Abstract
Background
Antiretroviral treatment failure is a global issue, particularly in developing countries such as Sub-Saharan Africa. Prior research findings were highly variable and inconsistent across areas. As a result, the goal of this systematic review and meta-analysis was to determine the pooled prevalence of treatment failure among children receiving antiretroviral medication in Sub-Saharan Africa.
Methods
To find qualifying papers, we searched databases (such as PubMed, Google Scholar, African Journals Online, Scopus, and the Cochrane Library). The data were retrieved using Microsoft Excel and exported to STATA Version 14 for analysis. To check for publication bias, we employed Egger and Begg’s regression tests. A random-effects model was used to assess the pooled prevalence of treatment failure due to high levels of variability.
Results
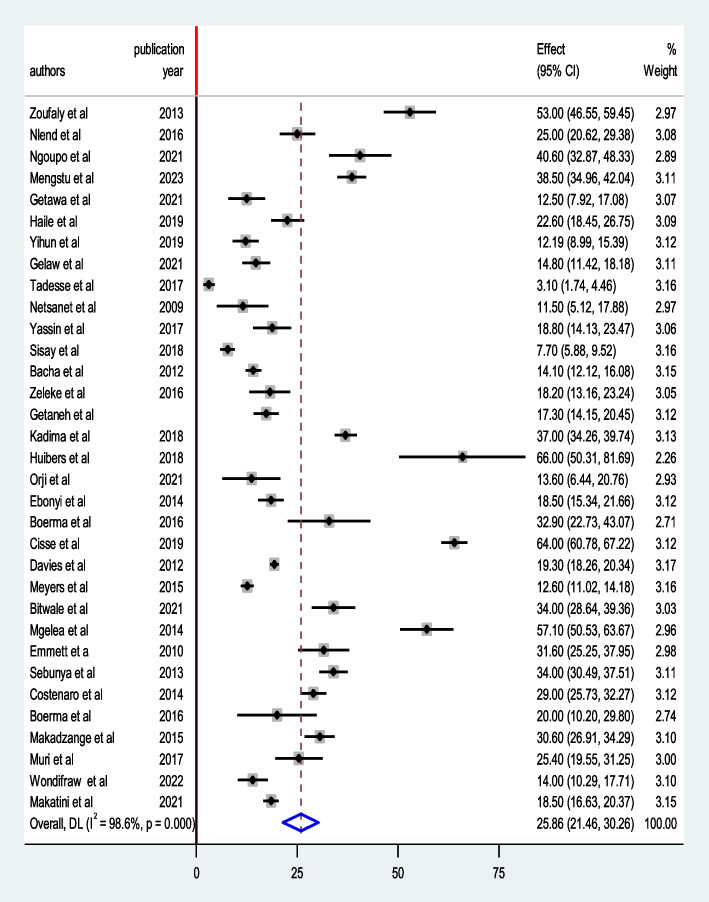
Following the removal of duplicated articles and quality screening, a total of 33 primary articles were determined to be appropriate for inclusion in the final analysis for this study. Overall, the pooled prevalence of treatment failure among HIV-infected children was 25.86% (95% CI: 21.46, 30.26). There is great variety across the included studies, with the majority of them being conducted in Ethiopia. Cameroon had the greatest pooled prevalence of treatment failure among HIV-infected children, at 39.41% (95% CI: 21.54, 57.28), while Ethiopia had the lowest, at 13.77% (95% CI: 10.08, 17.47).
Conclusions
The pooled estimate prevalence of treatment failure among HIV-infected children in Sub-Saharan Africa was high. The implementation of national and international policies and strategies on ART clinic care services should be given special focus in order to reduce treatment failure in children living with HIV/AIDS.
Trial registration
The protocol has been registered in the PROSPERO database under the registration number CRD-429011.
Keywords: HIV/AIDS, Treatment failure, Systematic review, Meta-analysis, Sub-Sahara Africa
Introduction
Acquired immune deficiency syndrome (AIDS) is a viral disease caused by the human immunodeficiency virus (HIV) that weakens the immune system and increases susceptibility to opportunistic infections [1]. The Human Immunodeficiency Virus (HIV) pandemic affects a large portion of the global population [2]. HIV has many routes of transmission including mother-to-child transmission [3]. Several activities have been implemented on prevention of mother to child transmission (PMTCT) intervention since, such as increasing institutional delivery, infant prophylaxis, Antiretroviral coverage, and proper feeding practices of infants [3–5]. In 2018, the number of people living with HIV worldwide exceeded 37.9 million.. Around 1.8 million of them were children (aged under 15 years) [6]. This year, 23.3 million HIV-positive individuals worldwide had access to antiretroviral therapy (ART) [7].
Antiretroviral therapy (ART) is critical for slowing the progression of HIV/AIDS, improving patient health, and ensuring long-term access to care for HIV-infected patients [8, 9]. Maintaining long-term ART adherence, viral load suppression, and preventing of antiretroviral therapy failure, on the other hand, remains a challenge for HIV-infected children [10]. Treatment failure can be classified as immunological, clinical, virological, or a mix of these [11].
Treatment failure can be more accurately and informatively determined by looking at virological failure, based on existing standards [11, 12]. In the absence of viral load monitoring, the move to second-line medication regimens is based on clinical criteria (i.e. opportunistic infections) or immunological criteria (patient cell differentiation CD4 T-cell count) [11]. According to the Joint United Nations Program on HIV/AIDS (UNAIDS) and partners, 90% of individuals on ART will have viral suppression by the end of 2030 worldwide [11, 13]. As a result, executing globally approved preventive measures and detecting treatment failure early is critical for treatment efficacy and meeting a stated strategic treatment goal of 2030 [14, 15].
The goal of this systematic review and meta-analysis was to estimate the rates of treatment failure among children on ART using available primary studies in Sub-Saharan Africa, which has one of the highest rates of HIV/AIDS worldwide. The estimated prevalence of treatment failure in Sub-Saharan Africa countries was inconsistent with wide ranges. In South Africa, for example, the prevalence of treatment failure ranged from 12.6% [16] to 19.3% [17], in Tanzania from 25.4% [18] to 57.1% [19], in Cameroon from 25% [20] to 53% [21], in Nigeria from 13.6% [22] to 32.9% [23], and in Ethiopia from 3.1% [24] to 22.6% [25].The findings of this study will allow countries to sustain treatment successes and hasten the decline of childhood treatment failure in the region, as well as assist decision makers and other concerned stakeholders in designing, implementing, and evaluating interventions to improve level of ART adherence.
Materials and methods
Study identification and reporting
We researched both published and unpublished studies regarding the prevalence of treatment failure in HIV-infected children. The results of this systematic review and meta-analyses were prepared and presented according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [26].
Study design, settings and search strategies
The pooled prevalence of treatment failure among HIV-infected children in Sub-Saharan African nations was estimated using a comprehensive review and meta-analysis. A comprehensive search was undertaken on PubMed, Google Scholar, African Journals Online, Scopus, and the Cochrane Library databases to find possibly relevant primary articles. Some internet repository library centers were explored for unpublished relevant literatures to this study. Furthermore, gray literatures have been identified through the review of reference lists and the involvement of subject matter experts. To search on the advanced PubMed search engine, MeSH (Medical Subject Headings), Boolean operators, and all fields within records were applied. Children, child, pediatrics, treatment failure, antiretroviral therapy, prevalence, proportion, and associated factors were among the search terms or phrases used.
The advanced PubMed database search strategy was performed using the following key terms.
(((((((((Prevalence[tw] OR proportion[tw])) OR ("Prevalence"[MeSH Terms] OR "magnitude"[MeSH Terms] OR "incidence"[MeSH Terms]))) AND (((Treatment failure[tw] OR immunological failure[tw] OR clinical failure [tw] OR virological failure[tw])) OR ("treatment failure"[MeSH Terms] OR "treatment"[All Fields] OR "Failures"[MeSH Terms] OR "Treatment Outcome"[MeSH Terms]))) AND (((factors[tw] OR determinants[tw] OR predictors[tw])) OR ("factors"[MeSH Terms] OR "risk factors"[MeSH Terms] OR "determinants"[MeSH Terms]))) AND (((Children[tw] OR pediatrics[tw] OR Infant[tw])) OR ("child"[MeSH Terms] OR "pediatrics"[MeSH Terms] OR "infant"[MeSH Terms]))) AND (((Antiretroviral therapy [tw] OR ART[tw])) OR (("anti-retroviral agents"[All Fields] OR "anti-retroviral agents"[MeSH Terms] OR "therapeutics"[MeSH Terms]))) AND (((Human Immunodeficiency Virus[tw] OR HIV[tw] OR AIDS[tw])) OR ("HIV"[MeSH Terms] OR "acquired immunodeficiency syndrome"[MeSH Terms]))) AND Sub-Sahara African[tw]. The search was done between April 7 and May 29, 2023. All papers published up to May 29, 2023 were included. Endnote X8 software manager was used to cite references and manage the searched literatures.
Outcome measurements
The outcome measure of interest in this study is HIV/AIDS treatment failure, which was defined by the WHO as immunological, clinical, and virological treatment failure [11].
Eligibility criteria
Inclusion criteria
Study area
Only studies conducted in sub-Saran Africa were included to produce single estimate of common effects.
Study design
All observational study designs reporting the prevalence of treatment failure were eligible for this meta-analysis.
Population
All HIV-infected children on antiretroviral treatment.
Language
Only articles reported in English language were incorporated.
Publication condition
Both published and unpublished studies were considered.
Exclusion criteria
We omitted primary studies that did not provide quantifiable treatment failure outcomes for children or did not pass our quality screening. Conference reports and papers that did not provide access to the full text were excluded. These researches were excluded due to the inability to evaluate the quality of papers in the absence of complete text.
Data selection process
Three authors (BG, KA, and BC) retrieved the relevant data from the included articles using a standardized data extraction format adapted from the Joanna Briggs Institute (JBI). Duplicate articles and articles with titles indicating that they did not deal with antiretroviral treatment failure were removed. The three reviewers additionally evaluated every article against the inclusion and exclusion criteria. Any differences during screening were resolved through dialogue. The data selection form comprised the first author’s name, publication year, country of study, study area, study design, sample size, response rate, and prevalence with 95% CI.
Quality assessment
The whole text of the articles was assessed for relevance based on titles, objectives, and method, and the quality of the included articles was rated by four investigators (BGW, CMT, AK, and YA) using the Newcastle–Ottawa Scale quality evaluation tool for observational studies [27]. Any disagreements between the four quality assessors were handled by repeating the procedures and involving a third reviewer before computing the final appraisal results. The Newcastle–Ottawa Scale scores of four independent reviewers were averaged to determine the quality of included articles.
Data processing and statistical analysis
The required data were retrieved from the primary articles using Microsoft Excel and imported to STATA software version 14 for further analysis. The random-effects model developed by DerSimonian and Laird was used to assess the overall pooled prevalence of treatment failure [28]. The p-values of the Cochrane Q and I2 test statistics [29] were used to determine heterogeneity. Subgroup analysis was performed to account for random variation in the original study’s point estimations and to analyze how failure varies across subgroup participants. Sensitivity analysis was used to look for outliers among the collected articles. Publication bias across studies was assessed using funnel plot and egger’s regression test. The Egger’s regression test results were not statistically significant for publication bias at the 5% significance level [30]. A forest plot format was used to present the point prevalence and 95% CIs. In this plot, the weight of study was indicated by the size of each box, while each crossed line referred to a 95% confidence interval. The effect size estimates were reported in the form of pooled prevalence.
Results
Study selection
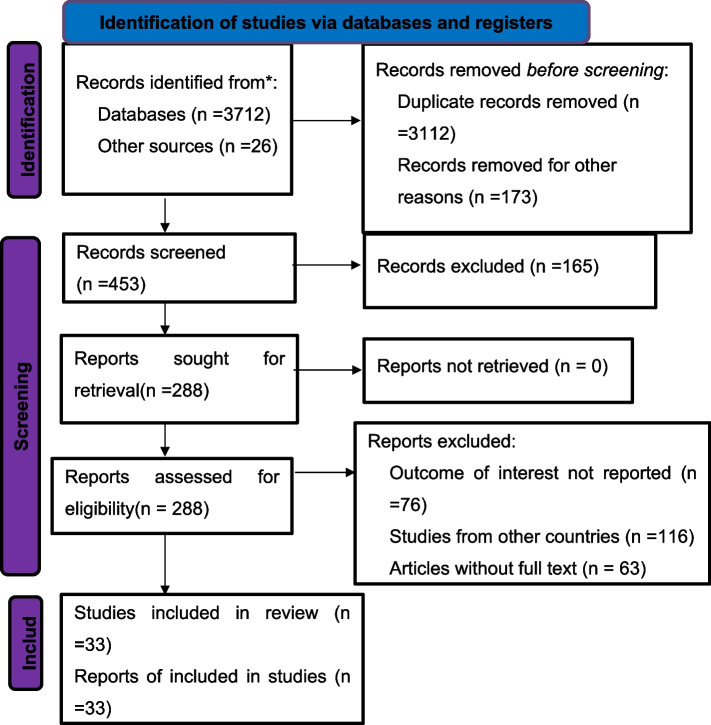
We retrieved 3,738 articles from PubMed, Google Scholar, African Journals Online, Cochrane Library, Scopus, and other sources. There were approximately 626 non-duplicated articles among the initial articles. After reviewing the titles and abstracts of the remaining articles, 228 were excluded. Then, 225 potentially full-text articles were evaluated for eligibility using pre-defined criteria, and 192 articles were dropped for various reasons. Finally, 33 articles satisfied the inclusion criteria and were included in the final meta-analysis to assess the prevalence of treatment failure (Fig. 1).
Fig. 1.
PRISMA 2020 flow chart of primary study selection for systematic review and meta-analysis of treatment failure among HIV infected children in Sub-Sahara Africa, 2009 to 2023
Characteristics of included studies
This study comprised 33 original publications with a total of 21,722 study participants published between 2009 and 2023 from various parts of Sub-Saharan African countries. Of the 33 included primary articles, 12 were from Ethiopia [24, 25, 31–40], 4 were from Tanzania [18, 19, 41, 42], 3 were from Cameroon [20, 21, 43], 3 were from Uganda [44–46], 3 were from Nigeria [22, 23, 47], 3 were from South Africa [16, 17, 48], and the remaining 5 were from Eritrea [49], Kenya [50], Malawi [51], Senegal [52], and Zimbabwe [53]. Twenty-two of the studies were cohort studies [16, 17, 22–25, 31–36, 38, 40, 43–49, 51], ten were cross-sectional studies [18–21, 37, 39, 41, 42, 50, 52, 53], and one was a case–control study design [50] (Table 1). The sample size of individual articles in our study ranged from 35 [51] to 5485 [17].
Table 1.
General characteristics of studies included in systematic review and meta-analysis of treatment failure among HIV-infected children in Sub-Sahara Africa, 2009–2023
| Authors | Study design | Publication year | Country | Sample | Prevalence(%) | Quality assessment |
|---|---|---|---|---|---|---|
| Zoufaly etal | Cross-sectional | 2013 | Cameroon | 230 | 53 | Good |
| Nlend etal | Cross-sectional | 2016 | Cameroon | 375 | 25 | Good |
| Ngoupo etal | Cohort | 2021 | Cameroon | 155 | 40.6 | Good |
| Mengstu etal | Cohort | 2023 | Eritrea | 724 | 38.5 | Good |
| Getawa et al | Cross-sectional | 2021 | Ethiopia | 200 | 12.5 | Good |
| Haile et al | Cohort | 2019 | Ethiopia | 391 | 22.6 | Good |
| Yihun et al | Cohort | 2019 | Ethiopia | 402 | 12.19 | Good |
| Gelaw et al | Cross-sectional | 2021 | Ethiopia | 424 | 14.8 | Good |
| Tadesse et al | Cohort | 2017 | Ethiopia | 628 | 3.1 | Good |
| Netsanet et al | Cohort | 2009 | Ethiopia | 96 | 11.5 | Good |
| Yassin et al | Cohort | 2017 | Ethiopia | 269 | 18.8 | Good |
| Sisay et al | Cohort | 2018 | Ethiopia | 824 | 7.7 | Good |
| Bacha et al | Cohort | 2012 | Ethiopia | 1,186 | 14.1 | Good |
| Zeleke et al | Cohort | 2016 | Ethiopia | 225 | 18.2 | Good |
| Getaneh etal | Cohort | Ethiopia | 554 | 17.3 | Good | |
| Kadima etal | Case–control | 2018 | Kenya | 1190 | 37 | Good |
| Huibers et al | Cohort | 2018 | Malawi | 35 | 66 | Good |
| Orji et al | Cohort | 2021 | Nigeria | 88 | 13.6 | Good |
| Ebonyi etal | Cohort | 2014 | Nigeria | 580 | 18.5 | Good |
| Boerma etal | Cohort | 2016 | Nigeria | 82 | 32.9 | Good |
| Cisse et al | Cross-sectional | 2019 | Senegal | 851 | 64 | Good |
| Davies et al | Cohort | 2012 | South Africa | 5485 | 19.3 | Good |
| Meyers et al | Cohort | 2015 | South Africa | 1692 | 12.6 | Good |
| Bitwale etal | Cross-sectional | 2021 | Tanzania | 300 | 34 | Good |
| Mgelea etal | Cross-sectional | 2014 | Tanzania | 218 | 57.1 | Good |
| Emmett et a | Cross-sectional | 2010 | Tanzania | 206 | 31.6 | Good |
| Sebunya et al | Cohort | 2013 | Ugandan | 701 | 34 | Good |
| Costenaro et al | Cohort | 2014 | Mozambique and Uganda | 740 | 29 | Good |
| Boerma etal | Cohort | 2017 | Ugandan | 64 | 20 | Good |
| Makadzange etal | Cross-sectional | 2015 | Zimbabwe | 599 | 30.6 | Good |
| Muri et al | Cross-sectional | 2017 | Tanzania | 213 | 25.4 | Good |
| Wondifraw et al | Cohort | 2022 | Ethiopia | 336 | 14 | Good |
| Makatini etal | Cohort | 2021 | South Africa | 1659 | 18.5 | Good |
Results of individual studies
Risk of bias in studies
The Egger’s test was used to check for publication bias, which revealed no statistically significant publication bias with a p-value of 0.78. We also used a funnel plot to assess publication bias for overall treatment failure (Fig. 2).
Fig. 2.
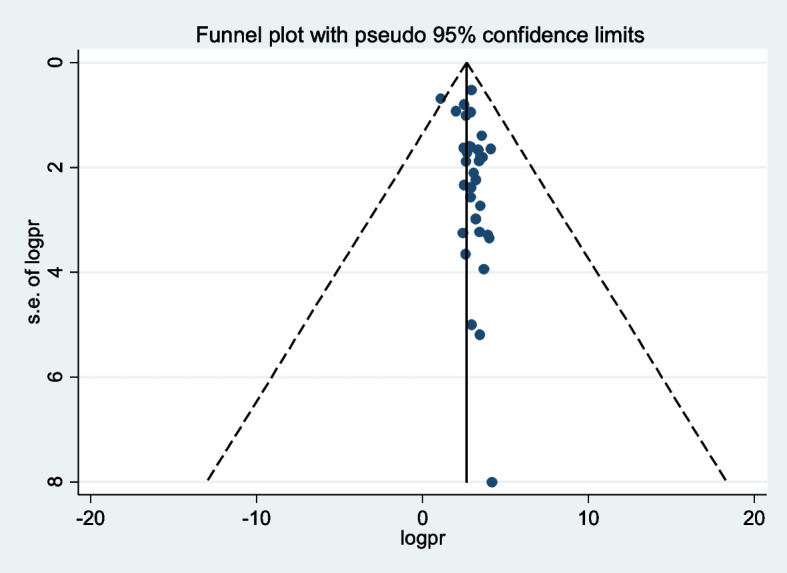
Funnel plot with 95% confidence limits of the pooled prevalence of treatment failure among HIV-infected children in Sub-Sahara Africa, 2009 to 2023
Heterogeneity and sensitivity analysis
The heterogeneity test (I2) result was 98.6%, p < 0.01, indicating that there is significant variety across the included primary articles. In the sensitivity analysis, all studies were within the confidence interval, and no single study contributed to publication bias.
Prevalence of HIV/AIDS treatment failure
The overall pooled prevalence of treatment failure among HIV-infected children in Sub-Saharan Africa was found to be 25.86% (95% CI: 21.46, 30.26, I2 = 98.6%, P < 0.01) (Fig. 3).
Fig. 3.
Forest plot of the pooled prevalence of treatment failure among HIV- infected children in Sub-Sahara Africa, 2009 to 2023
Subgroup analysis
Various criteria were used to assess the subgroup prevalence of treatment failure. These were the geographical settings, study design type, and publication year. Our subgroup analyses revealed that the largest proportion of treatment failure was seen among HIV-infected children residing in Cameroon (39.41% (95% CI: 21.54, 57.28, I2 = 98.1%, P < 0.01) and the lowest in Ethiopia (13.77% (95% CI: 10.08, 17.47, I2 = 94.9%, P < 0.01) (Table 2). Treatment failure among HIV-infected children was 34.76% (95% CI: 22.44, 47.06, I2 = 98.6%, P < 0.01), and 20.82% (95% CI: 17.02, 24.62, I2 = 97.7%, P < 0.01) in cross-sectional and cohort studies, respectively (Table 2). Furthermore, the prevalence of treatment failure among HIV-infected children was 27.87% (95% CI: 22.30, 33.45, I2 = 97.9%, P < 0.01) in studies published from 2009 to 2015, yet it was 25.29% (95% CI: 18.25, 32.33, I2 = 98.9%, P < 0.01) in articles published from 2016 to 2023 (Table 2).
Table 2.
Summary of subgroup analysis for the pooled prevalence of treatment failure among HIV-infected children in Sub-Sahara Africa, 2009 to 2023 (n = 33)
| Variables | Subgroup | Prevalence,% (95% CI, I2, P-value) |
|---|---|---|
| Country | Cameroon | 39.41 (21.54–57.28, 96.10, < 0.01) |
| Ethiopia | 13.77 (10.08–17.47, 94.9, < 0.01) | |
| Nigeria | 20.66 (12.44–28.88, 78.80, 0.009) | |
| South Africa | 16.81 (12.59–21.03, 96, < 0.01) | |
| Tanzania | 36.96 (24.15–49.77, 94.50, < 0.01) | |
| Other | 39.30 ( 29.91–48.69, 97.80, < 0.01) | |
| Study design | Cross-sectional | 34.76 (22.44–47.08, 98.60, < 0.01) |
| Cohort | 20.82 (17.02–24.62, 97.70, < 0.01) | |
| Publication year | 2009–2015 | 27.87 (22.30–33.45, 97.90, < 0.01) |
| 2016–2023 | 25.29 (18.25–32.33, 98.90, < 0.01) |
Discussion
Despite advancements in comprehensive HIV care facilities and ART coverage, HIV/AIDS transmission and the rate of treatment failure among HIV-infected children remain a global problem, particularly in Sub-Saharan African countries. Treatment failure is one of the leading causes of childhood morbidity and mortality in resource limited countries. This meta-analysis and systemic review was conducted to determine the pooled prevalence of treatment failure among HIV-infected children in Sub-Saharan African countries. The findings will help patients stay on first-line ART regimens longer, avoiding the more expensive and dangerous second-line ART regimens in developing countries.
The pooled prevalence of treatment failure among HIV-infected children in Sub-Saharan African countries was 25.86% (95% CI: 21.46, 30.26), as per the findings of this meta-analysis. Our findings are consistent with previous research on HIV-infected children in Mozambique and Uganda (29%) [45], Tanzania (25.4%) [18], Cameroon (25%) [20], and Ethiopia (22.6%) [25]. This suggests that simply initiating ART for HIV-infected children is insufficient, and that treatment failure prevention initiatives, regular therapeutic medication monitoring, and resistance testing in the area should be prioritized instead. A higher burden of HIV/AIDS treatment failure may be associated with lower socioeconomic level indices in Sub-Saharan African nations (such as unemployment, lack of education beyond a university, financial difficulties, and rental or unstable housing status).
Our finding is significantly greater than the findings of the other meta-analyses and observational studies. For example, the prevalence of treatment failure was found in Ethiopia (12.34%) [54], South Africa (12.6%) [16], Nigeria (13.6%) [22], and Uganda (20%) [46] based on prior studies. This figure, on the other hand, is significantly lower than studies conducted in Malawi (66%) [51], Tanzania (57.1%) [19], Eritrea (38.5%) [49], Kenya (37%) [50], Senegal (64%) [52], and Cameroon (53%) [21]. The aforementioned disparities could be attributed to differences in study technique and sample size utilized to diagnose treatment failure by individual studies conducted in each country. This could be due to variation in patient monitoring standards or the quality of medical services, socioeconomic status, and an increase in the number of patients on ART over time, all of which could have an impact on the capacity of HIV treatment failure diagnosis, monitoring, adherence, and treatment outcome in general.
There is statistically significant heterogeneity among the included primary studies in this systematic review and meta-analysis. Thus, we conducted subgroup analysis. As a result, Ethiopia had the lowest prevalence of HIV treatment failure (13.77% (95% CI: 10.08, 17.47) among Sub-Saharan African countries. Furthermore, Cameroon had the highest rate of treatment failure among HIV-infected Sub-Saharan African children, at 39.41% (95% CI: 21.54, 57.28). The disparity may be attributed to differences in study design, sample size, and the number of primary studies included in our review from each country.
In Ethiopia, HIV/AIDS patients treatment is decentralized, with the majority of ART services delivered at the primary health care facility level [55]. Additionally, health systems in countries such as Ethiopia may have an impact on the quality of care, patient monitoring, and treatment adherence [56]. In Ethiopia’s healthcare system, skilled health extension workers provide follow-up care to HIV/AIDS patients in the community, especially rural areas. Health extension workers provide health information on HIV/AIDS and other community health issues [57]. This may assist to reduce stigma and discrimination in the community, which may improve ART adherence and treatment outcomes.
In Sub-Saharan African countries, the burden of treatment failure among HIV-infected children remains significant. This could be correlated to ART delay [58], opportunistic infections [59], poor HIV care, late identification of HIV treatment failure [60], ART adverse response [61], dietary issues [62], and low ART adherence [63]. As a result, HIV-infected children require special attention during their ART follow-up visit because they are more prone to have treatment failure.
Limitations of the review
Many limits must be considered before interpreting the results of this review. This review covered only articles written in English, which may exclude some works. In addition, the majority of studies included in this investigation were from Ethiopia, resulting in a significant degree of variability that limits our ability to determine the precise prevalence of treatment failure across the country. Furthermore, because this meta-analysis covers a limited number of trials, the results may not accurately represent regional failure rates.
Conclusion
This meta-analysis found that the prevalence of treatment failure among HIV-infected children in Sub-Saharan African nations remained significantly high. It is suggested that children living with HIV require additional treatment adherence counseling, care, and support. Treatment failure assessment and interventions should be prioritized during HIV care services for children at ART clinics in each country.
Acknowledgements
We would like to give our special gratitude for the authors of primary studies which were included to this systematic review and meta-analysis.
Abbreviations
- AIDS
Acquired immune deficiency syndrome
- ART
Antiretroviral therapy
- CI
Confidence interval
- HIV
Human immunodeficiency virus
- WHO
World health organization
Authors’ contributions
BGW: Conception of study design, research protocol, data extraction, statistical analysis, data interpretation and prepare the initial drafts of the manuscript. ZAY, YA, KA, MW, CMT, BC, and AK: data extraction, quality assessment, statistical analysis, reviewing and editing the final draft of the manuscript. All authors have read and approved the final version of our manuscript.
Funding
The authors received no specific funding this review.
Availability of data and materials
All generated or analyzed data during this study will be available from the supporting information file.
Declarations
Ethics approval and consent to participate
Not applicable since no need of primary data collection.
Consent for publication
Not applicable.
Competing interests
The authors declared that they have no conflicts interests regarding the content of this study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kliegman SG B, SHAH,TASKER,WILSON. Nelson Textbook of Pediatrics. 21st edition. Philadelphia: Elsevier, United States of America: 2020.
- 2.Cohen MS, Hellmann N, Levy JA, DeCock K, Lange J. The spread, treatment, and prevention of HIV-1: evolution of a global pandemic. J Clin Investig. 2008;118(4):1244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization WH. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- 4.Iliff PJ, Piwoz EG, Tavengwa NV, Zunguza CD, Marinda ET, Nathoo KJ, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS. 2005;19(7):699–708. [DOI] [PubMed] [Google Scholar]
- 5.Adetokunboh OO, Oluwasanu M. Eliminating mother-to-child transmission of the human immunodeficiency virus in sub-Saharan Africa: The journey so far and what remains to be done. J Infect Public Health. 2016;9(4):396–407. [DOI] [PubMed] [Google Scholar]
- 6.Unaids G. HIV & AIDS Statistics—2019 Fact Sheet. Geneva: Switzerland; 2019. [Google Scholar]
- 7.Organization WH. Global status report on alcohol and health 2018: World Health Organization; 2019.
- 8.HIV/AIDS Strategic Plan 2015–2020 by the Federal Democratic Republic of Ethiopia Ministry of Health FHAPaCOAA, Ethiopia.
- 9.Unaids W. Global AIDS monitoring. Geneva: UNAIDS; 2017. [Google Scholar]
- 10. Judd A, Lodwick R, Noguera-Julian A, Gibb D, Butler K, Costagliola D, et al. Pursuing Later Treatment Options II (PLATO II) Project Team for the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) in EuroCoord. Higher rates of triple-class virological failure in perinatally HIV infected teenagers compared with heterosexually infected young adults in Europe. HIV MEDICINE. 2016. [DOI] [PMC free article] [PubMed]
- 11.Organization WH. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach: World Health Organization; 2016. [PubMed]
- 12.Levi J, Raymond A, Pozniak A, Vernazza P, Kohler P, Hill A. Can the UNAIDS 90–90-90 target be achieved? A systematic analysis of national HIV treatment cascades. BMJ Glob Health. 2016;1(2): e000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sidibé M, Loures L, Samb B. The UNAIDS 90–90–90 target: a clear choice for ending AIDS and for sustainable health and development. Journal of the International AIDS Society. 2016;19(1). [DOI] [PMC free article] [PubMed]
- 14.Organization WH. The global health sector strategy on HIV. 2014.
- 15.El-Khatib Z, Katzenstein D, Marrone G, Laher F, Mohapi L, Petzold M, et al. Adherence to drug-refill is a useful early warning indicator of virologic and immunologic failure among HIV patients on first-line ART in South Africa. PLoS ONE. 2011;6(3):e17518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyers T, Sawry S, Wong JY, Moultrie H, Pinillos F, Fairlie L, et al. Virologic failure among children taking lopinavir/ritonavir-containing first-line antiretroviral therapy in South Africa. Pediatr Infect Dis J. 2015;34(2):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies M-A, Moultrie H, Eley B, Rabie H, Van Cutsem G, Giddy J, et al. Virologic failure and second-line antiretroviral therapy in children in South Africa-The IeDEA Southern Africa Collaboration. J Acquired Immun Deficiency Syndromes (1999). 2011;56(3):270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muri L, Gamell A, Ntamatungiro AJ, Glass TR, Luwanda LB, Battegay M, et al. Development of HIV drug resistance and therapeutic failure in children and adolescents in rural Tanzania: an emerging public health concern. AIDS (London, England). 2017;31(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mgelea EM, Kisenge R, Aboud S. Detecting virological failure in HIV-infected Tanzanian children. S Afr Med J. 2014;104(10):696–9. [DOI] [PubMed] [Google Scholar]
- 20.Nlend AEN, Lyeb S, Moyo STN, Motaze AN. Viral Monitoring and Prevalence of Viral Failure in HIV-1 Infected Children under First Line Antiretroviral Therapy during the First 60 Months of Treatment in Yaoundé, Cameroon: A Serial Cross Sectional Analysis. Open Journal of Pediatrics. 2016;6(1):69–74. [Google Scholar]
- 21.Zoufaly A, Fillekes Q, Hammerl R, Nassimi N, Jochum J, Drexler JF, et al. Prevalence and determinants of virological failure in HIV-infected children on antiretroviral therapy in rural Cameroon: a cross-sectional study. Antivir Ther. 2013;18(5):681–90. [DOI] [PubMed] [Google Scholar]
- 22.Orji M-L, Onyire NB, Ojukwu JO, Oyim-Elechi CO. The outcome of intervention, characteristics, and determinants of treatment failure in HIV-infected adolescents on first-line antiretroviral therapy at a tertiary health institution. South-east Nigeria Nigerian Journal of Medicine. 2021;30(5):586–91. [Google Scholar]
- 23.Boerma RS, Boender TS, Sigaloff KC, Rinke de Wit TF, Van Hensbroek MB, Ndembi N, et al. High levels of pre‐treatment HIV drug resistance and treatment failure in Nigerian children. J Int AIDS Soc. 2016;19(1):21140. [DOI] [PMC free article] [PubMed]
- 24.Tadesse BT, Foster BA, Jerene D, Ruff A. Cohort profile: improving treatment of HIV-infected Ethiopian children through better detection of treatment failure in southern Ethiopia. BMJ Open. 2017;7(2):e013528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haile GS, Berha AB. Predictors of treatment failure, time to switch and reasons for switching to second line antiretroviral therapy in HIV infected children receiving first line anti-retroviral therapy at a Tertiary Care Hospital in Ethiopia. BMC Pediatr. 2019;19:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88: 105906. [DOI] [PubMed] [Google Scholar]
- 27.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. bmj. 2021;372. [DOI] [PMC free article] [PubMed]
- 28.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Archives of Public Health. 2014;72:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rücker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on I 2 in assessing heterogeneity may mislead. BMC Med Res Methodol. 2008;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Research synthesis methods. 2010;1(2):97–111. [DOI] [PubMed] [Google Scholar]
- 31.Bacha T, Tilahun B, Worku A. Predictors of treatment failure and time to detection and switching in HIV-infected Ethiopian children receiving first line anti-retroviral therapy. BMC Infect Dis. 2012;12(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sisay MM, Ayele TA, Gelaw YA, Tsegaye AT, Gelaye KA, Melak MF. Incidence and risk factors of first-line antiretroviral treatment failure among human immunodeficiency virus-infected children in Amhara regional state, Ethiopia: a retrospective follow-up study. BMJ Open. 2018;8(4):e019181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yihun BA, Kibret GD, Leshargie CT. Incidence and predictors of treatment failure among children on first-line antiretroviral therapy in Amhara Region Referral Hospitals, northwest Ethiopia 2018: A retrospective study. PLoS ONE. 2019;14(5): e0215300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Getaneh Y, Yizengaw A, Likie A, Getahun M, Feleke A, Kidane E, et al. Rate and predictors of Treatment Failure among pediatric population taking Highly Active Antiretroviral Therapy in Ethiopia. medRxiv. 2019:19005538.
- 35.Wondifraw EB ea. Predictors of first-line antiretroviral treatment failure among children on antiretroviral therapy at the University of Gondar comprehensive specialised hospital, North-west,Ethiopia: a 14-year long-term follow up study. BMJ Open. 2022,12(12):e064354. [DOI] [PMC free article] [PubMed]
- 36.Yassin S, Gebretekle GB. Magnitude and predictors of antiretroviral treatment failure among HIV-infected children in Fiche and Kuyu hospitals, Oromia region, Ethiopia: a retrospective cohort study. Pharmacol Res Perspect. 2017;5(1): e00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Getawa S, Fentahun A, Adane T, Melku M. Antiretroviral treatment failure and associated factors among HIV-infected children on antiretroviral therapy: a retrospective study. HIV/AIDS-Research and Palliative Care. 2021;(13):229–37. [DOI] [PMC free article] [PubMed]
- 38.Workneh N, Girma T, Woldie M. Immunologic and clinical outcomes of children on HAART: a Retrospective cohort analysis at Jimma University specialized hospital. Ethiop J Health Sci. 2009;19(2):75–82
- 39.Gelaw B, Mulatu G, Tesfa G, Marew C, Chekole B, Alebel A. Magnitude and associated factors of virological failure among children on ART in Bahir Dar Town public health facilities, Northwest Ethiopia: a facility based cross-sectional study. Ital J Pediatr. 2021;47:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeleke A. Prevalence of antiretroviral treatment failure and associated factors in HIV infected children on antiretroviral therapy at Gondar University Hospital, retrospective cohort study. International Journal of Medicine and Medical Sciences. 2016;8(11):125–32. [Google Scholar]
- 41.Bitwale NZ, Mnzava DP, Kimaro FD, Jacob T, Mpondo BC, Jumanne S. Prevalence and factors associated with virological treatment failure among children and adolescents on antiretroviral therapy attending HIV/AIDS care and treatment clinics in dodoma municipality, central tanzania. Journal of the Pediatric Infectious Diseases Society. 2021;10(2):131–40. [DOI] [PubMed] [Google Scholar]
- 42.Emmett SD, Cunningham CK, Mmbaga BT, Kinabo GD, Schimana W, Swai ME, et al. Predicting virologic failure among HIV-1-infected children receiving antiretroviral therapy in Tanzania: a cross-sectional study. J Acq Immun Deficiency Synd (1999). 2010;54(4):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tagnouokam-Ngoupo PA, Penda IC, Tchatchueng Mbougua JB, Tetang Ndiang S, Yuya Septoh F, Kenne A, et al. Virological failure and antiretroviral resistance among HIV-infected children after five years follow-up in the ANRS 12225-PEDIACAM cohort in Cameroon. PLoS ONE. 2021;16(3): e0248642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sebunya R, Musiime V, Kitaka SB, Ndeezi G. Incidence and risk factors for first line anti retroviral treatment failure among Ugandan children attending an urban HIV clinic. AIDS Res Ther. 2013;10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costenaro P, Penazzato M, Lundin R, Rossi G, Massavon W, Patel D, et al. Predictors of treatment failure in HIV-positive children receiving combination antiretroviral therapy: cohort data from Mozambique and Uganda. Journal of the Pediatric Infectious Diseases Society. 2015;4(1):39–48. [DOI] [PubMed] [Google Scholar]
- 46.Boerma RS, Kityo C, Boender TS, Kaudha E, Kayiwa J, Musiime V, et al. Second-line HIV treatment in Ugandan children: favorable outcomes and no protease inhibitor resistance. J Trop Pediatr. 2017;63(2):135–43. [DOI] [PubMed] [Google Scholar]
- 47.Ebonyi AO, Oguche S, Ejeliogu EU, Okpe SE, Agbaji OO, Sagay SA, et al. Risk factors for first-line antiretroviral treatment failure in HIV-1 infected children attending Jos University Teaching Hospital, Jos, North Central Nigeria. British Journal of Medicine and Medical Research. 2014;4(15):2983–94. [Google Scholar]
- 48.Makatini Z, Blackard J, Mda S, Miles P, Towobola O. High virological failure rates in HIV-1 perinatally infected children in South Africa: A retrospective cohort study. S Afr Med J. 2021;111(3):255–9. [DOI] [PubMed] [Google Scholar]
- 49.Mengistu ST, Ghebremeskel GG, Achila OO, Abrehe MB, Tewelde SF, Idris MM, et al. Prevalence and factors associated with pediatric HIV therapy failure in a tertiary hospital in Asmara, Eritrea: A 15-year retrospective cohort study. PLoS ONE. 2023;18(3):e0282642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kadima J, Patterson E, Mburu M, Blat C, Nyanduko M, Bukusi EA, et al. Adoption of routine virologic testing and predictors of virologic failure among HIV-infected children on antiretroviral treatment in western Kenya. PLoS ONE. 2018;13(11):e0200242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huibers MH, Moons P, Cornelissen M, Zorgdrager F, Maseko N, Gushu MB, et al. High prevalence of virological failure and HIV drug mutations in a first-line cohort of Malawian children. J Antimicrob Chemother. 2018;73(12):3471–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cissé A-M, Laborde-Balen G, Kébé-Fall K, Dramé A, Diop H, Diop K, et al. High level of treatment failure and drug resistance to first-line antiretroviral therapies among HIV-infected children receiving decentralized care in Senegal. BMC Pediatr. 2019;19:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Makadzange A, Higgins-Biddle M, Chimukangara B, Birri R, Gordon M, Mahlanza T, et al. Clinical, virologic, immunologic outcomes and emerging HIV drug resistance patterns in children and adolescents in public ART care in Zimbabwe. PLoS ONE. 2015;10(12):e0144057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gelaw B, Dessalegn L, Alem E, Tekalign T, Lankirew T, Eshetu K, et al. Prevalence and associated factors of treatment failure among children on ART in Ethiopia: A systematic review and meta-analysis. PLoS ONE. 2022;17(4):e0261611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Assefa Y, Jerene D, Lulseged S, Ooms G, Van Damme W. Rapid scale-up of antiretroviral treatment in Ethiopia: successes and system-wide effects. PLoS Med. 2009;6(4):e1000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H, Tesfaye R, Ramana GN, Chekagn CT. Ethiopia health extension program: an institutionalized community approach for universal health coverage: World Bank Publications; 2016.
- 57.Mwai GW, Mburu G, Torpey K, Frost P, Ford N, Seeley J. Role and outcomes of community health workers in HIV care in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2013;16(1):18586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nash D, Tymejczyk O, Gadisa T, Kulkarni SG, Hoffman S, Yigzaw M, et al. Factors associated with initiation of antiretroviral therapy in the advanced stages of HIV infection in six Ethiopian HIV clinics, 2012 to 2013. J Int AIDS Soc. 2016;19(1):20637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haileamlak A, Hagos T, Abebe W, Abraham L, Asefa H, Teklu AM. Predictors of Hospitalization among Children on ART in Ethiopia: a Cohort study. Ethiop J Health Sci. 2017;27(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deribew A, Biadgilign S, Berhanu D, Defar A, Deribe K, Tekle E, et al. Capacity of health facilities for diagnosis and treatment of HIV/AIDS in Ethiopia. BMC Health Serv Res. 2018;18:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abdissa SG, Fekade D, Feleke Y, Seboxa T, Diro E. Adverse drug reactions associated with antiretroviral treatment among adult Ethiopian patients in a tertiary hospital. Ethiop Med J. 2012;50(2):107–13. [PubMed] [Google Scholar]
- 62.Gebremichael DY, Hadush KT, Kebede EM, Zegeye RT. Food insecurity, nutritional status, and factors associated with malnutrition among people living with HIV/AIDS attending antiretroviral therapy at public health facilities in West Shewa Zone, Central Ethiopia. BioMed research international. 2018;2018(Article ID 1913534):9. [DOI] [PMC free article] [PubMed]
- 63.Endalamaw A, Tezera N, Eshetie S, Ambachew S, Habtewold TD. Adherence to highly active antiretroviral therapy among children in ethiopia: a systematic review and meta-analysis. AIDS Behav. 2018;22:2513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All generated or analyzed data during this study will be available from the supporting information file.