Abstract
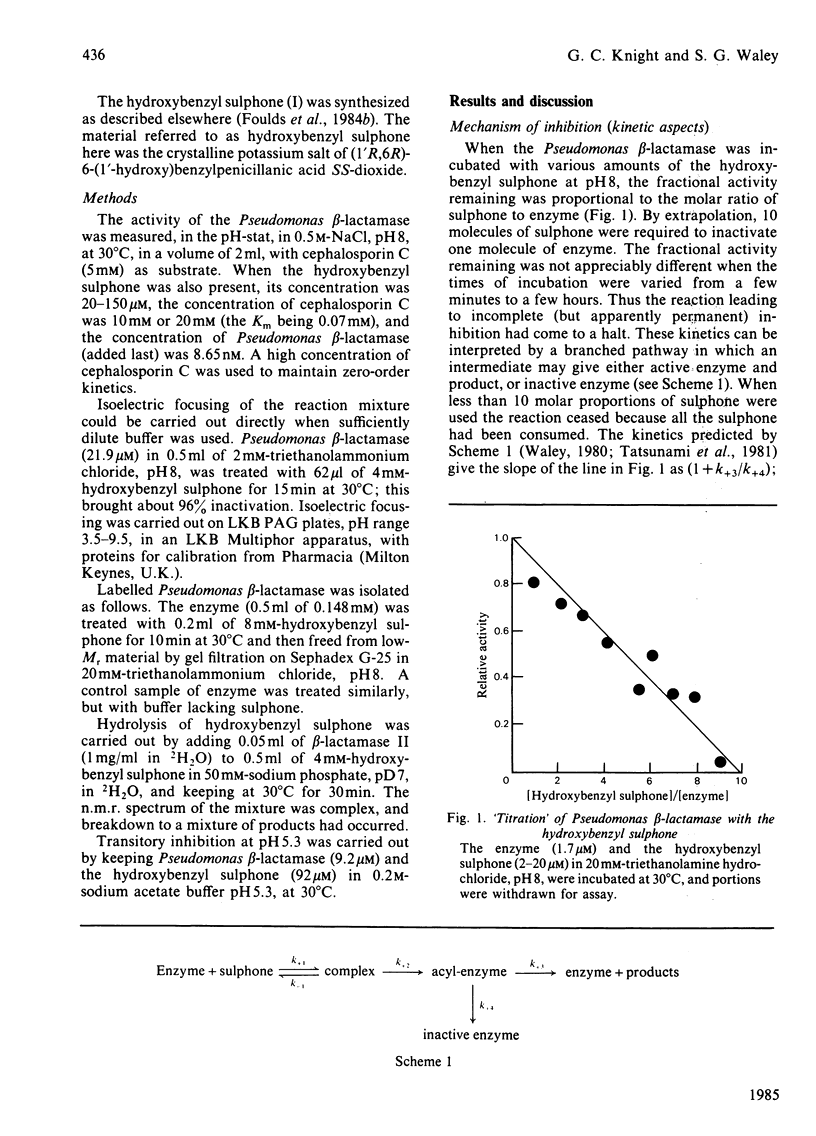
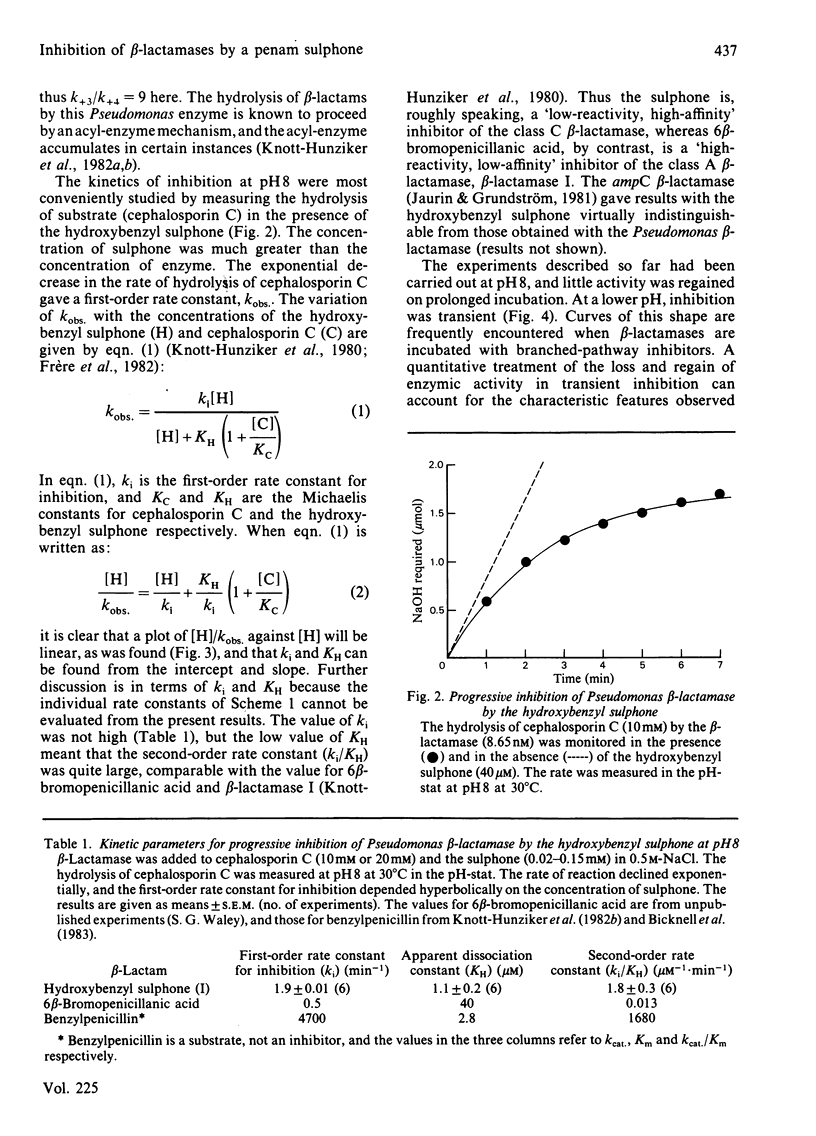
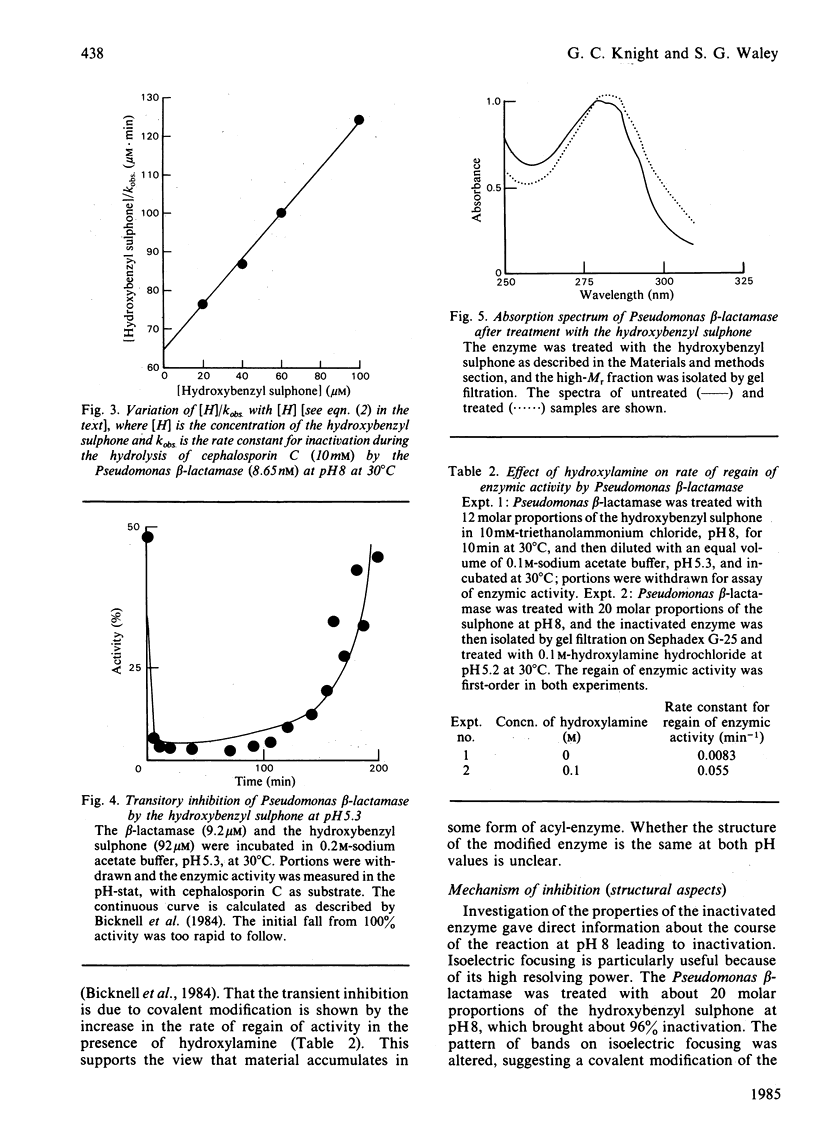
beta-Lactamases, enzymes that catalyse the hydrolysis of the beta-lactam ring in beta-lactam antibiotics, are divided into three classes, A, B and C, on the basis of the structures so far determined. There are relatively few effective inhibitors of class C beta-lactamases. A beta-lactam sulphone with a hydroxybenzyl side chain, namely (1'R,6R)-6-(1'-hydroxy)benzylpenicillanic acid SS-dioxide (I), has now been studied. The sulphone is a good mechanism-based inhibitor of class C beta-lactamases. At pH8, the inhibition of a Pseudomonas beta-lactamase is irreversible, and proceeds at a rate that is about one-tenth the rate of concurrent hydrolysis. The labelled enzyme has enhanced u.v. absorption and is probably an enamine. At a lower pH, however, inhibition is transitory.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- Baldwin G. S., Edwards G. F., Kiener P. A., Tully M. J., Waley S. G., Abraham E. P. Production of a variant of beta-lactamase II with selectively decreased cephalosporinase activity by a mutant of Bacillus cereus 569/H/9. Biochem J. 1980 Oct 1;191(1):111–116. doi: 10.1042/bj1910111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berks M., Redhead K., Abraham E. P. Isolation and properties of an inducible and a constitutive beta-lactamase from Pseudomonas aeruginosa. J Gen Microbiol. 1982 Jan;128(1):155–159. doi: 10.1099/00221287-128-1-155. [DOI] [PubMed] [Google Scholar]
- Bicknell R., Knott-Hunziker V., Waley S. G. The pH-dependence of class B and class C beta-lactamases. Biochem J. 1983 Jul 1;213(1):61–66. doi: 10.1042/bj2130061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. G., Knowles J. R. Penicillanic acid sulfone: an unexpected isotope effect in the interaction of 6 alpha- and 6 beta-monodeuterio and of 6,6-dideuterio derivatives with RTEM beta-lactamase from Escherichia coli. Biochemistry. 1981 Jun 23;20(13):3680–3687. doi: 10.1021/bi00516a003. [DOI] [PubMed] [Google Scholar]
- Brown A. G. New naturally occurring beta-lactam antibiotics and related compounds. J Antimicrob Chemother. 1981 Jan;7(1):15–48. doi: 10.1093/jac/7.1.15. [DOI] [PubMed] [Google Scholar]
- Cartwright S. J., Waley S. G. Purification of beta-lactamases by affinity chromatography on phenylboronic acid-agarose. Biochem J. 1984 Jul 15;221(2):505–512. doi: 10.1042/bj2210505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright S. J., Waley S. G. beta-Lactamase inhibitors. Med Res Rev. 1983 Oct-Dec;3(4):341–382. doi: 10.1002/med.2610030402. [DOI] [PubMed] [Google Scholar]
- Davies R. B., Abraham E. P. Separation, purification and properties of beta-lactamase I and beta-lactamase II from Bacillus cereus 569/H/9. Biochem J. 1974 Oct;143(1):115–127. doi: 10.1042/bj1430115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English A. R., Retsema J. A., Girard A. E., Lynch J. E., Barth W. E. CP-45,899, a beta-lactamase inhibitor that extends the antibacterial spectrum of beta-lactams: initial bacteriological characterization. Antimicrob Agents Chemother. 1978 Sep;14(3):414–419. doi: 10.1128/aac.14.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J., Charnas R. L., Bradley S. M., Knowles J. R. Inactivation of the RTEM beta-lactamase from Escherichia coli. Interaction of penam sulfones with enzyme. Biochemistry. 1981 May 12;20(10):2726–2731. doi: 10.1021/bi00513a004. [DOI] [PubMed] [Google Scholar]
- Fisher J., Charnas R. L., Knowles J. R. Kinetic studies on the inactivation of Escherichia coli RTEM beta-lactamase by clavulanic acid. Biochemistry. 1978 May 30;17(11):2180–2184. doi: 10.1021/bi00604a024. [DOI] [PubMed] [Google Scholar]
- Flett F., Curtis N. A., Richmond M. H. Mutant of Pseudomonas aeruginosa 18S that synthesizes type Id beta-lactamase constitutively. J Bacteriol. 1976 Sep;127(3):1585–1586. doi: 10.1128/jb.127.3.1585-1586.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frère J. M., Dormans C., Lenzini V. M., Duyckaerts C. Interaction of clavulanate with the beta-lactamases of Streptomyces albus G and Actinomadura R39. Biochem J. 1982 Dec 1;207(3):429–436. doi: 10.1042/bj2070429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaurin B., Grundström T. ampC cephalosporinase of Escherichia coli K-12 has a different evolutionary origin from that of beta-lactamases of the penicillinase type. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4897–4901. doi: 10.1073/pnas.78.8.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemal C., Knowles J. R. Penicillanic acid sulfone: interaction with RTEM beta-lactamase from Escherichia coli at different pH values. Biochemistry. 1981 Jun 23;20(13):3688–3695. doi: 10.1021/bi00516a004. [DOI] [PubMed] [Google Scholar]
- Knott-Hunziker V., Orlek B. S., Sammes P. G., Waley S. G. Kinetics of inactivation of beta-lactamase I by 6 beta-bromopenicillanic acid. Biochem J. 1980 Jun 1;187(3):797–802. doi: 10.1042/bj1870797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott-Hunziker V., Petursson S., Jayatilake G. S., Waley S. G., Jaurin B., Grundström T. Active sites of beta-lactamases. The chromosomal beta-lactamases of Pseudomonas aeruginosa and Escherichia coli. Biochem J. 1982 Mar 1;201(3):621–627. doi: 10.1042/bj2010621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott-Hunziker V., Petursson S., Waley S. G., Jaurin B., Grundström T. The acyl-enzyme mechanism of beta-lactamase action. The evidence for class C Beta-lactamases. Biochem J. 1982 Nov 1;207(2):315–322. doi: 10.1042/bj2070315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D., Jago M., Abraham E. P. Cephalosporinase and penicillinase activities of a beta-lactamase from Pseudomonas pyocyanea. Biochem J. 1965 Sep;96(3):739–752. doi: 10.1042/bj0960739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsunami S., Yago N., Hosoe M. Kinetics of suicide substrates. Steady-state treatments and computer-aided exact solutions. Biochim Biophys Acta. 1981 Dec 15;662(2):226–235. doi: 10.1016/0005-2744(81)90034-6. [DOI] [PubMed] [Google Scholar]
- Waley S. G. Kinetics of suicide substrates. Biochem J. 1980 Mar 1;185(3):771–773. doi: 10.1042/bj1850771. [DOI] [PMC free article] [PubMed] [Google Scholar]


