: food for thought, food for the future? Int J Biochem. 1979;10(2):103–108. doi: 10.1016/0020-711x(79)90104-6. [DOI] [PubMed] [Google Scholar]
- Folan M. A., Coughlan M. P. The cellulase complex in the culture filtrate of the thermophyllic fungus, Talaromyces emersonii. Int J Biochem. 1978;9(10):717–722. doi: 10.1016/0020-711x(78)90038-1. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Nakamura S. Multiple forms of glucose oxidase with different carbohydrate compositions. Biochim Biophys Acta. 1981 Jan 15;657(1):40–51. doi: 10.1016/0005-2744(81)90128-5. [DOI] [PubMed] [Google Scholar]
- Jourdian G. W., Dean L., Roseman S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J Biol Chem. 1971 Jan 25;246(2):430–435. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Margolis J., Kenrick K. G. Polyacrylamide gel electrophoresis in a continuous molecular sieve gradient. Anal Biochem. 1968 Oct 24;25(1):347–362. doi: 10.1016/0003-2697(68)90109-7. [DOI] [PubMed] [Google Scholar]
- McHale A., Coughlan M. P. A convenient zymogram stain for cellulases. Biochem J. 1981 Oct 1;199(1):267–268. doi: 10.1042/bj1990267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale A., Coughlan M. P. Synergistic hydrolysis of cellulose by components of the extracellular cellulase system of Talaromyces emersonii. FEBS Lett. 1980 Aug 11;117(1):319–322. doi: 10.1016/0014-5793(80)80971-9. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Shoemaker S. P., Brown R. D., Jr Characterization of endo-1,4-beta-D-glucanases purified from Trichoderma viride. Biochim Biophys Acta. 1978 Mar 14;523(1):147–161. doi: 10.1016/0005-2744(78)90017-7. [DOI] [PubMed] [Google Scholar]
- Sternberg D., Vijayakumar P., Reese E. T. beta-Glucosidase: microbial production and effect on enzymatic hydrolysis of cellulose. Can J Microbiol. 1977 Feb;23(2):139–147. doi: 10.1139/m77-020. [DOI] [PubMed] [Google Scholar]
- Wood T. M., McCrae S. I. The cellulase of Trichoderma koningii. Purification and properties of some endoglucanase components with special reference to their action on cellulose when acting alone and in synergism with the cellobiohydrolase. Biochem J. 1978 Apr 1;171(1):61–72. doi: 10.1042/bj1710061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. M., McCrae S. I. The purification and properties of the C 1 component of Trichoderma koningii cellulase. Biochem J. 1972 Aug;128(5):1183–1192. doi: 10.1042/bj1281183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. M. Properties and mode of action of cellulases. Biotechnol Bioeng Symp. 1975;(5):111–133. [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]


