Abstract
This is the case of a previously healthy 15-month-old girl who initially presented to her primary pediatrician with a 2-week history of intermittent periorbital edema. The edema had improved by the time of the visit, and a urine specimen was unable to be obtained in the clinic. A routine fingerstick demonstrated anemia to 8.8 mg/dL, so the patient was started on ferrous sulfate. She then returned to the emergency department 1 month later with severe periorbital edema and pallor but no other significant symptoms. On physical examination, she was tachycardic with striking periorbital edema and an otherwise normal physical examination. She was noted to have a severe microcytic anemia (hemoglobin of 3.9 mg/dL and mean corpuscular volume of 53.1 fL) and hypoalbuminemia (albumin of 1.9 g/dL and total protein of 3.3 g/dL). The remainder of her electrolytes and liver function test results were within normal limits. A urinalysis was sent, which was negative for protein. Our panel of experts reviews her case to determine a unifying diagnosis for both her severe anemia and her hypoalbuminemia.
CASE HISTORY WITH SUBSPECIALTY INPUT
Dr Audrey Kamzan (Pediatric Hospitalist Medicine, Moderator)
A 15-month-old girl presented to her primary pediatrician’s office with a 2-week history of intermittent unilateral periorbital edema. She had not had any recent illness, fever, or changes in urine output. Her physical examination in the clinic was normal with no evidence of edema at the time. Her past medical history and birth history were unremarkable; she was born term without any complications. Both of the patient’s parents were born in Croatia, but the patient was born in the United States, and there was no history of consanguinity. Two months ago, she traveled with her parents to Croatia. There was no significant family history. On review of systems, she had no history of fever, weight change, recent illness, diarrhea, bloody or melanotic stools, or change in activity level.
At this visit, it was also noted that a screening hemoglobin had not yet been done; a fingerstick hemoglobin was performed and was 8.8 mg/dL.
Dr Lydia Kim, what were your initial concerns as an outpatient pediatrician? What is on the differential for this patient with periorbital edema and anemia?
Dr Lydia Kim (General Pediatrics)
The mother describes intermittent swelling around 1 eye. With no other symptoms, and given the intermittent nature of the swelling, I might explore any atopic history the child might have. She also fits into the classic age range for nephrotic syndrome, so I would want to check a urinalysis for protein.
The patient also had anemia on her screening hemoglobin test. Iron deficiency anemia (IDA) is the most common cause of anemia in her age group and is most frequently attributable to poor nutritional intake, so I would be sure to obtain a detailed dietary history.1 With an otherwise normal history and physical examination, I would likely treat her empirically with dietary changes and oral iron therapy then recheck her hemoglobin level. A rise in hemoglobin of 1 g/dL after 1 month of adequate iron supplementation is considered diagnostic for IDA.2 I would also send for a lead level test. Given the concurrent periorbital edema, I would have a low threshold for a broader workup. Renal disease can also cause anemia, again leading me to pursue urine testing.
Dr Kamzan
The patient’s parents mentioned that they have had a difficult time weaning her off the bottle, and she was drinking large quantities of cow’s milk. A bag was placed for urine collection, but the patient did not void spontaneously in the clinic, so urine studies could not be performed. Given that the edema had already resolved, a catheterized specimen was not felt to be necessary. A lead level was obtained and was <3 μg/dL. The patient was prescribed ferrous sulfate for treatment of her anemia, and she was scheduled for a follow-up appointment in 1 month for a hemoglobin recheck. The patient was instructed to return sooner if the periorbital edema recurred. Three weeks later, the patient again developed periorbital edema and presented to our emergency department (ED) for evaluation.
In the ED, the parents noted that the periorbital edema was now bilateral and, over the last 7 days, had progressively worsened. Her urine output was normal, and there was no change in her eating or drinking habits. The results of her review of systems remained negative for other symptoms.
On ED assessment, the patient was tachycardic with a heart rate of 187 beats per minute. Her respiratory rate was 40 breaths per minute with an oxygen saturation of 96% on room air. Her weight was 13.3 kg (>99th percentile), and her height was 83.8 cm (95th–98th percentile). On examination, the patient was well developed and fussy but consolable. She was markedly pale with striking periorbital edema. She appeared well hydrated with moist mucous membranes. Her lungs were clear to auscultation bilaterally with normal work of breathing. Her cardiac examination demonstrated tachycardia but no murmur or gallop. Her abdominal examination was normal; her abdomen was soft and nontender, and no hepatosplenomegaly was noted. She was noted to have 2+ pretibial and pedal pitting edema bilaterally.
Initial bloodwork and urine were obtained by the ED physician. A complete blood count was significant for a hemoglobin of 3.9 mg/dL and hematocrit of 15.4 with a mean corpuscular volume of 53.1 fL. Her white blood cell count was 15.7 × 103/μL, and her platelet count was 611 000/μL. Her laboratories were also notable for an albumin of 1.9 g/dL and a total protein of 3.3 g/dL. The remainder of her electrolytes and liver function test results were within normal limits. A urinalysis was sent, which demonstrated a pH of 6.5, a specific gravity of 1.011, 3 red blood cells, and 2 white blood cells and was otherwise negative, including for protein.
Given her severe anemia and hypoalbuminemia, a pediatric hospitalist consult was obtained. Dr Newcomer, as the pediatric consultant in the ED, what were your primary concerns at this point? What additional workup did you recommend?
Dr Andy Newcomer (Pediatric Hospitalist Medicine)
This patient has a chronic, severe microcytic anemia as well as hypoalbuminemia. My differential diagnosis at this point was broad, but my first concern was to stabilize this patient with severe anemia. She is hemodynamically stable but certainly symptomatic from her anemia given her tachycardia. My goal was to begin slowly correcting her anemia to avoid transfusion-associated circulatory overload (TACO). TACO is an incompletely understood complication of blood transfusions that can result in tachycardia, tachypnea, and hypoxia and is associated with significant morbidity and mortality. One proposed mechanism is an increase in central venous pressure, which leads to heart failure and pulmonary edema.3 Children <3 years of age are at increased risk, especially in the setting of fluid overload, hypoalbuminemia, or cardiac or renal impairment.4 Although slower transfusions and diuretics have become routine practice for severe anemia in many centers, the evidence behind these interventions is weak, especially in pediatrics.5 However, in light of this patient’s risk factors (<3 years of age and low albumin), we felt it prudent to give 2 low-volume (5 mL/kg) transfusions back to back over a total of 8 hours.
Given her hypoalbuminemia, a chest radiograph was done before transfusion to rule out baseline pulmonary edema and the need for diuretics. The patient’s chest radiograph demonstrated a normal cardiac silhouette and normal lung fields with no evidence of pulmonary edema or pleural effusion. The patient was admitted to our pediatric ward for further workup and management.
Dr Kamzan
What did you think was causing her microcytic anemia? What additional workup did you recommend?
Dr Newcomer
The most common causes of microcytic anemia are IDA and α or β thalassemia. Less common causes include other hemoglobinopathies, lead poisoning, sideroblastic anemia, and chronic inflammation. The American Academy of Pediatrics guidelines for the workup of severe IDA recommend, at a minimum, adding serum ferritin and C-reactive protein (CRP) to the hemoglobin measurement.6 Serum ferritin is a sensitive marker for iron stores in a healthy individual but may also be elevated in the setting of anemia of chronic disease. A normal CRP is needed to help rule out those confounders and has the added benefit of helping to diagnose anemia of chronic disease.6 Low serum iron level, reticulocyte count, and elevated total iron-binding capacity, as well as an elevated red blood cell distribution width, would also support the diagnosis of IDA. If iron studies were unrevealing, I would consider a workup for thalassemia. This patient’s background should raise concerns for thalassemia because the incidence of β thalassemia in Croatia, where both parents were born, is estimated to be 0.8% to 1%.7,8 This patient already had 1 normal lead level, which was confirmed on review of outpatient records, so there is no need to send this test again.
On the basis of the above differential, a more extensive laboratory workup was ordered (Table 1).
TABLE 1.
Laboratory Data
| Test | Reference Range | Laboratory Value |
|---|---|---|
| Albumin, g/dL | 3.7–5.1 | 1.9 |
| Total protein, g/dL | 6.2–8.6 | 3.3 |
| Complete blood count | ||
| White blood cell count, K/μL | 6–17.5 | 15.7 |
| Platelets, K/μL | 143–398 | 611 |
| Hemoglobin, mg/dL | 10.5–13.5 | 3.9 |
| Hematocrit, % | 33–39 | 15.4 |
| Mean corpuscular volume, fL | 70–86 | 53.1 |
| Red cell distribution width, fL | 36.9–48.3 | 56.2 |
| Ferritin, ng/mL | 8–180 | a |
| Iron studies | ||
| Serum iron, μg/dL | 23–182 | <8 |
| Iron-binding capacity, μg/dL | 240–520 | b |
| Saturation, % | — | b |
| CRP, mg/dL | <0.8 | <0.3 |
| Lead, μg/dL | 0–9 | <3.0 |
| Reticulocyte count, % | 0.99–1.82 | 0.14 |
| Stool studies | ||
| A1AT, mg/dL | <55 | 695 |
| Calprotectin, μg/g | ≤50 | 223 |
| FOBT | Negative | Negative |
| Urinalysis | ||
| pH | 5.0–8.0 | 6.5 |
| Specific gravity | 1.005–1.030 | 1.011 |
| Red blood cells, cells per hpf | 0–2 | 0 |
| White blood cells, cells per hpf | 0–4 | 0 |
| Protein | Negative | Negative |
Hpf, high-power field; —, not applicable.
Test not performed because of insufficient quantity of blood.
Unable to calculate because of low serum iron.
Dr Kamzan
What did you learn from this more extensive workup?
Dr Newcomer
The patient’s reticulocyte count was low at 0.14%. A serum iron level was also low (<8 μg/dL). A fecal occult blood test (FOBT) was sent to rule out gastrointestinal bleeding as the source of low iron; multiple FOBTs were sent, and all had a negative result for occult blood.
The concerns regarding the patient’s high cow’s milk intake, sometimes in excess of 32 oz per day, were again elicited on history. The parents also had not been able to get her to take the ferrous sulfate as prescribed. At this time, iron deficiency due to dietary causes remained highest on our differential. The blood transfusion provided her a large dose of iron intravenously, and she was started on oral iron as well. The parents were also counseled regarding augmenting the patient’s dietary iron intake and limiting cow’s milk intake.
Dr Kamzan
Dr Federman, does this patient’s severe anemia seem consistent with excessive milk intake? Are there any other diagnoses that should be considered?
Dr Noah Federman (Pediatric Hematologist-Oncologist)
In general, the evaluation and management of a child with suspected or confirmed uncomplicated IDA does not require the help of a pediatric hematologist. I agree with the workup, management, and differential thus far as well as the slow rate of red blood cell transfusion and concerns for TACO in this patient. I was asked for consultation in this case because of the severity of anemia and hypoalbuminemia potentially related to dietary cow’s milk intake. Although the anemia in this case is severe, it is not uncommon in toddlers with excessive milk intake and poor dietary iron consumption. Excessive ingestion of cow’s milk in children results in IDA due to both poor iron absorption from low bioavailability and, in some cases, occult blood loss from intestinal irritation.9
It is always important to consider other causes of severe anemia. One should consider red blood cell destruction (eg, hemolytic anemia). A reticulocyte count and a direct antiglobulin test are fairly good screening tests for red cell production and immune-mediated causes of red cell destruction, respectively. In this case, the reticulocyte count was low, and the direct antiglobulin test result was negative, which is consistent with absent or low red blood cell production, not an immune-mediated process. Finally, I always consider a diagnosis of an underlying malignancy in these cases; a careful history, physical examination, and review of the peripheral blood smear are critical. The presence of >1 cell line abnormality and/or hepatosplenomegaly should raise concern for a malignant etiology.
Dr Kamzan
Dr Federman, you reviewed the peripheral smear. What were your thoughts at this time?
Dr Federman
As a hematologist, I find that review of the peripheral smear is critical to understanding the underlying diagnosis and potentially ruling out other diagnoses. It is important to remember to perform a peripheral smear before red cell transfusion in order to avoid confounding the interpretation. The smear in this case was classic for severe IDA, showing marked hypochromic, microcytic red cells of varying sizes, indicating the chronicity of the IDA. There were increased platelets of normal size and morphology; thrombocytosis in the presentation of IDA is common. The etiology of the thrombocytosis in IDA is not entirely understood but may be due to thrombopoietin stimulation and/or acting as an acute phase reactant. The white blood cells were also normal in morphology, and more importantly, there were no leukemic blast cells appreciated.
In review of the peripheral smear, it is also important to assess for evidence of other red blood cell disorders, including the presence of spherocytes, target cells, anisocytes, teardrop cells, elliptocytes, and many others, that could be indicative of an underlying hemoglobinopathy or red blood cell membranopathy and resultant hemolysis. None of these were present on my review.
Dr Kamzan
Dr Newcomer, what about her hypoalbuminemia? How can you explain that?
Dr Newcomer
It is useful to think of the causes of hypoalbuminemia as due to either decreased albumin production (poor nutrition or liver dysfunction resulting in decreased synthesis) or an increase in losses (typically through urine or stool). For a child with edema and hypoalbuminemia, urinary causes of protein loss, such as nephrotic syndrome, would be at the top of my differential; I was surprised by her normal urinalysis result. Another possible cause of IDA and hypoalbuminemia is kwashiorkor, a severe form of protein energy malnutrition with edema. It is typically seen in low- and middle-income countries, but there have been cases reported in the United States.10 This patient was at the 90th percentile for both weight and height parameters, and a review of the patient’s outpatient records demonstrated that she had always been >50th percentile for weight and height, making malnutrition unlikely.
With regard to her liver function, her transaminases and alkaline phosphatase were within normal limits, and her coagulation studies were also normal, confirming adequate synthetic function. Finally, we began to consider stool losses of protein, protein-losing enteropathy (PLE), as a possible cause of her hypoalbuminemia.
Dr Kamzan
At this time, excessive milk ingestion seems to be the most likely cause of the patient’s severe IDA. However, we have been unable to elucidate the cause of the patient’s hypoalbuminemia. Dr Wozniak, does this case seem consistent with PLE? What are the most common causes of PLE in children?
Dr Laura Wozniak (Pediatric Gastroenterology)
PLE is characterized by abnormal protein loss from the gastrointestinal tract, resulting in hypoproteinemia and edema. It is caused by protein leakage across the gut or diminished uptake by intestinal lymphatics.11 Peripheral edema as well as ascites, pleural effusions, and pericardial effusions can be seen. The clinical manifestations vary depending on the underlying cause, but most patients present with diarrhea. This patient did not have diarrhea, but because we have already ruled out most other common causes of hypoalbuminemia, the diagnosis of PLE should be considered.
Dr Kamzan
What are the most common causes of PLE in children?
Dr Wozniak
There are a number of primary and secondary causes of PLE (Table 2), each of which presents differently. Some examples include inflammatory bowel disease (IBD), celiac disease, and gastrointestinal infections. However, many of these are less likely given the lack of diarrhea.
TABLE 2.
Causes of PLE
| Mucosal Injury With Inflammation and/or Ulcerations | Gastrointestinal tumors Graft-versus-host disease Infection: Bacterial: Clostridium difficile, Salmonella, Shigella, Campylobacter, Helicobacter pylori, Clostridium perfringens Parasitic: Strongyloides stercoralis, Giardia lamblia Viral: rotavirus, measles, cytomegalovirus IBD: Ulcerative colitis Crohn disease Necrotizing enterocolitis Nonsteroidal anti-inflammatory drug enteropathy |
| Mucosal injury without inflammation and/or ulcerations | Celiac disease Eosinophilic gastroenteritis Food-induced enteropathy Hypertrophic gastropathy Small intestinal bacterial overgrowth Vasculitic disorders: Systemic lupus erythematosus Henoch-Schonlein purpura |
| Lymphatic abnormalities | Primary intestinal lymphangiectasia12 Secondary intestinal lymphangiectasia Obstructive: Crohn disease, sarcoidosis, lymphoma Elevated lymph pressure: congestive heart failure, constrictive pericarditis, Fontan procedure for single ventricle, portal hypertension Syndromal: Hennekam, Klippel-Trenaunay, Noonan, Turner |
Dr Kamzan
Is there further workup for the patient’s hypoalbuminemia that you would recommend?
Dr Wozniak
At this point, I would recommend further testing for PLE. The diagnosis of PLE can be established by measuring fecal α−1 antitrypsin (A1AT), a serum protein that is not ingested in the diet. An elevated fecal A1AT level suggests increased enteral protein loss. In most cases, the etiology of PLE can be established from a careful history, physical examination, and laboratory workup; endoscopy is rarely necessary to diagnose the underlying cause.
While we wait for the stool A1AT results, I might also consider a preliminary workup for causes of PLE. In our patient, IBD seems unlikely given that she had no diarrhea and no stool inflammatory cells. However, a stool calprotectin, as well as an erythrocyte sedimentation rate and CRP, would help screen for underlying IBD. Elevated fecal calprotectin, a granulocyte protein that plays a role in neutrophil defense, can also be seen in PLE but is not specific to the diagnosis.12 In addition, I would recommend celiac titers to rule out celiac disease: antitissue transglutaminase, antiendomysial antibodies, and a total immunoglobulin A level. A cardiac etiology seems unlikely given that the patient had a normal heart size on chest radiograph. Likewise, portal hypertension seems unlikely given that she has no ascites, splenomegaly, or thrombocytopenia. However, because venous outflow obstruction (eg, hepatic or portal vein thrombosis) can, on rare occasions, lead to PLE, I would recommend an abdominal ultrasound with Doppler.
It is important to note that although milk-protein allergy can lead to anemia and potentially PLE as well, I had a low suspicion in this case. This patient was older (patients typically present within the first year), and the stool was heme-negative. Both of these make milk-protein allergy unlikely.
Dr Kamzan
What were the results of the workup for PLE?
Dr Wozniak
The patient’s stool A1AT level was elevated to 695 mg/dL (reference range: <55 mg/dL). There were no stool cells present, and an FOBT had a negative result. The fecal calprotectin was 223 μg/g; sources differ on the expected normal values for this age group, so this may be considered either mildly elevated or normal for age.13 Celiac titer results were negative, and an abdominal ultrasound with Doppler was within normal limits. A review of the literature was performed, during which we discovered several case reports identifying an association between severe IDA and PLE.14–16 The causal link between the 2 findings is unclear, but in published reports, iron therapy and correction of the IDA led to complete resolution of the PLE.17,18 Given the expectation that the PLE would likely resolve with iron therapy, as well as the low suspicion for other inflammatory diseases based on the clinical presentation and workup, immediate endoscopy was not indicated. However, close follow-up of this patient is important; if the PLE did not resolve with iron therapy, endoscopy would be the next step.
Dr Kamzan
Dr Newcomer, what were the next steps you took in managing this patient?
Dr Newcomer
The patient continued to have significant periorbital edema, so she was given 2 mL/kg of 25% albumin followed by 0.5 mg/kg of furosemide. This intervention dramatically improved her edema. The patient was discharged with close follow-up with her primary pediatrician and a plan to repeat a complete blood count and albumin within 1 week.
Dr Kamzan
What happened to the patient after discharge?
Dr Kim
After discharge, the patient’s parents limited cow’s milk in the child’s diet (but she continues to consume dairy products in moderation). They gave the patient the ferrous sulfate as prescribed, and her albumin and hemoglobin continued to uptrend (Fig 1). A repeat A1AT level was obtained 2 weeks after discharge, which at that time was undetectable, and the patient’s edema never returned. Given the improvement in edema with only treatment of her iron deficiency, the patient was presumed to have PLE related to underlying IDA.
FIGURE 1.
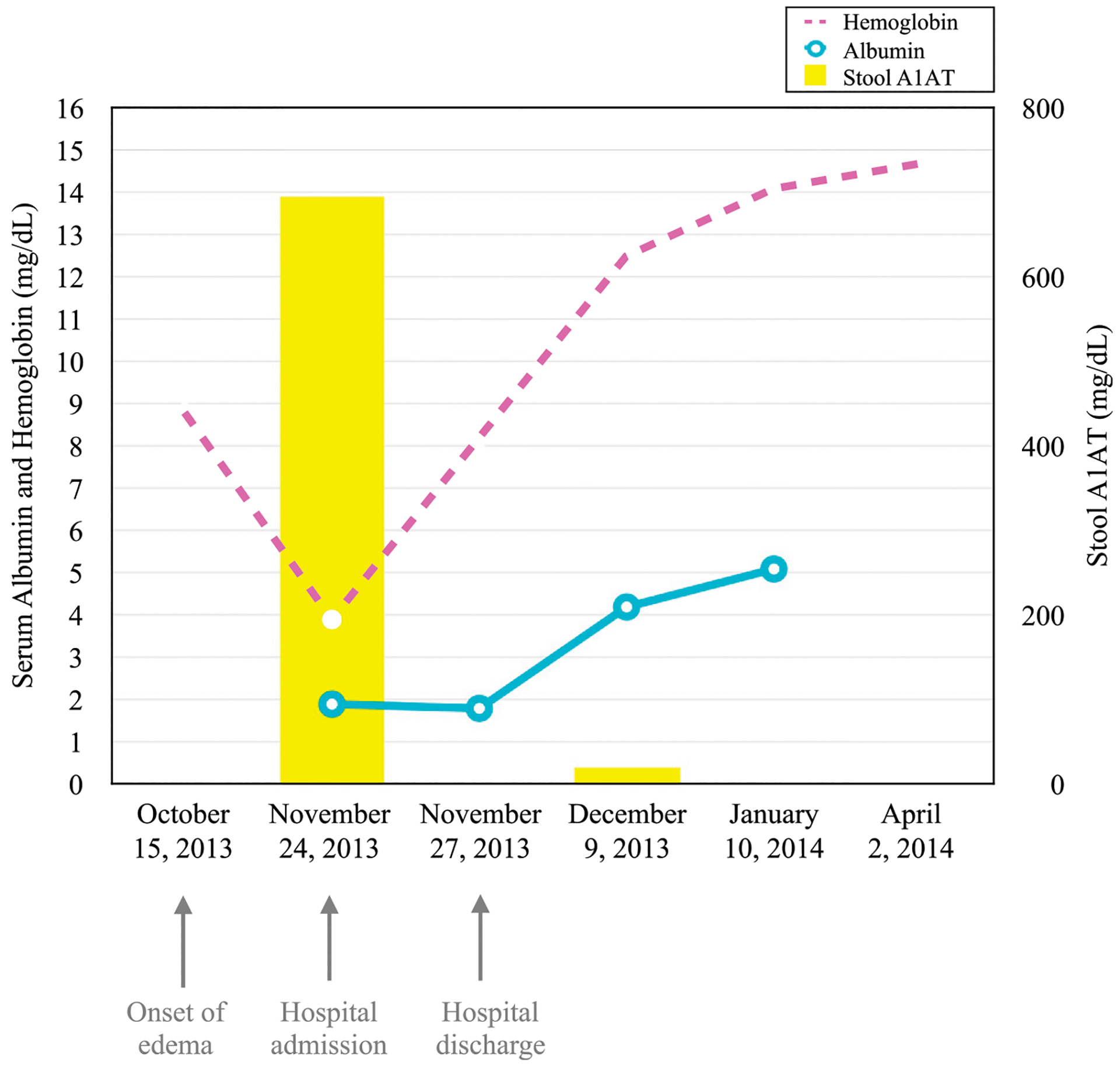
Case laboratory values over time.
DISCUSSION
This is a case of a common disease process (IDA) presenting with an unusual sequela (PLE). The patient’s elevated stool A1AT levels ultimately confirmed the diagnosis of PLE despite the lack of diarrhea or any other suggestive symptoms. An association between severe IDA and PLE has been described in the literature, but the mechanism is poorly understood.14–18 Some cases may be explained by milk-protein allergy. However, there have been reported cases in which there was no clinical or laboratory evidence of milk-protein allergy and intestinal biopsy results were normal, showing no change in villus structure or quantity of intraepithelial lymphocytes.15 In published reports of these cases, iron therapy corrected both the IDA and the hypoalbuminemia without switching to a dairy-free diet.17,18 Further research is needed to elucidate the causative mechanism of PLE in these cases. As described in the literature, simply by treating this patient’s underlying IDA with iron supplementation and limiting cow’s milk intake, we were able to achieve complete resolution of her PLE and resulting hypoalbuminemia. Importantly, the patient was able to continue to consume dairy products in moderation without recurrence of her PLE or IDA, so we do not feel that milk-protein allergy played a role in her disease process.
IDA is common in the United States and remains the world’s most common single-nutrient deficiency among children.19 Therefore, we suspect there may be an under-recognized burden of PLE in these patients. In toddlers, nutritional concerns are the most common causes of IDA. Specifically, excessive cow’s milk intake is known to be an important cause due to the calcium content blocking the absorption of iron. It is not uncommon for the resulting anemia to be profound, as we saw in this case. There were significant safety issues involved with treating this patient’s anemia; our team was concerned that the threat of TACO may have been further heightened by the decreased oncotic pressure due to low protein, so we pursued a plan of slow transfusion.
For the general pediatrician consulted about a toddler with periorbital edema, nephrotic syndrome should be high on the differential; we were surprised to find her urine to be negative for protein. Our multidisciplinary team was essential in making the diagnosis by employing a systematic approach to the hypoalbuminemia workup. We conceptualized the causes of hypoalbuminemia into 2 groups: decreased production of protein (such as malnutrition and liver disease) and increased losses (in the urine or stool). By ruling out most other causes of hypoalbuminemia, we were left with PLE as the presumptive diagnosis while awaiting the A1AT results. We felt there was likely to be a common cause between the IDA and PLE in this otherwise healthy child. A review of the literature led to the discovery of the many case reports describing the association between PLE and IDA. General pediatricians, who are on the front lines of diagnosing and treating IDA, would benefit from increased awareness of this association to improve recognition and prevention of PLE as a complication.
FUNDING:
Dr Federman is supported by the National Institutes of Health National Center for Advancing Translational Sciences (grant UL1TR001881).
ABBREVIATIONS
- A1AT
α−1 antitrypsin
- CRP
C-reactive protein
- ED
emergency department
- FOBT
fecal occult blood test
- IBD
inflammatory bowel disease
- IDA
iron deficiency anemia
- PLE
protein-losing enteropathy
- TACO
transfusion-associated circulatory overload
Footnotes
FINANCIAL DISCLOSURE: Dr Federman has served as a scientific advisory board member for Loxo Oncology and Bayer; the other authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
REFERENCES
- 1.Oski FA. Iron deficiency in infancy and childhood. N Engl J Med. 1993;329(3): 190–193 [DOI] [PubMed] [Google Scholar]
- 2.Reeves JD. Prediction of therapeutic response to iron. In: Oski FA, Pearson HA, eds. Iron Nutrition Revisited–Infancy, Childhood, Adolescence: Report of the Eighty-Second Ross Conference on Pediatric Research. Columbus, OH: Ross Laboratories; 1981:114–125 [Google Scholar]
- 3.Andreu G Transfusion-associated circulatory overload and transfusion-related acute lung injury: diagnosis, pathophysiology, management and prevention. ISBT Sci Ser. 2009;4:63–71 [Google Scholar]
- 4.Alam A, Lin Y, Lima A, Hansen M, Callum JL. The prevention of transfusion-associated circulatory overload. Transfus Med Rev. 2013;27(2):105–112 [DOI] [PubMed] [Google Scholar]
- 5.Agrawal AK, Hsu E, Quirolo K, Neumayr LD, Flori HR. Red blood cell transfusion in pediatric patients with severe chronic anemia: how slow is necessary? Pediatr Blood Cancer. 2012; 58(3):466–468 [DOI] [PubMed] [Google Scholar]
- 6.Baker RD, Greer FR; Committee on Nutrition, American Academy of Pediatrics. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age). Pediatrics. 2010; 126(5):1040–1050 [DOI] [PubMed] [Google Scholar]
- 7.Weatherall DJ, Clegg JB. The Thalassaemia Syndromes. 2001
- 8.Efremov GD. Hemoglobinopathies in Yugoslavia: an update. Hemoglobin. 1992;16(6):531–544 [DOI] [PubMed] [Google Scholar]
- 9.Ziegler EE, Fomon SJ, Nelson SE, et al. Cow milk feeding in infancy: further observations on blood loss from the gastrointestinal tract. J Pediatr. 1990; 116(1):11–18 [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Severe malnutrition among young children–Georgia, January 1997-June 1999. MMWR Morb Mortal Wkly Rep. 2001;50(12):224–227 [PubMed] [Google Scholar]
- 11.Braamskamp MJ, Dolman KM, Tabbers MM. Clinical practice. Protein-losing enteropathy in children. Eur J Pediatr. 2010;169(10):1179–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyllie R, Hyams JS, Kay M, eds. Pediatric Gastrointestinal and Liver Disease, 3rd ed. Philadelphia, PA: Saunders; 2006 [Google Scholar]
- 13.Herrera OR, Christensen ML, Helms RA. Calprotectin: clinical applications in pediatrics. J Pediatr Pharmacol Ther. 2016;21(4):308–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naiman JL, Oski FA, Diamond LK, Vawter GF, Shwachman H. The gastrointestinal effects of iron deficiency anemia. Pediatrics. 1964;33:83–99 [PubMed] [Google Scholar]
- 15.Lundström U, Perkkiö M, Savilahti E, Siimes M. Iron deficiency anaemia with hypoproteinaemia. Arch Dis Child. 1983; 58(6):438–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogelaar JL, Loar RW, Bram RJ, Fischer PR, Kaushik R. Anasarca, hypoalbuminemia, and anemia: what is the correlation? Clin Pediatr (Phila). 2014;53(7):710–712 [DOI] [PubMed] [Google Scholar]
- 17.Salstrom JL, Kent M, Liang X, Wang M. Toddlers with anasarca and severe anemia: a lesson in preventive medicine. Curr Opin Pediatr. 2012;24(1): 129–133 [DOI] [PubMed] [Google Scholar]
- 18.Nickerson HJ, Silberman T, Park RW, DeVries EO, Broste SK. Treatment of iron deficiency anemia and associated protein-losing enteropathy in children. J Pediatr Hematol Oncol. 2000;22(1): 50–54 [DOI] [PubMed] [Google Scholar]
- 19.United Nations Administrative Committee on Coordination/Sub-Committee on Nutrition and International Food Policy Research Institute. Fourth Report of the World Nutrition Situation. Geneva, Switzerland: United Nations Administrative Committee on Coordination, Subcommittee on Nutrition; 2000 [Google Scholar]


