Abstract
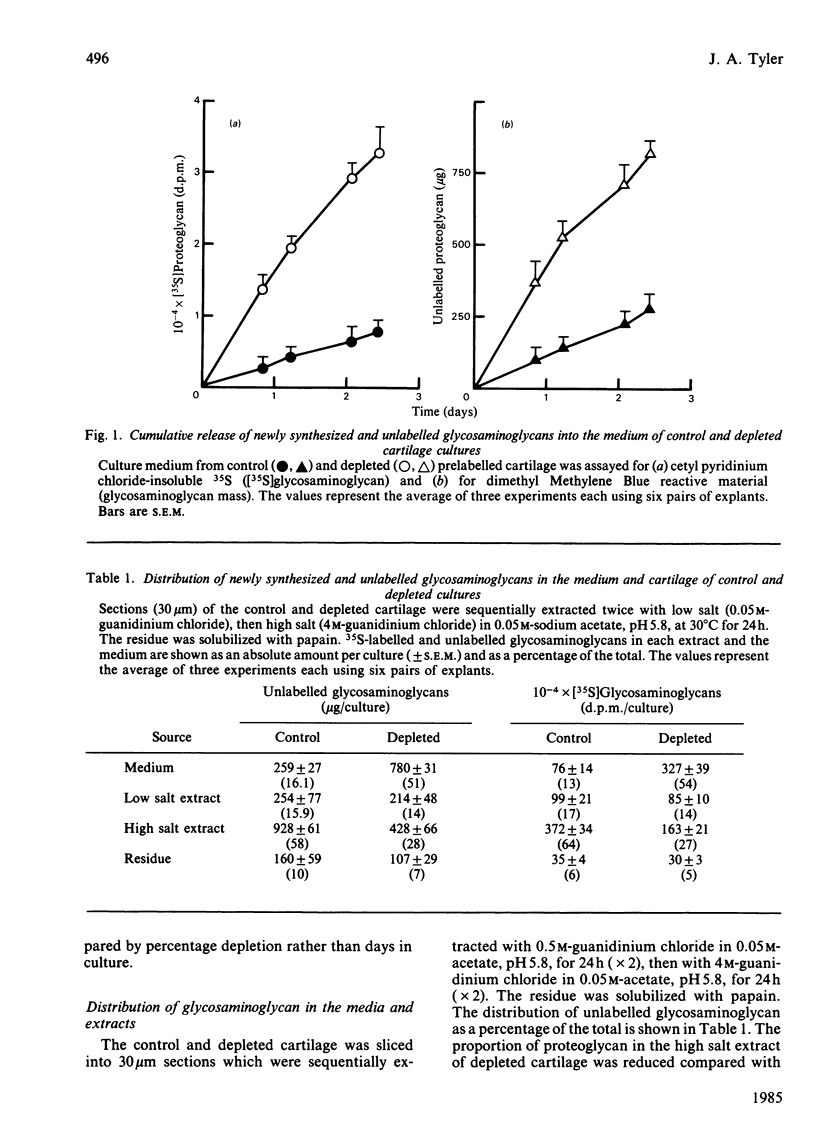
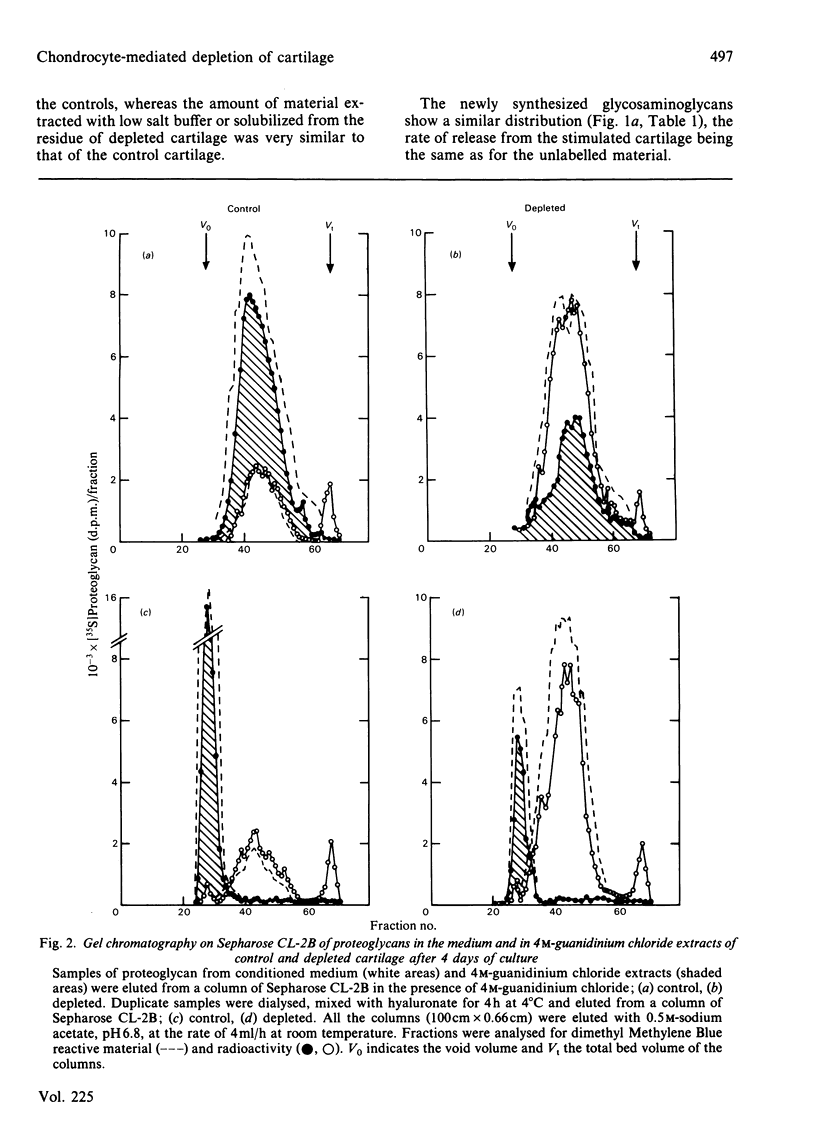
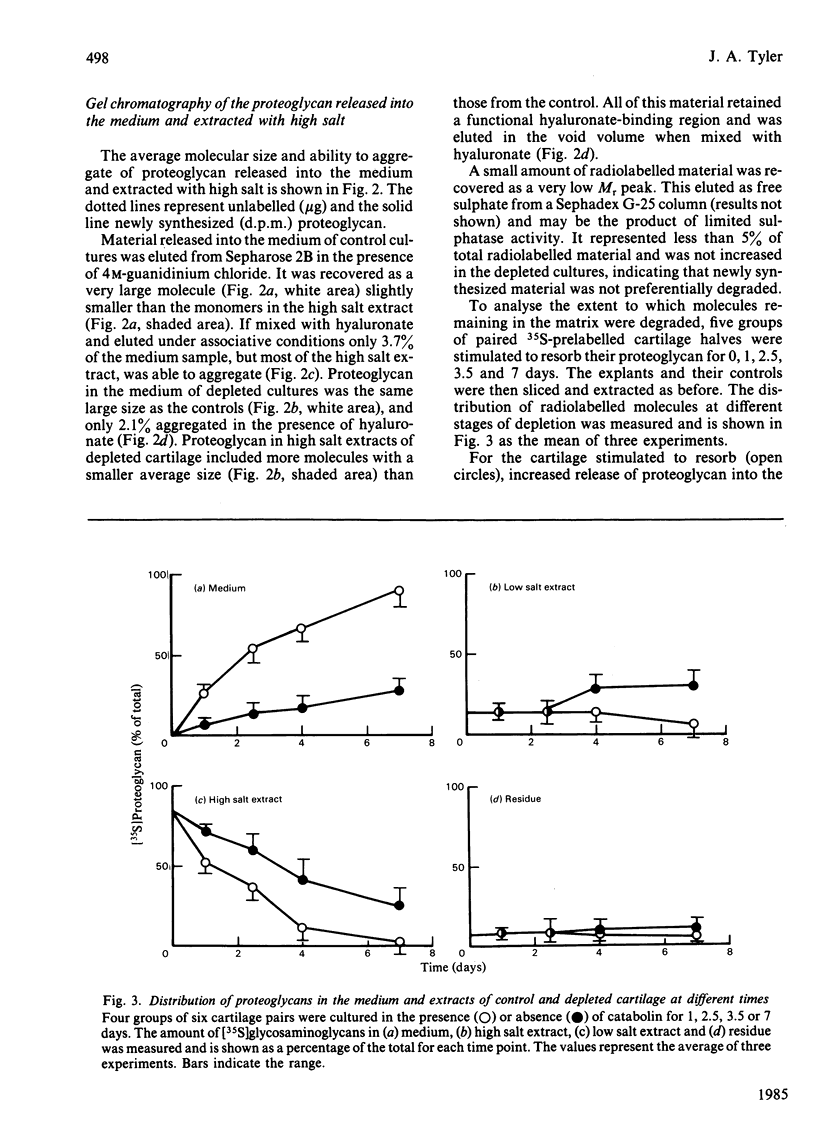
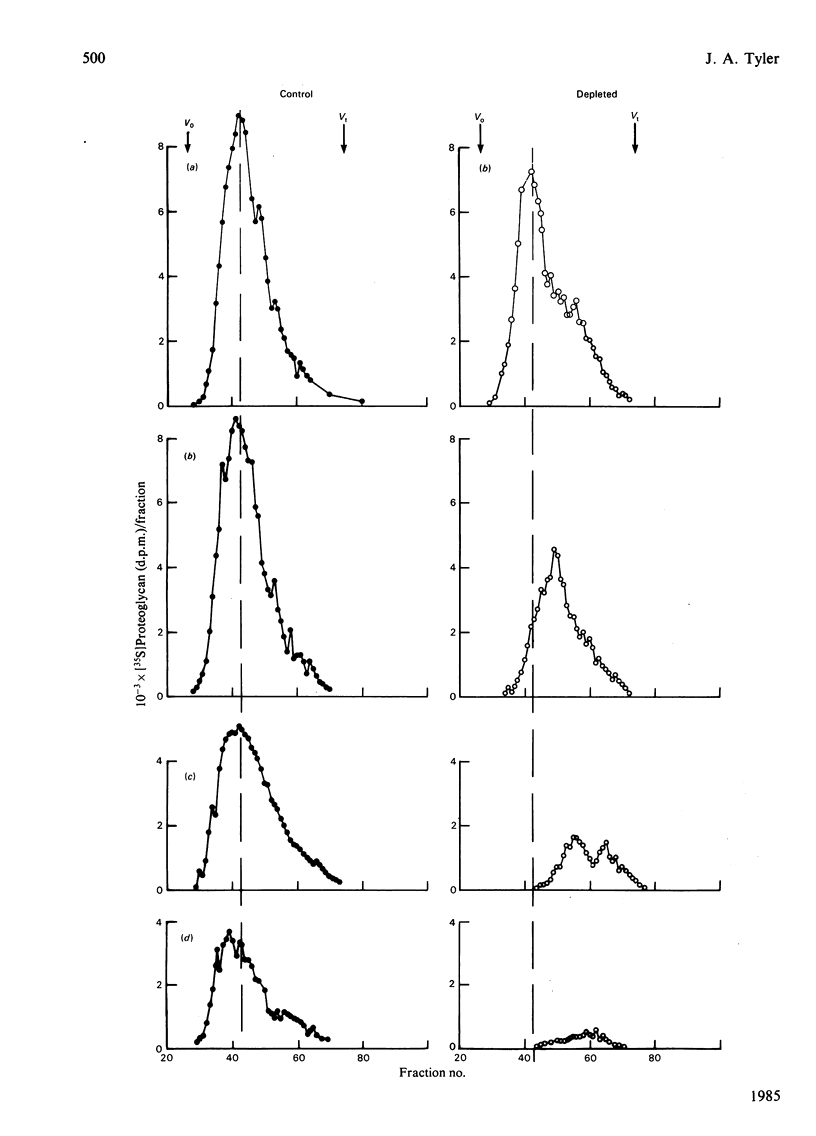
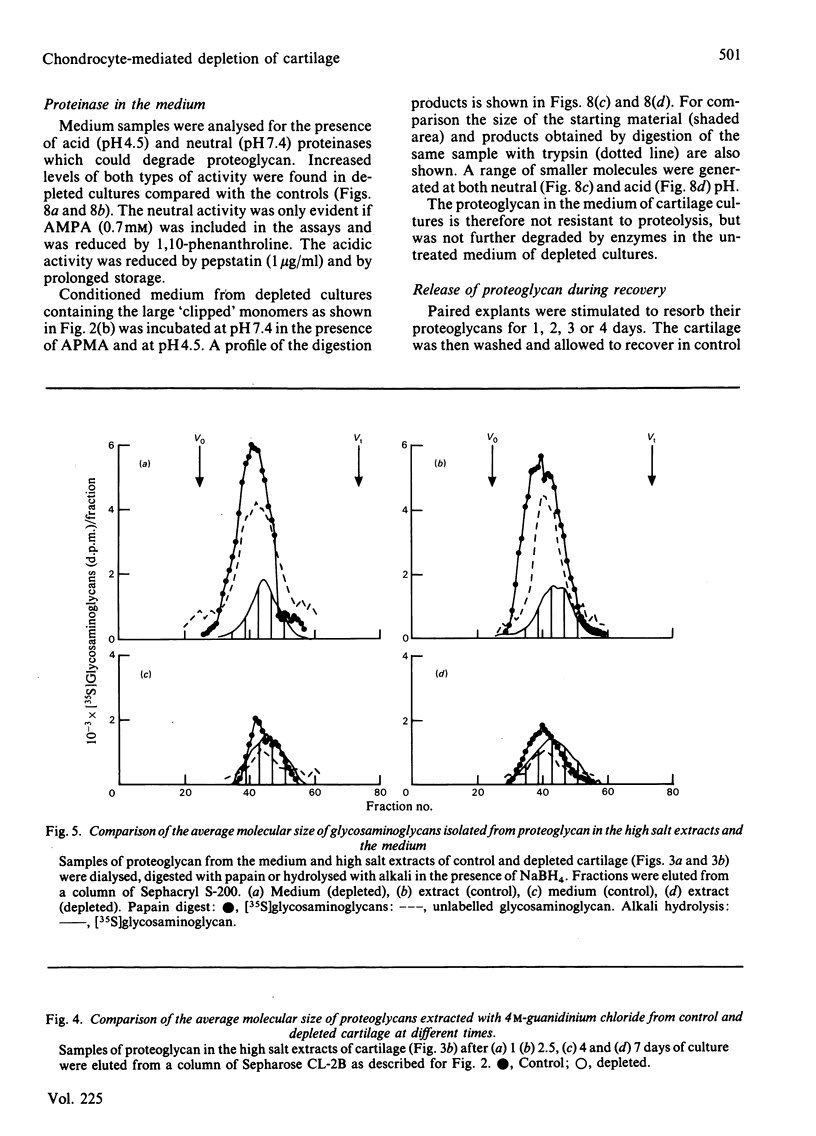
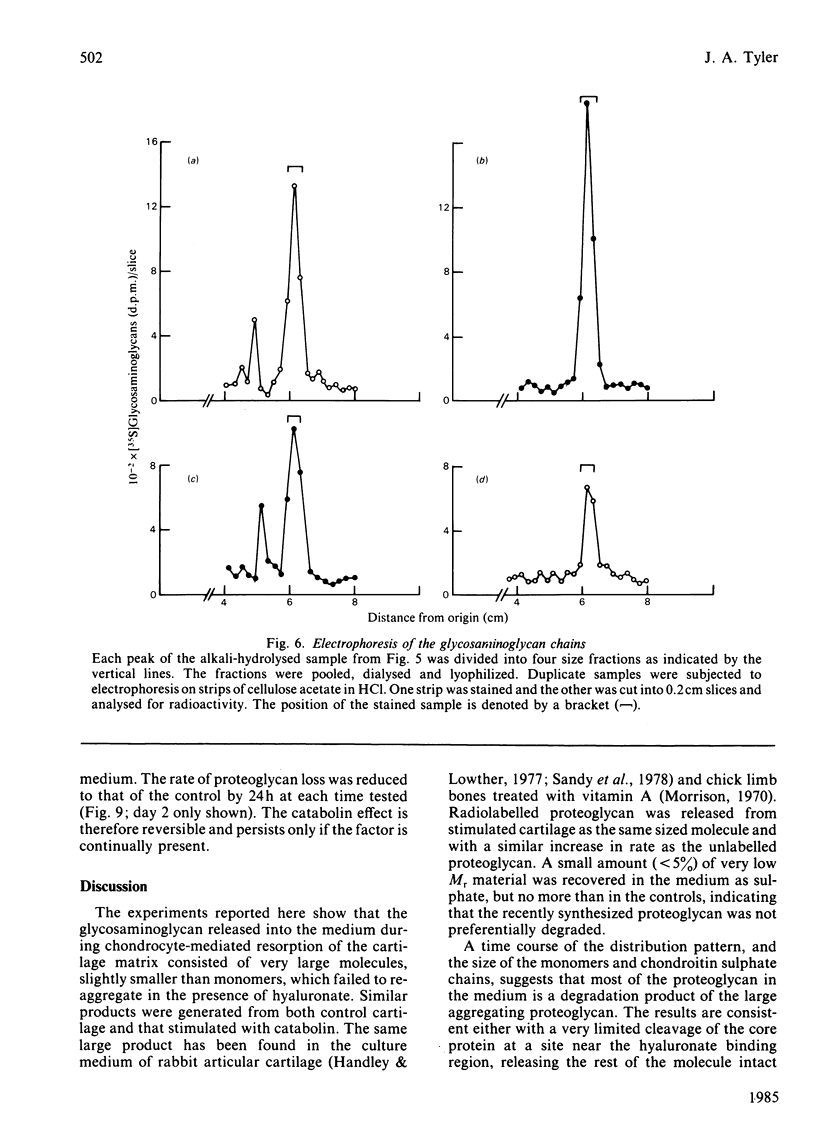
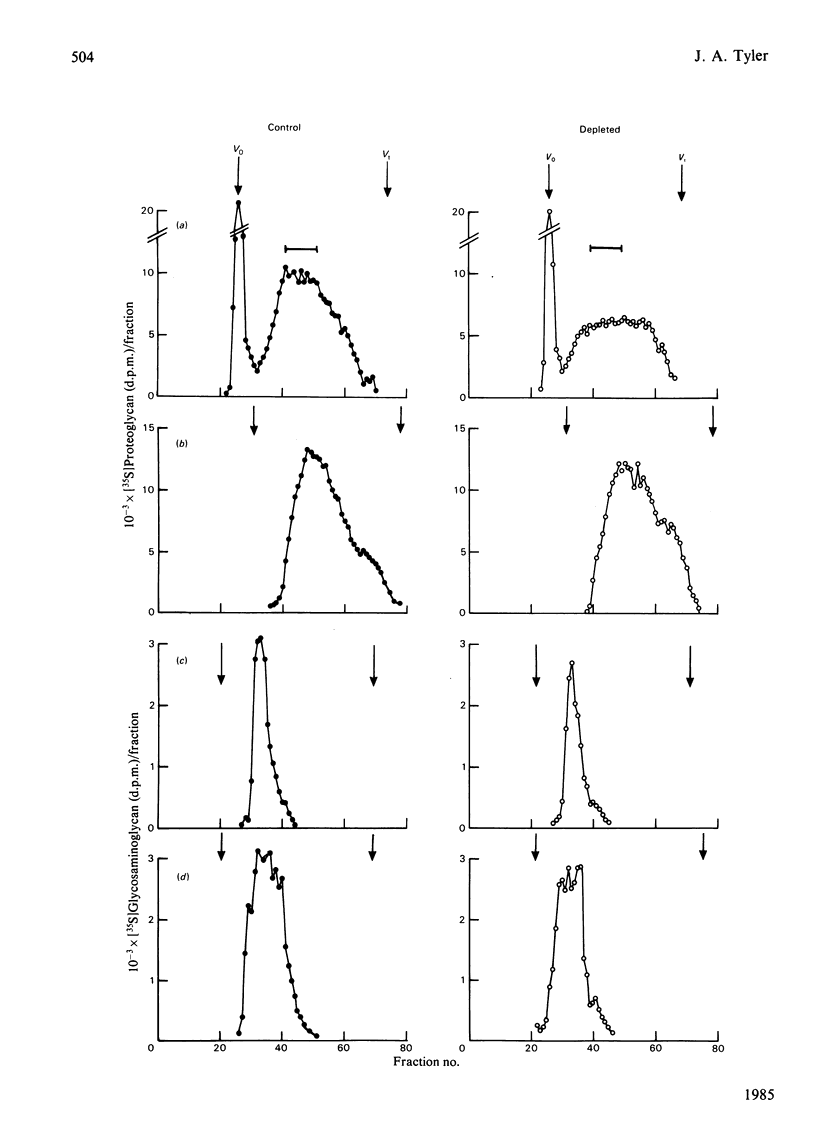
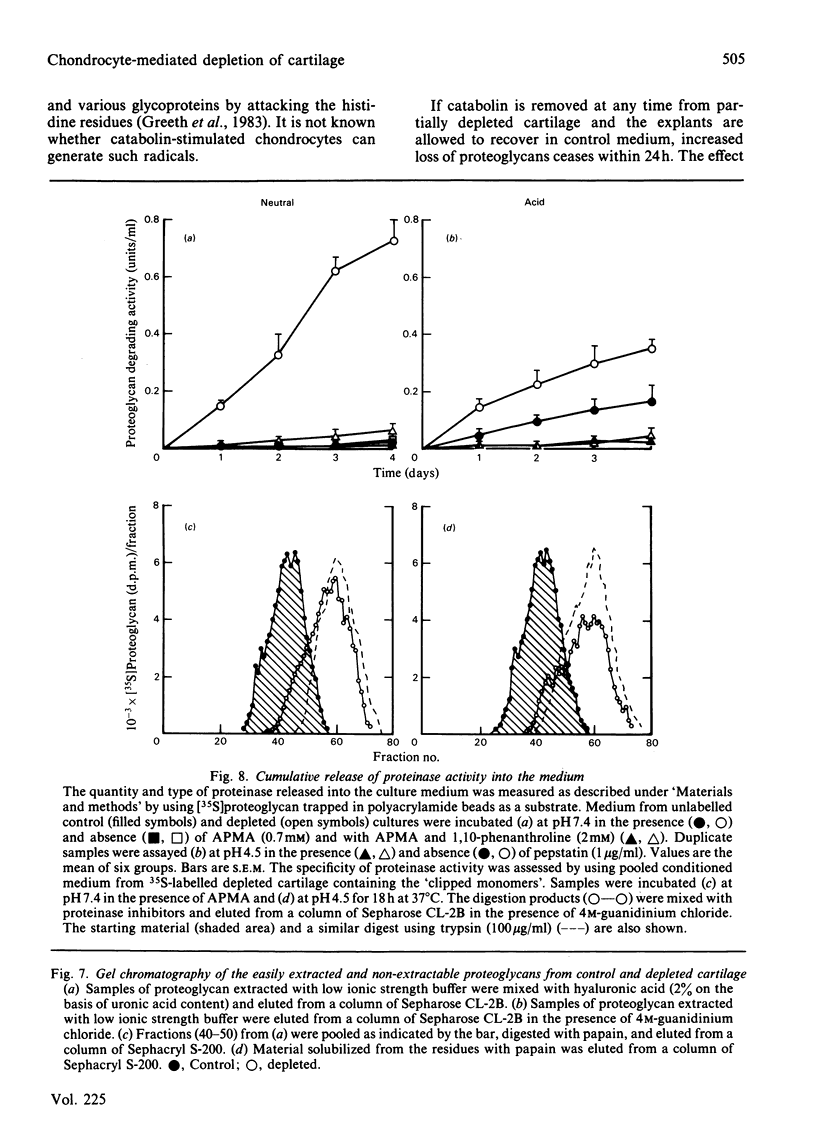
The degradation of proteoglycan was examined in cultured slices of pig articular cartilage. Pig leucocyte catabolin (10 ng/ml) was used to stimulate the chondrocytes and induce a 4-fold increase in the rate of proteoglycan loss from the matrix for 4 days. Material in the medium of both control and depleted cultures was mostly a degradation product of the aggregating proteoglycan. It was recovered as a very large molecule slightly smaller than the monomers extracted with 4M-guanidinium chloride and lacked a functional hyaluronate binding region. The size and charge were consistent with a very limited cleavage or conformational change of the core protein near the hyaluronate binding region releasing the C-terminal portion of the molecule intact from the aggregate. The 'clipped' monomer diffuses very rapidly through the matrix into the medium. The amount of proteoglycan extracted with 4M-guanidinium chloride decreased during culture from both the controls and depleted cartilage, and the average size of the molecules initially remained the same. However, the proportion of molecules with a smaller average size increased with time and was predominant in explants that had lost more than 70% of their proteoglycan. All of this material was able to form aggregates when mixed with hyaluronate, and glycosaminoglycans were the same size and charge as normal, indicating either that the core protein had been cleaved in many places or that larger molecules were preferentially released. A large proportion of the easily extracted and non-extractable proteoglycan remained in the partially depleted cartilage and the molecules were the same size and charge as those found in the controls. There was no evidence of detectable glycosidase activity and only very limited sulphatase activity. A similar rate of breakdown and final distribution pattern was found for newly synthesized proteoglycan. Increased amounts of latent neutral metalloproteinases and acid proteinase activities were present in the medium of depleted cartilage. These were not thought to be involved in the breakdown of proteoglycan. Increased release of proteoglycan ceased within 24h of removal of the catabolin, indicating that the effect was reversible and persisted only while the stimulus was present.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Cathepsin D. Purification of isoenzymes from human and chicken liver. Biochem J. 1970 Apr;117(3):601–607. doi: 10.1042/bj1170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Cartwright E. C., Campbell I. K., Britz M. L., Sandy J. D., Lowther D. A. Characterization of latent and active forms of cartilage proteinases produced by normal immature rabbit articular cartilage in tissue culture. Arthritis Rheum. 1983 Aug;26(8):984–993. doi: 10.1002/art.1780260807. [DOI] [PubMed] [Google Scholar]
- Cleland R. L., Stoolmiller A. C., Rodén L., Laurent T. C. Partial characterization of reaction products formed by the degradation of hyaluronic acid with ascorbic acid. Biochim Biophys Acta. 1969 Dec 30;192(3):385–394. doi: 10.1016/0304-4165(69)90387-0. [DOI] [PubMed] [Google Scholar]
- Creeth J. M., Cooper B., Donald A. S., Clamp J. R. Studies of the limited degradation of mucus glycoproteins. The effect of dilute hydrogen peroxide. Biochem J. 1983 May 1;211(2):323–332. doi: 10.1042/bj2110323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. D., Woessner J. F., Jr Extracts of human articular cartilage contain an inhibitor of tissue metalloproteinases. Biochem J. 1984 Feb 15;218(1):277–280. doi: 10.1042/bj2180277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Barrett A. J., Weston P. D. Cathepsin D. Characteristics of immunoinhibition and the confirmation of a role in cartilage breakdown. Biochem J. 1971 Jun;123(1):1–13. doi: 10.1042/bj1230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Blow A. M., Barrett A. J., Martin P. E. Proteoglycan-degrading enzymes. A radiochemical assay method and the detection of a new enzyme cathepsin F. Biochem J. 1977 Dec 1;167(3):775–785. doi: 10.1042/bj1670775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Dingle T. T. The site of cartilage matrix degradation. Biochem J. 1980 Aug 15;190(2):431–438. doi: 10.1042/bj1900431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Saklatvala J., Hembry R., Tyler J., Fell H. B., Jubb R. A cartilage catabolic factor from synovium. Biochem J. 1979 Oct 15;184(1):177–180. doi: 10.1042/bj1840177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELL H. B., MELLANBY E., PELC S. R. Influence of excess vitamin A on the sulphate metabolism of bone rudiments grown in vitro. J Physiol. 1956 Oct 29;134(1):179–188. doi: 10.1113/jphysiol.1956.sp005633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Fell H. B., Jubb R. W. The effect of synovial tissue on the breakdown of articular cartilage in organ culture. Arthritis Rheum. 1977 Sep-Oct;20(7):1359–1371. doi: 10.1002/art.1780200710. [DOI] [PubMed] [Google Scholar]
- Galloway W. A., Murphy G., Sandy J. D., Gavrilovic J., Cawston T. E., Reynolds J. J. Purification and characterization of a rabbit bone metalloproteinase that degrades proteoglycan and other connective-tissue components. Biochem J. 1983 Mar 1;209(3):741–752. doi: 10.1042/bj2090741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Production of superoxide, hydrogen peroxide and hydroxyl radicals by phagocytic cells: a cause of chronic inflammatory disease? Cell Biol Int Rep. 1982 Jun;6(6):529–542. doi: 10.1016/0309-1651(82)90175-8. [DOI] [PubMed] [Google Scholar]
- Handley C. J., Lowther D. A. Extracellular matrix metabolism by chondrocytes. III. Modulation of proteoglycan synthesis by extracellular levels of proteoglycan in cartilage cells in culture. Biochim Biophys Acta. 1977 Nov 7;500(1):132–139. doi: 10.1016/0304-4165(77)90053-8. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Ewins R. J., Muir H. Cartilage proteoglycans. Structure and heterogeneity of the protein core and the effects of specific protein modifications on the binding to hyaluronate. Biochem J. 1976 Jul 1;157(1):127–143. doi: 10.1042/bj1570127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Fitton-Jackson S., Muir H. Replacement of proteoglycans in embryonic chick cartilage in organ culture after treatment with testicular hyaluronidase. Biochem J. 1972 Aug;129(1):101–112. doi: 10.1042/bj1290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Characterization of chondroitin sulfate isolated from trypsin-chymotrypsin digests of cartilage proteoglycans. Arch Biochem Biophys. 1974 Nov;165(1):427–441. doi: 10.1016/0003-9861(74)90182-9. [DOI] [PubMed] [Google Scholar]
- Heinegård D. Hyaluronidase digestion and alkaline treatment of bovine tracheal cartilage proteoglycans. Isolation and characterisation of different keratan sulfate proteins. Biochim Biophys Acta. 1972 Nov 28;285(1):193–207. doi: 10.1016/0005-2795(72)90191-2. [DOI] [PubMed] [Google Scholar]
- Heinegård D. Polydispersity of cartilage proteoglycans. Structural variations with size and buoyant density of the molecules. J Biol Chem. 1977 Mar 25;252(6):1980–1989. [PubMed] [Google Scholar]
- Jasin H. E. Bacterial lipopolysaccharides induce in vitro degradation of cartilage matrix through chondrocyte activation. J Clin Invest. 1983 Dec;72(6):2014–2019. doi: 10.1172/JCI111166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasin H. E., Dingle J. T. Human mononuclear cell factors mediate cartilage matrix degradation through chondrocyte activation. J Clin Invest. 1981 Sep;68(3):571–581. doi: 10.1172/JCI110290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubb R. W., Fell H. B. The breakdown of collagen by chondrocytes. J Pathol. 1980 Mar;130(3):159–167. doi: 10.1002/path.1711300304. [DOI] [PubMed] [Google Scholar]
- Killackey J. J., Roughley P. J., Mort J. S. Proteinase inhibitors of human articular cartilage. Coll Relat Res. 1983 Sep;3(5):419–430. doi: 10.1016/s0174-173x(83)80022-3. [DOI] [PubMed] [Google Scholar]
- Klämfeldt A., Jones I. L., McGuire M. B. Enhanced breakdown of bovine articular cartilage proteoglycans by conditioned synovial medium in vitro. The effect of glucocorticoids and protein synthesis inhibitors. Scand J Rheumatol. 1982;11(4):230–234. doi: 10.3109/03009748209098196. [DOI] [PubMed] [Google Scholar]
- Knight J. A., Stephens R. W., Bushell G. R., Ghosh P., Taylor T. K. Neutral protease inhibitors from human intervertebral disc and femoral head articular cartilage. Biochim Biophys Acta. 1979 May 1;584(2):304–310. doi: 10.1016/0304-4165(79)90276-9. [DOI] [PubMed] [Google Scholar]
- Kuettner K. E., Hiti J., Eisenstein R., Harper E. Collagenase inhibition by cationic proteins derived from cartilage and aorta. Biochem Biophys Res Commun. 1976 Sep 7;72(1):40–46. doi: 10.1016/0006-291x(76)90957-8. [DOI] [PubMed] [Google Scholar]
- LUCY J. A., DINGLE J. T., FELL H. B. Studies on the mode of action of excess of vitamin A. 2. A possible role of intracellular proteases in the degradation of cartilage matrix. Biochem J. 1961 Jun;79:500–508. doi: 10.1042/bj0790500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millroy S. J., Poole A. R. Pig articular cartilage in organ culture. Effect of enzymatic depletion of the matrix on response of chondrocytes to complement-sufficient antiserum against pig erythrocytes. Ann Rheum Dis. 1974 Nov;33(6):500–508. doi: 10.1136/ard.33.6.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales T. I., Wahl L. M., Hascall V. C. The effect of bacterial lipopolysaccharides on the biosynthesis and release of proteoglycans from calf articular cartilage cultures. J Biol Chem. 1984 Jun 10;259(11):6720–6729. [PubMed] [Google Scholar]
- Morrison R. I., Barrett A. J., Dingle J. T., Prior D. Cathepsins BI and D. Action on human cartilage proteoglycans. Biochim Biophys Acta. 1973 Apr 12;302(2):411–419. doi: 10.1016/0005-2744(73)90170-8. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cambray G. J., Virani N., Page-Thomas D. P., Reynolds J. J. The production in culture of metalloproteinases and an inhibitor by joint tissues from normal rabbits, and from rabbits with a model arthritis. II. Articular cartilage. Rheumatol Int. 1981;1(1):17–20. doi: 10.1007/BF00541218. [DOI] [PubMed] [Google Scholar]
- Poole A. R., Hembry R. M., Dingle J. T. Cathepsin D in cartilage: the immunohistochemical demonstration of extracellular enzyme in normal and pathological conditions. J Cell Sci. 1974 Jan;14(1):139–161. doi: 10.1242/jcs.14.1.139. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Crowe R. M. Isolation of a protease inhibitor from tissues resistant to tumor invasion. Hoppe Seylers Z Physiol Chem. 1977 Dec;358(12):1525–1531. doi: 10.1515/bchm2.1977.358.2.1525. [DOI] [PubMed] [Google Scholar]
- Roughley P. J., Barrett A. J. The degradation of cartilage proteoglycans by tissue proteinases. Proteoglycan structure and its susceptibility to proteolysis. Biochem J. 1977 Dec 1;167(3):629–637. doi: 10.1042/bj1670629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J., Murphy G., Barrett A. J. Proteinase inhibitors of bovine nasal cartilage. Biochem J. 1978 Mar 1;169(3):721–724. doi: 10.1042/bj1690721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J. The degradation of cartilage proteoglycans by tissue proteinases. Proteoglycan heterogeneity and the pathway of proteolytic degradation. Biochem J. 1977 Dec 1;167(3):639–646. doi: 10.1042/bj1670639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royce P. M., Lowther D. A. Fluorimetric determination of DNA in papain digests of cartilage, using ethidium bromide. Connect Tissue Res. 1979;6(4):215–221. doi: 10.3109/03008207909152323. [DOI] [PubMed] [Google Scholar]
- Saklatvala J., Curry V. A., Sarsfield S. J. Purification to homogeneity of pig leucocyte catabolin, a protein that causes cartilage resorption in vitro. Biochem J. 1983 Nov 1;215(2):385–392. doi: 10.1042/bj2150385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J., Curry V. A., Sarsfield S. J. Purification to homogeneity of pig leucocyte catabolin, a protein that causes cartilage resorption in vitro. Biochem J. 1983 Nov 1;215(2):385–392. doi: 10.1042/bj2150385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J., Sarsfield S. J. Lymphocytes induce resorption of cartilage by producing catabolin. Biochem J. 1982 Jan 15;202(1):275–278. doi: 10.1042/bj2020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Brown H. L., Lowther D. A. Control of proteoglycan synthesis. Studies on the activation of synthesis observed during culture of articular cartilages. Biochem J. 1980 Apr 15;188(1):119–130. doi: 10.1042/bj1880119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Brown H. L., Lowther D. A. Degradation of proteoglycan in articular cartilage. Biochim Biophys Acta. 1978 Nov 1;543(4):536–544. doi: 10.1016/0304-4165(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Schmut O., Hofmann H. Studies on the generation of hydrogen peroxide during some non-enzymic reactions changing the hyaluronic acid molecule. Biochim Biophys Acta. 1975 Dec 5;411(2):231–235. doi: 10.1016/0304-4165(75)90303-7. [DOI] [PubMed] [Google Scholar]
- Simůnek Z., Muir H. Proteoglycans of the knee-joint cartilage of young normal and lame pigs. Biochem J. 1972 Nov;130(1):181–187. doi: 10.1042/bj1300181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg J., Sledge C. B., Noble J., Stirrat C. R. A tissue-culture model of cartilage breakdown in rheumatoid arthritis. Quantitative aspects of proteoglycan release. Biochem J. 1979 May 15;180(2):403–412. doi: 10.1042/bj1800403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tougaard L. The degree of mineralization in bone tissue. The phosphorus-hydroxyproline ratio determined on small amounts of bone tissue. Scand J Clin Lab Invest. 1973 Dec;32(4):351–355. doi: 10.3109/00365517309084358. [DOI] [PubMed] [Google Scholar]
- Tsiganos C. P., Muir H. Studies on protein-polysaccharides from pig laryngeal cartilage. Heterogeneity, fractionation and characterization. Biochem J. 1969 Aug;113(5):885–894. doi: 10.1042/bj1130885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. A., Fell H. B., Lawrence C. E. The effect of cortisol on porcine articular tissues in organ culture. J Pathol. 1982 Aug;137(4):335–351. doi: 10.1002/path.1711370408. [DOI] [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of the molecular weight and molecular-weight distribution of chondroitin sulphate. J Chromatogr. 1971 Jul 8;59(1):87–97. doi: 10.1016/s0021-9673(01)80009-1. [DOI] [PubMed] [Google Scholar]
- Wasteson A., Lindahl U., Hallén A. Mode of degradation of the chondroitin sulphate proteoglycan in rat costal cartilage. Biochem J. 1972 Dec;130(3):729–738. doi: 10.1042/bj1300729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson A., Lindahl U. The distribution of sulphate residues in the chondroitin sulphate chain. Biochem J. 1971 Dec;125(3):903–908. doi: 10.1042/bj1250903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner J. F., Jr Purification of cathepsin D from cartilage and uterus and its action on the protein-polysaccharide complex of cartilage. J Biol Chem. 1973 Mar 10;248(5):1634–1642. [PubMed] [Google Scholar]


