Abstract
Binge drinking is rising among aged adults (>65 years of age), however the contribution of alcohol misuse to neurodegenerative disease development is not well understood. Both advanced age and repeated binge ethanol exposure increase neuroinflammation, which is an important component of neurodegeneration and cognitive dysfunction. Surprisingly, the distinct effects of binge ethanol exposure on neuroinflammation and associated degeneration in the aged brain have not been well characterized. Here, we establish a model of intermittent binge ethanol exposure in young and aged female mice to investigate the effects of advanced age and binge ethanol on these outcomes. Following intermittent binge ethanol exposure, expression of pro-inflammatory mediators (tnf-α, il-1β, ccl2) was distinctly increased in isolated hippocampal tissue by the combination of advanced age and ethanol. Binge ethanol exposure also increased measures of senescence, the nod like receptor pyrin domain containing 3 (NLRP3) inflammasome, and microglia reactivity in the brains of aged mice compared to young. Binge ethanol exposure also promoted neuropathology in the hippocampus of aged mice, including tau hyperphosphorylation and neuronal death. We further identified advanced age-related deficits in contextual memory that were further negatively impacted by ethanol exposure. These data suggest binge drinking superimposed with advanced age promotes early markers of neurodegenerative disease development and cognitive decline, which may be driven by heightened neuroinflammatory responses to ethanol. Taken together, we propose this novel exposure model of intermittent binge ethanol can be used to identify therapeutic targets to prevent advanced age- and ethanol-related neurodegeneration.
Keywords: Alcohol, Dementia, Inflamm-aging, Microglia activation, Neurodegenerative disease
1. Introduction
Alcohol misuse is becoming increasingly common among aged (>65 years) adults (Breslow et al., 2017). Recent surveys suggest 10 % of aged adults participate in binge drinking (Han et al., 2019), a drinking pattern defined as reaching a blood alcohol content of 0.08 g/dl or higher (Patrick and Azar, 2018). Although the impairments in ethanol metabolism and clearance that occur with advanced age cause heightened and prolonged effects of ethanol (Hahn and Burch, 1983), aged adult drinkers still report participating in classically defined binge drinking behaviors (i.e., consuming 4–5 drinks on one occasion) (Malani et al., 2021). Advanced age alone is one of the biggest risk factors for chronic diseases, such as neurodegenerative disease (Hou et al., 2019). Binge drinking during adolescence (Barnett et al., 2022) and middle age (Järvenpää et al., 2005) increases the risk for cognitive impairment and neurodegeneration in advanced age. However, the effects of binge alcohol use on the brain of aged subjects and resulting development of neurodegeneration is not well defined. By the year 2050, 90 million people in the United States will be 65 years of age or older and the number of people living with neurodegenerative diseases is projected to triple during that time (Hebert et al., 2013). Therefore, it is imperative that we elucidate how lifestyle factors, including binge alcohol use, contribute to age-related neurodegeneration and cognitive decline. Importantly, over the past two decades binge drinking has been particularly rising among aged adult women (Breslow et al., 1997). Because women already have an increased risk for dementia development compared to men (Beam et al., 2018), investigating the impact of binge alcohol use on the aged brain merits further investigation.
Neuroimmune dysfunction is a key component of both advanced age (MacPherson et al., 2018) and binge alcohol related- neurodegeneration (Crews et al., 2015). The aged brain undergoes chronic, basal, low-grade inflammation in a phenomenon known as “inflamm-aging (Franceschi et al., 2007).” Inflamm-aging of the central nervous system (CNS) is specifically characterized by activation of microglia, resident CNS macrophages, and resulting elevations in pro-inflammatory mediators. Inflamm-aging is a complex phenotype, and the triggering events remain unclear (Salvioli et al., 2013). Many hypothesize age-related elevations in inflammation are the result, alone or in combination, of accumulated danger associated molecular patterns (DAMPs) (Fonken et al., 2016; Feldman et al., 2015), aberrant activation of the nod-like receptor pyrin domain containing 3 (NLRP3) inflammasome (Sebastian-Valverde and Pasinetti, 2020; Youm et al., 2013), and/or cellular senescence (Sikora et al., 2021). Regardless of the precipitating event, clinical data suggest chronic inflammation increases susceptibility to dementia and neurodegeneration. For example, elevated levels of pro-inflammatory cytokines, tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β) and interleukin 6 (IL-6) in the blood (Lin et al., 2018; Feng et al., 2023) or cerebrospinal fluid (Hu et al., 2019; Taipa et al., 2019) in aged adults are positively correlated with cognitive impairment. The brains of healthy aged subjects demonstrates a robust upregulation of genes involved in innate immunity and microglia activation that are heightened in disease (Cribbs et al., 2012), indicating innate immune dysfunction is a critical factor that precedes the development of severe neurodegeneration. Aged male and female mice also present with heightened neuroinflammation at baseline compared to young (Youm et al., 2013). Aggravating neuroinflammation in aged rodents through external factors, like traumatic brain injury (Barker et al., 2023), anesthesia (Liu et al., 2021), and lipopolysaccharide (Liu et al., 2021; Huang et al., 2008), leads to more severe immune mediated neuronal damage and associated cognitive dysfunction. These studies further underscore the detrimental impact of unresolved and persistent immune activation to neurodegeneration.
A multitude of studies have implicated neuroinflammation as a driver of ethanol-induced neurodegeneration in young male rodent models (Zou and Crews, 2014; Zou and Crews, 2010; Zhao et al., 2013; Yang et al., 2014; Qin et al., 2008; Guerri and Pascual, 2019), especially within the hippocampus, a vital brain region for learning and memory (Gilbert and Brushfield, 2009). Ethanol crosses the blood brain barrier and induces direct neurotoxicity via the release of DAMPs (Zou and Crews, 2014), activation of pro-inflammatory signaling pathways, such as NLRP3 inflammasome, and generation of cytotoxic factors, like TNF-α (Zou and Crews, 2010). It is hypothesized that presence of DAMPs and cytokines perpetuate ethanol-related neuroinflammation after ethanol is cleared from the brain, and leading to secondary, inflammation mediated neuronal injury (Vallés et al., 2004; Tajuddin et al., 2014). Although all CNS cell types can produce pro-inflammatory cytokines after ethanol exposure (Crews et al., 2015), microglia are thought to be essential perpetuators of neuroinflammation (Chastain and Sarkar, 2014). For example, depletion of microglia in brain slice cultures harvested from young rats ex vivo attenuates ethanol-induced production of pro-inflammatory cytokine production and associated neuronal death (Coleman et al., 2020). Despite the similar neuroimmune mechanisms involved in binge ethanol and advanced age-related neurodegeneration, few studies address the influence of binge ethanol exposure on the aged brain.
Our limited understanding about the consequences of ethanol exposure on neurodegeneration is further complicated by the gap in knowledge on both the sex-specific influences of ethanol, and differences between mice and rat models. The majority of mouse studies that evaluate ethanol-related neuroinflammation have been performed in male mice (Pascual et al., 2015; Qin and Crews, 2012; Crews, 2012). Findings from Lowe et al. corroborate the neuroinflammatory effect of ethanol in young adult C57BL/6J female mice and show an increase in cytokines, chemokines and chemokine receptors (tnfα, il1β, ccl2, ccr2, ccr5) in the cerebellum and hippocampus after chronic ethanol feeding (Lowe et al., 2020). Contradictory findings supporting an anti-inflammatory effect of binge ethanol in young adult female C7BL/6J mice have also been reported (Niedzwiedz-Massey et al., 2023). Additional discrepancies arise from rat models; several studies using adolescent rats suggest both chronic and binge ethanol exposure causes cytokine production and neuronal injury (Li et al., 2019; Schneider et al., 2017; Obernier et al., 2002), whereas others suggest acute ethanol exposure blunts cytokine responses in rats (Doremus-Fitzwater et al., 2015). It is currently unclear if varying results between studies arise from differences of age, species, sex, or ethanol exposure method. In addition, few studies comprehensively explore neuroinflammation, tissue damage, and associated cognitive impairment. Given these discrepancies, the goal of the present study is to develop a model of binge ethanol exposure using young and aged female mice that will allow us to holistically investigate outcomes associated with neurodegeneration.
Binge ethanol-induced neuroinflammation and degeneration in young male mice and rats is typically elicited through daily gavages with ethanol doses between 5 and 15 g/kg/day (Qin and Crews, 2012; Majchrowicz, 1975; Qin et al., 2021); exposures that may not be tolerated by aged animals. To begin to elucidate how the brains of aged female mice may respond to binge ethanol, we have established a novel model of intermittent binge ethanol exposure superimposed with advanced age. Our model uses more moderate levels of ethanol that are well tolerated by aged mice. Using our paradigm, we show that advanced age amplifies neuroinflammatory response following multiple days of binge ethanol in female mice. We then show that advanced age and ethanol-related neuroinflammation are associated with signs of neurodegenerative disease and cognitive dysfunction. These findings have critical implications for the current female aging population that binge drink and can be used for future studies to elucidate the specific neuroinflammatory factors that may be responsible for the damaging effects of binge ethanol on the aged brain.
2. Materials & methods
2.1. Animals and intermittent binge ethanol protocol
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Colorado Anschutz Medical Campus. Female C57BL/6N mice were obtained from the National Institutes of Aging (NIA) aged rodent colony and maintained for at least two weeks in an in-house animal facility prior to use. Young (3–4-month-old) and aged (18–20-month-old) mice were randomized (2–3 mice/cage) into vehicle and ethanol treatment groups. Experimental cohorts were weight-matched within age groups. Mice received intragastric gavages of vehicle (water) or ethanol (3 g/kg in young and aged mice or 5 g/kg in young mice) at 2p.m. every-other-day over the course of 18 days, totaling 10 exposures. All mice were allowed ad libitum access to chow and water during the treatment period and housed on a 12- hour/12-hour light/dark cycle. Eighteen hours after the final gavage, mice were anesthetized and sacrificed. Brain tissue was extracted, and meninges were removed. Left hemispheres were fixed in formalin or frozen in optimal cutting temperature (OCT) compound and sectioned for histology or spatial transcriptomics, respectively. The hippocampus was excised from right hemispheres and placed in RNAlater (Ambion, Austin, TX) for RNA isolation.
2.2. Plasma ethanol measurements
Blood samples were collected at 30, 60, 120, 240 minutes after the final gavage to isolate plasma and determine blood ethanol levels as previously described (Anton et al., 2023). Fresh plasma samples were assayed for ethanol using a commercially available enzymatic assay kit (Ethanol L3K kit, Sekisui Diagnostics, Burlington, MA).
2.3. RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
RNA was isolated from hippocampal tissue stored in RNA later using RNeasy Mini Kit (Qiagen) per the manufacturer’s instructions. 1 μg of hippocampal RNA was reversed transcribed using SuperScript IV VILO master mix and analyzed with PowerSYBR qRT-PCR kits (ThermoFisher, Grand Island, NY) on a QuantStudio Real-Time PCR analyzer (Applied Biosystems, La Jolla, CA). Relative messenger RNA (mRNA) expression was determined using gene-specific primers (Supplemental Table 1). Statistical analyses were performed on the ΔCt values (average Ct of gene of interest – average Ct of housekeeping gene) (Anton et al., 2023). All qRT-PCR was performed using two housekeeping genes (18S, β-actin); trends were similar when data were normalized to both 18S and β-actin. mRNA expression values are represented as fold-change over young control groups using 18S as the housekeeping gene.
2.4. Multi-spectral imaging
Left hemispheres from each mouse were explanted, fixed in 10 % formalin and embedded with paraffin. Serial sections were cut using a microtome in 10-μm-thick sections between bregma −2.0 to −2.2 to capture the dorsal hippocampus and adhered to a glass slide. Slides were dewaxed with xylene, heat-treated in either pH 6 or pH 9 antigen retrieval buffer for 15 minutes in a pressure cooker, and blocked in antibody diluent (PerkinElmer, Waltham, MA). Sections were then sequentially stained for GFAP (1:500, 556,330 BD Pharmigen), NeuN (1:1000 ABN78 Millipore), Iba-1 (1:500, 19,741 Wako), NLRP3 (1:50, D4D8T Cell Signaling), IL-1β (1:200 ab9722 abcam), and HMGB1 (1:1000 D3E5 Cell Signaling) primary antibodies followed by HRP- conjugated secondary polymer (anti-rabbit, anti-mouse [PerkinElmer]) and HRP-reactive OPAL fluorescent reagents (PerkinElmer). To image nuclei, slides were stained with spectral DAPI and coverslips were applied with Prolong Diamond mounting media (Thermo Fisher Scientific). Whole slides were scanned using a Vectra Polaris System (Akoya Bio). After spectral unmixing, the CA3 region from each scan was manually annotated on QuPath (version 4.0) according to the Allen Mouse Brain Atlas. Qupath was used to measure percent positive area of NLRP3, microglia soma area. Cell segmenting was performed to identify neuronal nuclei and quantify nuclear HMGB1 H-score. CA3 regions were exported to ImageJ to measure staining intensity of all other markers. Two CA3 sections per mouse brain were utilized for each multi-spectral imaging panel; representative values were obtained after averaging the two sections from each mouse.
2.5. Immunohistochemistry
Brains were sectioned (10 μM) as described above. Three paraffin embedded brains sections per mouse were deparaffinized and stained with an antibody against PHF-1 (EPR14222, Abcam); slides were then counterstained with hematoxylin. Images were acquired using an upright Olympus BX43 Microscope (Waltham, Massachusetts). No specific immunostaining was seen in sections incubated with PBS/blocking buffer rather than the primary antibody (data not shown). Data are represented as the average intensity of PHF-1 staining in CA3 from all three sections/mouse. Terminal deoxynucleotidyl transferase-mediated deoxyuridine nick end labeling (TUNEL) staining was performed using ApopTag Red In Situ Apoptosis Detection Kit (S7165, Millipore Sigma) according to the manufacturer’s instructions. Slides were mounted using Prolong Diamond Antifade with DAPI (P36971, Invitrogen) and images were acquired using a confocal microscope (Nikon). For all staining panels, images were coded at the time of collection for a blinded analysis, and positive staining was quantified using Image J software. Number of TUNEL-positive cells per 4x field were enumerated using ImageJ.
2.6. Radial arm water maze
Three independent experiments were used for behavioral experiments. The Radial Arm Water Maze (RAWM) was performed as previously described (Joksimovic et al., 2023). The RAWM apparatus consists of a circular pool (100 cm in diameter and 35 cm in height) with inserts forming six arms 60 degrees apart. One of these arms features a platform submerged 1 cm below the water surface. The room-temperature water was made opaque by adding a nontoxic water-soluble white dye, which also enhances the contrast between the mouse and the background for optimal tracking. Before ethanol or vehicle exposure, the animals were trained for 2 consecutive days (10 swim trials per day in 5 sessions of 2 trials each). Each swim trial lasted a maximum of 60 s, and unsuccessful mice were guided to the platform to facilitate learning about the existence of the escape platform. After each trial, mice were gently dried with towels, put to a warm enclosure for 5–10 min, and then returned to their home cages at the end of each session. To assess learning of the platform location before ethanol or vehicle exposure, we quantified the latency to reach the platform and the number of incorrect arm entries. Young and aged animals were stratified based on pre-treatment performance and then assigned to ethanol or vehicle groups. Treatment groups were matched based on initial RAWM performance for both young and aged mice.
Twenty-four hours after final ethanol or vehicle gavage (20 days after training) all mice were re-introduced to the RAWM, but the location of the platform was switched to a different arm. All animals were tested on the RAWM for 1 day (10 swim trials per day in 5 sessions of 2 trials each). To assess memory, number of visits into the pre-treatment platform arm were counted and normalized to the total number of incorrect arm entries per trial. To evaluate acquisition of the new platform location, latency to reach the platform in the new location, and number of visits into the incorrect arms were measured.
2.7. Contextual fear conditioning
Two days after the final gavage (Day 21) mice were subjected to the contextual fear conditioning assay (Phillips and LeDoux, 1992). All animals were placed in a novel chamber (Med Associates, St. Albans, VT, USA, Modular Mouse Test Chamber) and allowed to freely explore for 5 min. Mice received an electric footshock at 3 minute and 4 minute (2 s each, 0.7 mA, constant electric current). All mice were then returned to their home cages. Twenty-four hours later, mice were reintroduced to the same chamber and allowed to freely explore for 5 minutes without any electric shock. Time spent “freezing” (the absence of movement except for respiration) was recorded using FreezeScan (Cleversys). Greater freezing time on the second day of contextual fear conditioning indicates memory of the adverse experience within the context of the chamber.
2.8. Statistical analysis
Values reported are means ± SEM. A total of seven cohorts of mice were used: 4 cohorts to generate biochemical data and 3 cohorts to gather behavioral data. The data were analyzed with RStudio (4.2) using repeated measure analysis of variance (ANOVA) or 2-way ANOVA to compare differences between groups and Tukey’s post-hoc analysis was used to adjust for multiple comparisons. General linearized models were utilized to control for cohort effects when more than two cohorts were used for each outcome. Grubb’s tests were performed on all data sets to identify statistical outliers. Statistical significance was determined by a p-value of 0.05 or lower. Individual p-values are reported in the figure legends.
3. Results
3.1. Intermittent binge ethanol exposure amplifies neuroinflammation in the aged brain
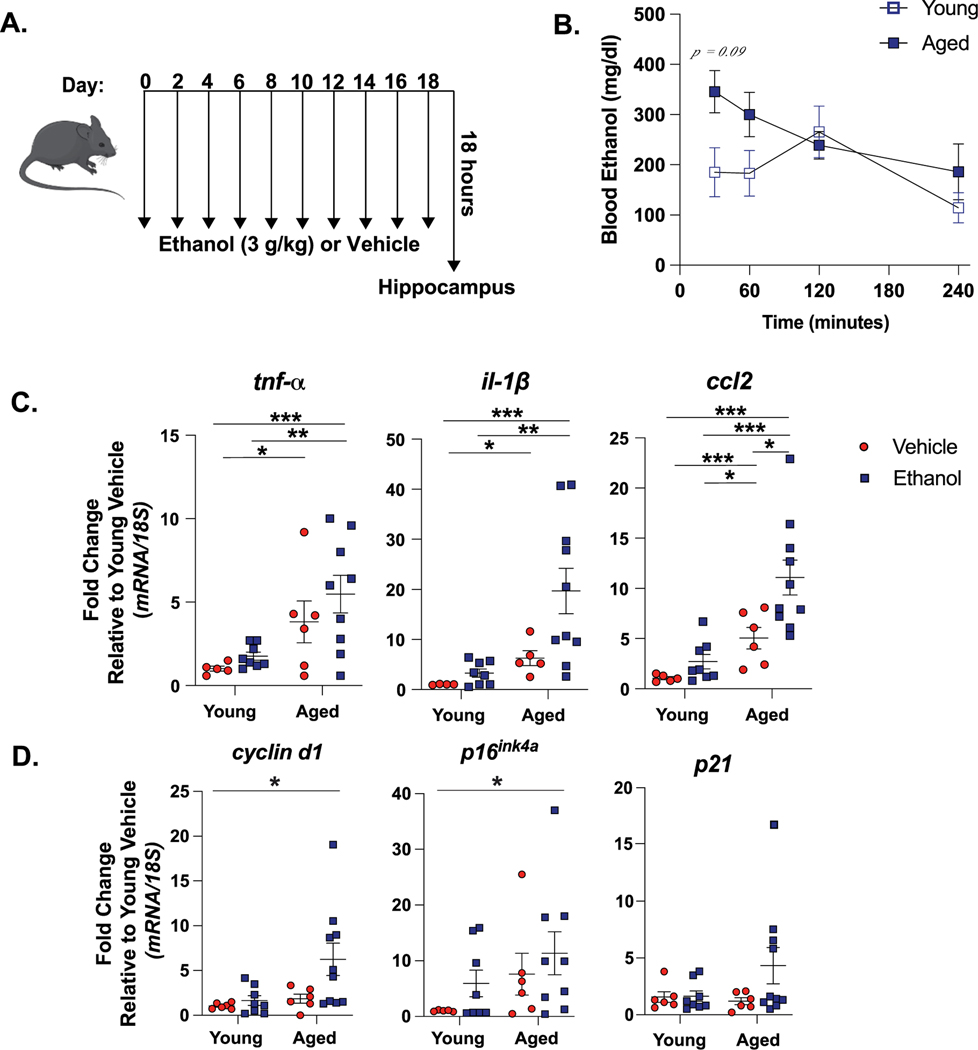
To define neuroinflammatory responses to binge ethanol, young and aged mice were exposed to our novel intermittent binge ethanol exposure paradigm (Fig. 1A). To determine if there were age-dependent differences in ethanol metabolism, we measured blood ethanol levels between 30 and 240 minutes after the final ethanol exposure. There was a trending increase in blood ethanol concentrations (BECs) in aged mice 30 minutes after the final gavage (387.1 mg/dl) compared to BECs of young mice (271.0 mg/dl) at the 30-minute timepoint (p = 0.09) (Fig. 1B). This trend toward age-related increase in BECs mimics the induction of blood alcohol levels observed in aged adults compared to young after consuming equal amounts of alcohol (Hahn and Burch, 1983).
Fig. 1. Hippocampal Measures of Inflammation and Senescence are Increased by Advanced Age in Binge Ethanol Exposed Mice.
(A) Young and aged mice were exposed to intragastric gavages of ethanol (3 g/kg) or vehicle every other day for 10 total exposures. (B) Blood ethanol levels were measured between 30 and 240 minutes after final ethanol gavage. Values represent the mean ± SEM, n = 6 per group, per time point. Age-related elevations in BECs trended toward significance 30 minutes after final exposure (p = 0.09), determine by a repeated measures ANOVA. Hippocampal RNA was isolated 18 hours after final exposure and expression of (C) pro-inflammatory cytokine (tnf-α, il-1β, ccl2) (D) senescence marker (cyclin d1, p16ink4a, p21) mRNA was detected using via qRT-PCR. All values are expressed as fold-change compared to young vehicle treated mice. Individual data points are represented, n = 6–10 per group. Mean and SEM are reported, values were significantly different from each other determined by 2-way ANOVA with Tukey’s post hoc test, * p < 0.05 ** p < 0.01, *** p < 0.001.
Production of pro-inflammatory cytokines, including TNF-α and IL-1β, promote neuronal injury independently in older individuals (Freeman and Ting, 2016) and following ethanol exposure (Zou and Crews, 2010; Lippai et al., 2013), while chemokines, like C-C motif chemokine ligand 2 (CCL2), facilitate macrophage activation and recruitment and promote neuroinflammation (Lucin and Wyss-Coray, 2009). To understand how advanced age may aggravate ethanol-induced neuroinflammation, we measured hippocampal pro-inflammatory cytokine production 18 hours after the final ethanol or vehicle exposure. Advanced age increased the mRNA expression of hippocampal cytokines and chemokines over young controls, supporting an “inflamm-aging” phenotype that has been observed in other mouse models of advanced age (Youm et al., 2013; Labrousse et al., 2012) (main effect of age for TNF-α F(1,29) = 20.18; IL-1β F(1,29) = 33.09; CCL-2F(1,29) = 78.53, all p < 0.001). Cytokine expression was further heightened in ethanol exposed aged mice compared to young (main effect of ethanol for TNF-α F(1,29) = 6.3; IL-1β F(1,29) = 5.5; CCL-2F(1,29) = 18.12, all p < 0.05. No age x ethanol interaction for all) (Fig. 1C). We next determined if elevations in pro-inflammatory cytokine mRNA in the hippocampi of aged ethanol exposed mice compared to young ethanol exposed mice were a result of heightened BECs. We repeated our intermittent binge ethanol paradigm with the addition of a young mouse group receiving 5 g/kg ethanol gavages (Supplemental Figure 1A). This dose of ethanol increased peak BECs in young mice similar to aged mice receiving 3 g/kg ethanol gavages (Supplemental Figure 1B). Despite similar BECs, the expression of hippocampal pro-inflammatory cytokine mRNA was increased in ethanol exposed aged mice compared to young mice following higher (5 g/kg) concentrations of ethanol (Supplemental Figure 1C). Peak BEC concentrations did not correlate with hippocampal cytokine expression (data not shown), suggesting these measures are not interdependent. We then measured withdrawal and intoxication in these animals, as the severity of both of these outcomes are associated with heightened neuroinflammation in young mice (Freeman et al., 2012; Walter and Crews, 2017). Both ethanol-related loss of righting reflex and ethanol withdrawal-induced convulsions were observed in 5 g/kg treated young mice, but not in young or aged mice treated with 3 g/kg ethanol (Supplemental Figure 1D), suggesting ethanol-related sedation or convulsions do not account of differences in neuroinflammation among young and aged mice given 3 g/kg ethanol. Together these data indicate heightened neuroinflammation from ethanol exposure in aged mice is not dependent on differences in ethanol metabolism.
We next evaluated markers of senescence in the hippocampus of our animals, because senescence is an important factor of advanced age-related neuroinflammation (Sikora et al., 2021), and occurs in the peripheral organs of young animals and aged animals after ad libitum ethanol consumption (Anton et al., 2023; Wan et al., 2017). Cellular senescence is characterized by the halted turnover of aging cells, associated production of pro-inflammatory cytokines (i.e., senescence associated secretory phenotype [SASP]), greater expression of growth arrest proteins (p16, p21), and cell cycle regulators (cyclin D1) (Sikora et al., 2021). Advanced age increased cyclind1 and p16ink4a mRNA expression in the hippocampus (main effect of age for CyclinD1: F(1,29) = 8.91, P16ink4a: F(1,29) = 7.75, all p < 0.01, no main effect of ethanol or age x ethanol interaction for either). Tukey’s pot-hoc test revealed the combination of advanced age and ethanol exposure increased the expression of cyclin d1 and p16ink4a levels compared to young controls (p < 0.05). However, we did not observe differences in p21 mRNA expression in the hippocampus due to age or ethanol. The increase in senescence markers in aged mice, but not young, may begin to suggest that binge ethanol exacerbates advance age-related senescence in the hippocampus (Fig. 1D).
3.2. Binge ethanol during advanced age potentiates markers of NLRP3 inflammasome activation and microglia reactivity
The NLRP3 inflammasome is an immunological sensor that can be activated by a variety of advanced age-related factors, including increased accumulation of DAMPs and pro-inflammatory cytokines (Latz and Duewell, 2018). These factors promote oligomerization of NLRP3 proteins which form a complex with ASC and caspase-1, ultimately leading to generation of IL-1β (Jin and Flavell, 2010). The NLRP3 inflammasome implicated in the neurodegeneration and cognitive decline that occurs with advanced age alone (Youm et al., 2013; De Biase et al., 2020), and enhanced activation of NLRP3 in the brains of aged mice from peripheral factors (eg., lipopolysaccharide) can accelerate neurodegeneration and impair cognition (Beyer et al., 2020). Although NLRP3 inflammasome activation occurs in the brains of young mice following chronic ethanol consumption (Alfonso-Loeches et al., 2016), the effect binge ethanol exposure has on NLRP3 activation in the brains of older subjects has not been described.
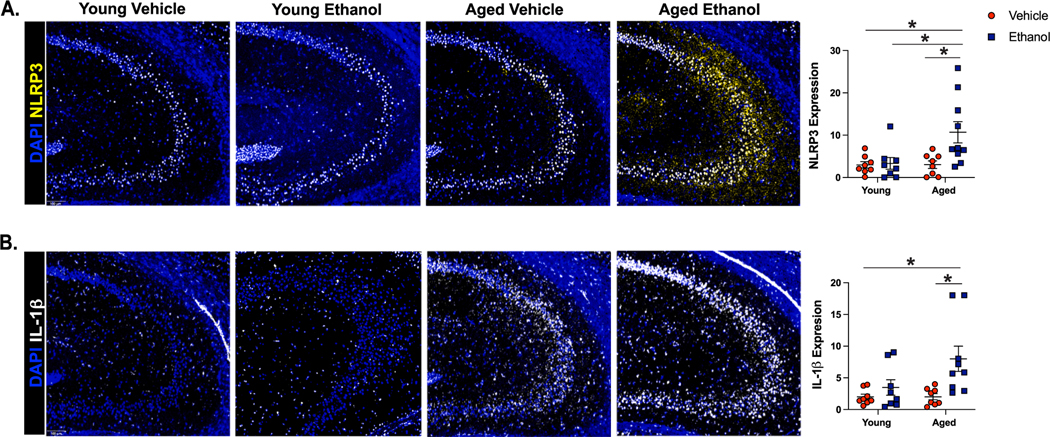
We utilized multi-spectral imaging to quantify markers of NLRP3 activation in cornu ammoni 3 (CA3) of the hippocampus in our murine model (Fig. 2). The CA3 region was chosen because of its function in memory formation (Gilbert and Brushfield, 2009), and because this region was impacted by advanced age and ethanol exposure in our panel. Young and aged mice had differential NLRP3 and IL-1β responses to ethanol (NLRP3: main effect of age F(1,29) = 4.65 and ethanol F(1,29) = 5.39, interaction of age x ethanol F(1,29) = 4.49, p < 0.05 for all. IL1β: main effect of ethanol F(1,29) = 8.45, p < 0.05. Trending main effect of age F(1,29) = 3.14, p = 0.08 and trending interaction of age x ethanol F (1,29) = 3.05, p = 0.09). Binge ethanol exposure increased the percent area stained for NLRP3 in aged mice compared to all other treatment groups, and increased the expression of IL-1β compared to young and aged controls (Fig. 2A,B). However, ethanol exposure did not change NLRP3 or IL-1β expression in CA3 of young mice. High mobility group box 1 (HMGB1) is a critical DAMP to activate the NLRP3 inflammasome upon ethanol exposure in the brains of young mice (Zou and Crews, 2014). No observable differences in HMGB1 nuclear localization were found in CA3 between experimental groups (Supplemental Figure 2A). Together, these results demonstrate that advanced age promotes binge ethanol-induced NLRP3 inflammasome activation and IL-1β production in the hippocampus.
Fig. 2. Aged Mice Express Amplified Markers of NLRP3 Inflammasome Activation in the Hippocampus After Binge Ethanol Exposure Compared to Young.
Young (3–4 months old) and aged (18–20 months old) female C57BL/6N mice were exposed to intragastric gavages of ethanol (3 g/kg) or vehicle every other day for 10 total exposures. Multispectral imaging was performed on left hemispheres of young and aged ethanol and vehicle treated mice. Two sections from each brain hemisphere was imaged using a whole slice scanning system and representative images at 10x magnification are shown. Expression of percent positive area for (A) NLRP3 and (B) IL-1β in CA3 of the hippocampus were quantified using QuPath software; data are represented as the average of two sections/brain. N = 8–10 per group, means and SEM are reported. Values were significantly different from each other determined by 2-way ANOVA with Tukey’s post hoc test, ** p < 0.01, *** p < 0.001.
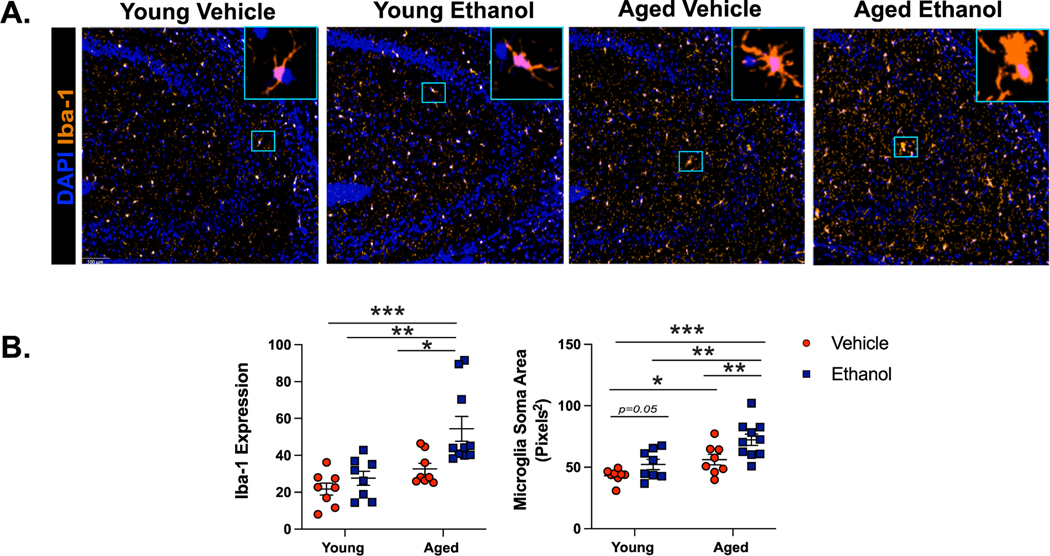
Along with NLRP3 inflammasome activation, dysregulated microglia polarization to a pro-inflammatory state precedes neurodegeneration during advanced age (Wendimu et al., 2022). In the brains of young mice, microglia become reactive from binge ethanol exposure (Fernandez-Lizarbe et al., 2009) and are the primary source of pro-inflammatory cytokines in brain slice cultures that are exposed to ethanol (Coleman et al., 2020). In response to ethanol, pro-inflammatory microglia are characterized by an increase in ionized calcium binding adaptor 1 (Iba-1) staining and enlarged cell body size (Crews, 2008). In our mouse model, ethanol exposure and advanced age promoted these activation markers independently (Iba-1: main effect of age F(1,29) = 6.22 and main effect of ethanol F(1,29) = 7.5, p < 0.05. No age x ethanol interaction). The combination of advanced age and ethanol exposure led to the greatest increase in the expression of Iba-1 compared to young controls, as demonstrated by Tukey’s pot-hoc testing (p < 0.05) (Fig. 3A,B). Microglia in CA3 of young ethanol exposed mice exhibited increased soma size compared to young controls, which was further increased due to advanced age and ethanol exposure (main effect of age F(1,29) = 30.6 and main effect of ethanol F(1,29) = 23.6, all p < 0.001. No age x ethanol interaction) (Fig. 3A,B). There were no differences in microglia number in the CA3 region regardless of advanced age or ethanol exposure (data not shown). Because astrocytes are another subset of glial cells that can contribute to the production of pro-inflammatory mediators in the brains of young ethanol exposed mice (Vallés et al., 2004), we also characterized changes in astrocyte reactivity via glial fibrillary acidic protein (GFAP) staining. We did not observe significant changes due to advanced age and/or ethanol exposure (Supplemental Figure 2B). These results indicate microglia from the aged brain are particularly primed to become reactive 18 hours after intermittent binge ethanol exposure.
Fig. 3. Binge Ethanol Increases Markers of Microglia Reactivity in the Brains of Aged Mice.
Young (3–4 months old) and aged (18–20 months old) female C57BL/ 6N mice were exposed to intragastric gavages of ethanol (3 g/kg) or vehicle every other day for 10 total exposures. Multispectral imaging was performed on left hemispheres of young and aged ethanol and vehicle treated mice. Sections from each brain hemisphere were imaged using a whole slice scanning system and representative images at 10x magnification are shown. (A) Expression of Iba-1 staining in CA3 of the hippocampus from all treatment groups with representative images of microglia morphology shown to the right of each image at 20x magnification. (B) Quantification of Iba-1 staining intensity and average microglia soma area, n = 8–10 per group. Means and SEM are reported, values were significantly different from each other determined by 2-way ANOVA with Tukey’s post hoc test, * p < 0.05 ** p < 0.01, *** p < 0.001.
3.3. Intermittent binge ethanol exposure promotes neurodegenerative disease-like pathology in the aged brain
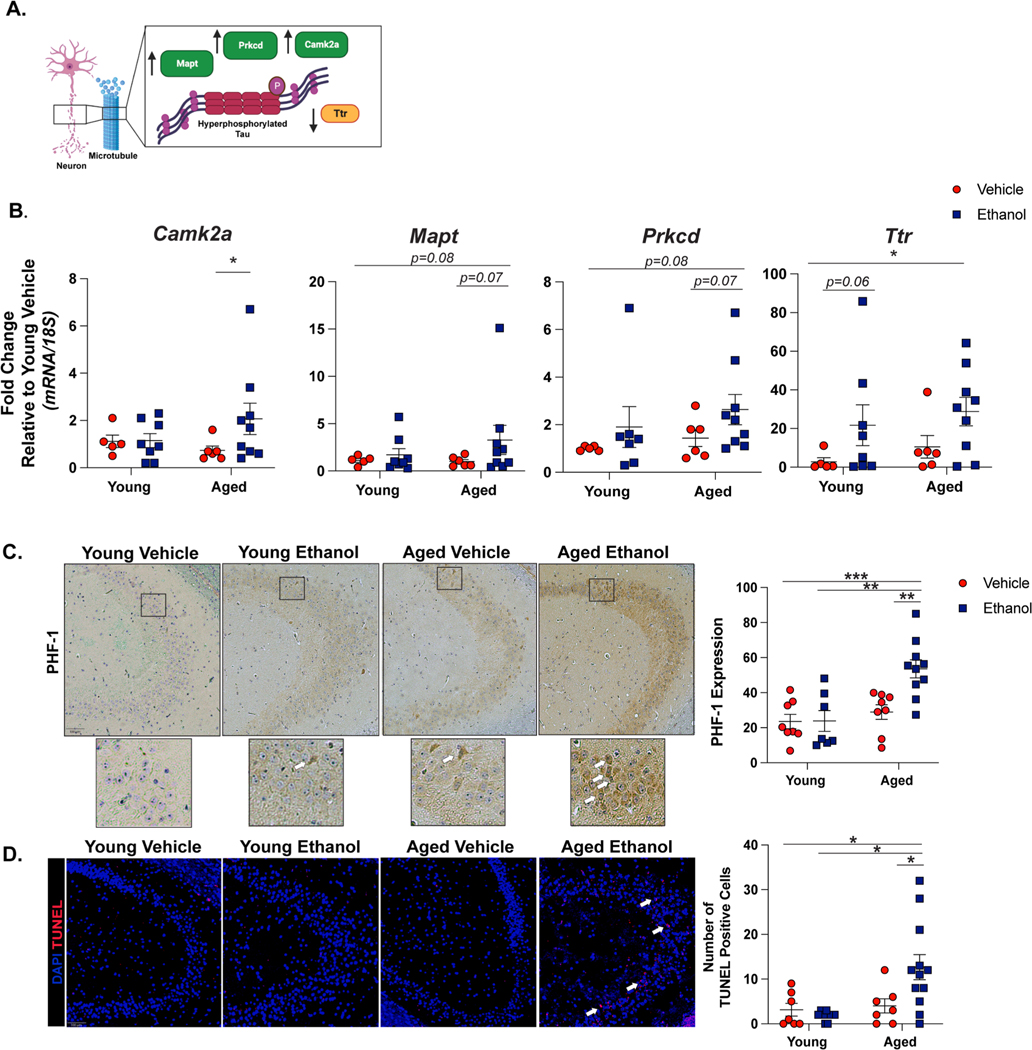
To understand if binge ethanol influences neuropathology in the brains of aged mice, we performed a pilot study utilizing spatial transcriptomics with brain tissue from our model (n = 1 per group). In CA3 of the aged ethanol sample, we observed increased expression of mapt, prkcd, camk2a but decreased ttr compared to all other treatment groups (data not shown). This gene expression pattern is consistent with tau hyperphosphorylation (Yoshimura et al., 2003; Correas et al., 1992; Saponaro et al., 2020) (Fig. 4A). To further investigate if there were advanced age and ethanol-related changes in neuropathological markers, we performed qRT-PCR on these target genes in the hippocampus of three experimental cohorts of mice. With the exception of ttr, these markers followed similar expression patterns as indicated by tauopathy models (Yoshimura et al., 2003; Correas et al., 1992). Ethanol increased the expression of camk2a (main effect of ethanol F(1,29) = 4.75, p < 0.05, no main effect of age or interaction effect) and post-hoc testing revealed increased camk2a expression in the hippocampus of aged ethanol exposed mice compared to aged controls (p < 0.05). We also observed trending increases in mapt and prkcd due to ethanol exposure (mapt: main effect of ethanol F(1,29) = 3.18, p = 0.08. prkcd: main effect of ethanol F(1,29) = 2.7, p = 0.1, no main effect of age or interaction effect for either). Although ttr was previously described to be downregulated with tauopathy, we observed that ethanol exposure increased ttr expression (main effect ethanol F(1,29) = 4.9, p < 0.05, no main effect of age or interaction effect). Post-hoc testing showed increased ttr in aged mice compared to young controls, and a trending increase in young ethanol exposed mice over control (Fig. 4B). Immunohistochemistry was performed to further characterize the neuropathological effect of ethanol and to validate the trends in tau hyperphosphorylation suggested by our qRT-PCR data. Phosphorylation of tau at the PHF-1 epitope is associated with neurodegeneration and cognitive impairment in post-mortem brain tissue of Alzheimer’s disease patients (Santa-Maria et al., 2012) and in mouse models of advanced aging (Torres et al., 2021). Here, we show that binge ethanol amplifies PHF-1 staining in the CA3 region of aged, but not young, mice (main effect of age F(1,29) = 13.53, main effect of ethanol F(1,29) = 5.4, interaction of age x ethanol: F(1,29) = 4.38, p > 0.05 for all). Additionally, the majority of positive PHF-1 staining was found the neuronal ribbon of CA3 (Fig. 4C). Because tau hyperphosphorylation and the aforementioned markers of neuroinflammation are implicated in neuronal death we next measured the number of apoptotic cells via Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling (TUNEL). Similar to PHF-1 staining, an increased number of TUNEL positive cells were found in CA3 region of brains from aged, ethanol exposed mice compared to all other treatment groups (interaction effect of age x ethanol: F(1,29) = 7.06, main effect of age: F(1,29) = 5.15, p < 0.05 for both. No main effect of ethanol). Positive cell staining occurred within the neuronal cell layer of CA3, suggesting neuronal apoptosis (Fig. 4D).
Fig. 4. Binge Ethanol Promotes Tau Hyperphosphorylation and Neuronal Death in the Brains of Aged Mice.
Young and aged mice were subjected to intermittent gavages of ethanol (3 g/kg) or vehicle every other day, totaling 10 exposures. 18 hours after final gavage, hippocampal tissue was isolated for qRT-PCR or immunohistochemistry. (A) Schematic of the role of target genes involved in tau hyperphosphorylation. Made with Biorender.com. (B) Hippocampal RNA was isolated 18 hours after final exposure and expression of tau hyperphosphorylation (camk2a, mapt, prkcd, ttr mRNA was detected using via qRT-PCR. Individual data points are represented, n = 6–10 per group. Values were significantly different from each other determined by 2-way ANOVA with Tukey’s post hoc test, * p < 0.05. (C) Paraffin-embedded were deparaffinized followed by immunodetection of the tau phosphorylation site PHF-1 and nuclei were counterstained with hematoxylin. Images were acquired using a 10x objective and positive staining was quantified using ImageJ. Representative images from CA3 of the hippocampus are shown with excerpts of staining shown at 20x magnification below. (D) Apoptotic cells were visualized in paraffin-embedded hippocampal sections. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)-positive cells were enumerated and are represented as total number of positive cells in a 10x field. N = 8–10 per group. Means and SEM are reported, values were significantly different from each other determined by 2-way ANOVA with Tukey’s post hoc test, * p < 0.05, **p < 0.01, ***p < 0.001.
3.4. Binge ethanol exposure exacerbates advanced age-related memory deficits
Given the sensitivity of aged mice to ethanol-induced neuroinflammation, damage, and neurodegenerative disease markers, we next evaluated hippocampal-dependent cognition in our mouse model. We tested young and aged mice in the radial arm water maze (RAWM), a validated measure of spatial memory and learning in rodents. All mice were introduced to the maze for 2 days prior to the initial vehicle or ethanol gavage (Fig. 5A) and given 20 trials over 2 days to learn the location of the escape platform (Fig. 5B). The number of incorrect arm entries per trial was measured to assess baseline differences in performance between young and aged mice. Although aged mice made more incorrect arm entries compared to young, these differences were not significant. Both young and aged mice made fewer incorrect arm entries over time, demonstrating that all animals were able to learn the platform location independent of age (Fig. 5C).
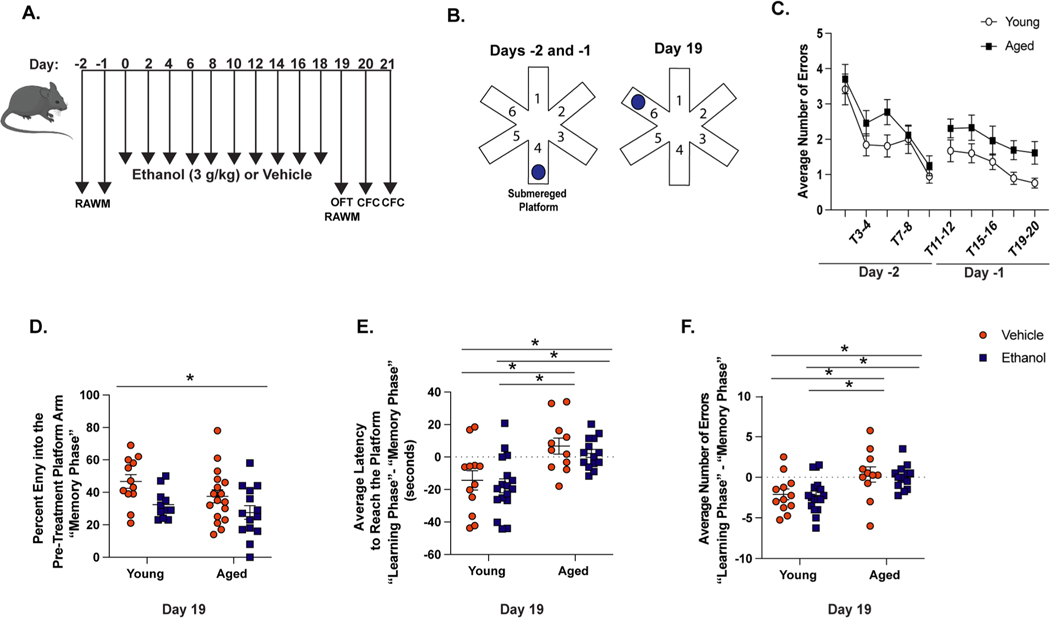
Fig. 5. Advanced Age-Related Deficits in Spatial Learning and Memory.
(A) Testing timeline of behavioral assays. Mice were trained to find the submerged platform in the radial arm water maze (RAWM) for 2 days leading up to our intermittent ethanol exposure paradigm. Twenty-four hours after the final exposure (Day 19), mice were re-introduced to the RAWM. All animals were also subjected to the open field task (OFT) 18 hours after the final ethanol exposure (Day 19) and contextual fear conditioning (CFC) on Days 20 and 21. (B) Mice were trained to find the submerged platform in arm 4 of the maze leading up to ethanol or vehicle exposure (Days −2 and −1). After the exposure paradigm, mice were re-introduced to the maze whereby the location of the platform was switched to arm 6 (Day 19). (C) Mice were trained on the RAWM prior to the exposure paradigm and the average number of incorrect arm entries made by young and aged mice on Days −2 and −1 were recorded. (D) The percent of arm entries into the pre-treatment platform arm during T1–4 (“memory phase”) compared to total incorrect arm entries. The difference in the latency to reach the new platform (E) and number incorrect arm entries (F) between T7–10 (“learning phase”) and T1–4 (“memory phase”) for each group. N = 11–17 per group, mean and SEM are reported. Values were significantly different from each other determined by 2-way ANOVA with Tukey’s post hoc test, * p < 0.05.
Young and aged mice were then subjected to our intermittent binge ethanol paradigm. Twenty-four hours after final exposure, mice were re-introduced to the RAWM (Fig. 5A), with a new platform location (Fig. 5B). A different platform location allowed us to measure both memory of the pre-treatment platform location during the first four trials of the maze (T1–4, i.e. “memory” phase), and learning of the new platform location during the last four trials of the maze (T7–10, i.e. “learning” phase). We grouped the trials this way because mice were placed into the pre-treatment platform arm during trial 5, ensuring mice explored the empty arm. During the “memory phase”, young vehicle animals had the highest percent of entry into the pre-treatment platform (46.7%), suggesting memory of the previous platform location. Aged ethanol exposed animals exhibited significantly reduced entries into the pre-treatment platform arm only after intermittent binge ethanol exposure compared to young controls (27.6%, p < 0.01) (main effect of ethanol F(1,50) = 0.8, p < 0.05, no main effect of age or age x ethanol interaction). Reduced exploration of the pre-treatment platform arm suggests memory impairment (Fig. 5D).
To understand if age or ethanol exposure influenced learning of the new platform location, we evaluated the animals’ ability to find the new platform over time. Reductions in the latency to find the new platform or number of incorrect arm entries during the “learning phase” compared to the “memory phase” of each group suggests learning of the new platform. Regardless of ethanol exposure, young mice displayed shorter latency to reach the new platform location (Fig. 5E) and fewer incorrect arm entries (Fig. 5F) during the “learning phase” of the RAWM compared to their performance during the “memory phase.” Aged mice did not improve their ability to find the new platform location. They displayed slightly longer latencies to reach the new platform location (Fig. 5E) and more incorrect arm entries over time (Fig. 5F). Importantly, the difference in incorrect arm entries and latency of aged mice between the “learning” and “memory” phases were significantly greater than that of young, independent of ethanol exposure (main of effect of age for incorrect arm entries: F(1,50) = 14.17 and latency: F(1,50) = 20.92, p < 0.001, no main effect of ethanol or age x ethanol interaction effect on either outcome). These data suggest an advanced age-related impairment in spatial learning during the RAWM. To address a potential limitation of the RAWM, we performed an Open Field task in all mice to account for differences in activity and associated anxiety prior to the RAWM (Fig. 5A). Mice were allowed to freely explore the open field apparatus for 10 minutes. We did not exhibit any differences in velocity or time spent in the center of the platform based on age or treatment status (Supplemental Figure 5). This result helps to inform the behaviors observed in the RAWM and indicate that the deficits seen due to advanced age may be result of hippocampal dysfunction.
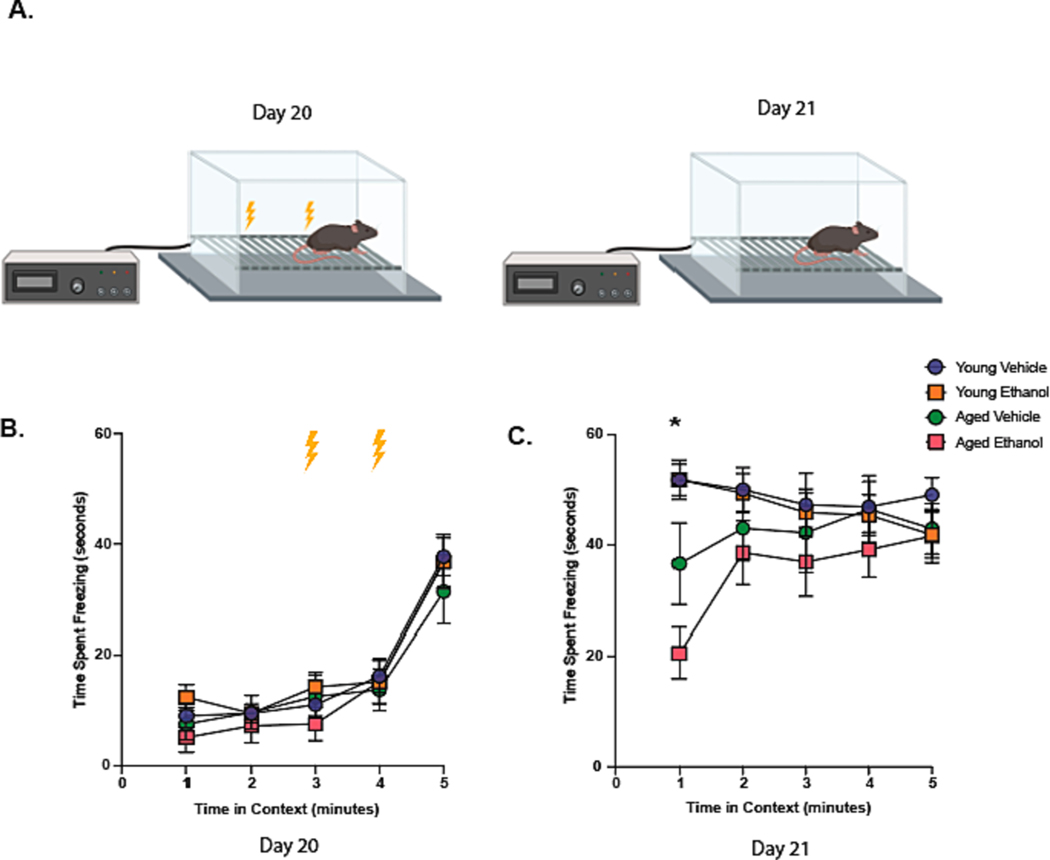
To further explore the cognitive deficits suggested by RAWM, we subjected animals to the contextual fear conditioning (CFC) paradigm two and three days after final ethanol exposure (Days 20 and 21) (Fig. 5A). On Day 20, all animals received a foot shock at the 3 and 4-minute mark (Fig. 6A). Freezing behavior in response to foot shocks did not differ between treatment groups on Day 20 (Fig. 6B). Animals were returned to the same testing chamber (same context) twenty-four hours later (Fig. 6A) and assessed for freezing behavior. Reduction in freezing behavior indicates reduced retrieval of the contextual fear memory formed on Day 20. Ethanol exposure significantly decreased freezing behavior in an advanced age-dependent manner during the first minute of exploration in the chamber (time x treatment group interaction F(12,132) = 3.25, p < 0.001, no main effect of treatment group or time) (Fig. 6C). These data suggest binge ethanol exposure impairs hippocampal-dependent cognition during advanced age.
Fig. 6. Binge Ethanol Exposure in Advanced Age Contributes to Impaired Contextual Memory.
(A) The contextual fear conditioning (CFC) paradigm used on Days 20 and 21. (B) The average time of freezing behavior (seconds) was measured over 5 minutes on Day 20 of the CFC. Lightning bolts indicate time of shock. (C) Average freezing behavior of all treatment groups over 5 minutes on Day 21. Animals were not subjected to foot shocks on Day 21. Mean and SEM are reported, values were significantly different from each other determined by repeated measures ANOVA with Geisser-Greenhouse correction and Tukey’s post hoc test * p < 0.001 of the aged ethanol group compared to all other treatment groups determined by repeated measures ANOVA with Geisser-Greenhouse correction and Tukey’s post hoc test.
4. Discussion
Binge alcohol consumption among aged adults is a growing public health concern and warrants investigation on the contribution of alcohol misuse to the development of age-related neurodegenerative disease (Breslow et al., 2017; Han et al., 2019). We previously lacked sufficient animal models to explore this topic. Although others have shown ethanol has modest influences on neuronal cytokine production (Kane et al., 2013) or microglia activation in aged mice compared to young (Grifasi et al., 2019), these studies did not holistically characterize neuroinflammation or explore the impact of ethanol exposure on neuronal death and cognitive impairment in the aged brain. Here, we find that the neuroinflammatory landscape of the hippocampus is heightened by ethanol in the aged female mouse brain. Particularly, we observed an increase of SASP factors, NLRP3 and associated cytokines, and microglia hyperreactivity. Binge ethanol exposure in the aged mice also promoted the expression of pathological hallmarks of neurodegeneration including tau hyperphosphorylation and neuronal death. Advanced age and ethanol related neuroinflammation and injury were associated with contextual memory impairments for up to 3 days after intermittent binge ethanol exposure.
We utilized 3 g/kg ethanol gavages in our model, a dose that is consistent with the amount of ethanol young and aged mice are reported to drink in ad libitum binge exposure models (Grifasi et al., 2019). The timing after ethanol exposure is critical to capture peak neuroinflammatory responses (Walter and Crews, 2017), and the use of gavage allowed us to control for time of ethanol consumption between young and aged mice in order to isolate hippocampal tissue at the 18 hour point. This bolus dose also induced BECs of mice that are consistent with other models of ethanol-related neuronal injury and pro-inflammatory cytokine production (Qin et al., 2008; Liu et al., 2019), whereas voluntary ethanol consumption models lead to lower BECs in young and aged male and female mice and are reported to elicit more moderate outcomes on neuroinflammation (Grifasi et al., 2019). Although the range of BECs we report in young and aged mice (280–380 mg/dl) are higher than the standard definition of binge-drinking in humans (reaching a blood alcohol level of 80 mg/dl or higher) (Patrick and Azar, 2018), mice metabolize ethanol approximately 5.5 fold faster than humans (Jeanblanc et al., 2019) and require up to 5 g/kg ethanol to mimic an average binge drinking episode in humans (Pruett et al., 2020).
In our model, aged mice had heightened mRNA expression of hippocampal pro-inflammatory cytokine mRNA (il-1β, tnf-α, and ccl2) after binge ethanol exposure compared to young. These data are consistent with other studies that suggest aged mice respond to systemic insults with greater CNS cytokine production (Godbout et al., 2005) and fit with our past reports that ethanol consumption during advanced age encourages inflammation in peripheral organs (Anton et al., 2023; McMahan et al., 2021). Prior to our study, it remained unclear if sensitivity to ethanol and associated neuroinflammation in aged mice was dependent on age-related alterations in blood ethanol levels and/or a result of more severe ethanol intoxication or withdrawal. We present data that demonstrate aged mice have persistent increases in hippocampal cytokine levels over young mice when blood ethanol levels are matched, suggesting differences in pharmacokinetics of ethanol does not solely drive toxicity in the aged brain. When given equal doses of ethanol, young and aged mice did not exhibit differences in withdrawal measurements or ethanol-related ataxia. These data suggest the underlying complexities of aging are likely a predominate prerequisite to potentiate neuroinflammatory responses to ethanol as opposed to heightened and sustained BECs.
Increased expression of pro-inflammatory modulators in older subjects is thought be a result of cellular senescence (Sikora et al., 2021), as senescent cells release more cytokines at baseline and under stress (i.e., SASP) (Davalli et al., 2016). Although the specific mechanism(s) that cause SASP are not well understood, evidence suggests an important axis involving the NLRP3 inflammasome (Gritsenko et al., 2020). Activation of the NLRP3 inflammasome and generation of IL-1β promotes secretion of other cytokines involved in SASP (i.e., IL-6, IL-8, TNF-α) and upregulates expression of p16 and p21 in peripheral organs and in CNS cells ex vivo (Romero et al., 2022; Shang et al., 2020). NLRP3 deficiency in aged mice both normalizes IL-1β levels in the hippocampus and diminishes production of other SASP cytokines (Youm et al., 2013). On the other end of the axis, reducing the number of senescent cells using senolytics also normalizes NLRP3 activation brain tissue (Kwon et al., 2021). These data suggest inflammasome activation is closely tied to cellular senescence and resulting cytokine production. We find advanced age and ethanol amplified expression of NLRP3 and cleaved IL-1β in CA3 of the hippocampus, suggesting NLRP3 activation may be an important component of age-related elevations in neuroinflammatory cytokines. We also find binge ethanol increases mRNA expression of cell cycle arrest proteins including (Cyclin D1 and p16ink4a) in the aged brain. Although these are hallmark markers of senescence in the CNS and periphery (Huang et al., 2022), our results could be confounded by ethanol-induced disruptions in neural stem cell proliferation in the hippocampus (Di Rocco et al., 2019). To fully elucidate outcomes on senescence in this model, future studies should verify the specific cell type(s) within in the hippocampus that achieve senescence using markers that are not dependent on the cell cycle, like beta-galactosidase (Huang et al., 2022).
Microglia propagate neuroinflammation after binge ethanol exposure in the brains of young mice (Fernandez-Lizarbe et al., 2009; Wang et al., 2018). Unlike peripheral macrophages that undergo suppressed immune responses with advanced age (Boehmer et al., 2005), microglia of the aged brain become “primed,” and exhibit heightened and prolonged production pro-inflammatory mediators in response to stimuli (Norden and Godbout, 2013). Importantly, non-resolving microglia polarization to a pro-inflammatory state is an early indicator of advanced age-related neurodegeneration (Hansen et al., 2018). These data led us to hypothesize microglia would be susceptible to activation from binge ethanol exposure in aged mice. Indeed, we observed ethanol exposure increased markers of microglia activation including Iba-1 expression and enlarged microglia soma size in CA3 of aged mice. These data suggest another potential mechanism by which the brains of aged mice may produce more pro-inflammatory cytokines after binge ethanol consumption compared to brains of young mice. These data support past studies that suggest ethanol induces microglia reactivity in mice and rats in vivo (Fernandez-Lizarbe et al., 2009; Pascual et al., 2007) and ex vivo (Fernandez-Lizarbe et al., 2009; Kalinin et al., 2018; Coleman et al., 2020). However, there is conflicting evidence that direct ethanol exposure suppresses pro-inflammatory signaling after ethanol withdrawal in mouse microglia in vivo (Guergues et al., 2020) and after direct ethanol exposure in rat microglia ex vivo (Bell-Temin et al., 2013). Additional investigation into the effects of ethanol exposure on primary microglia across the lifespan, and throughout different stages of ethanol exposure and withdrawal, is necessary to fully characterize the impact of ethanol on microglia phenotype.
Although we failed to see differences in astrocyte reactivity in our model via GFAP staining this does not necessarily exclude age and ethanol-related changes on astrocyte phenotype. Given the important role of astrocytes in ethanol metabolism in the brain (Jin et al., 2021), it will be important for future studies to dissect the molecular mechanisms within and between microglia and astrocytes that coordinate the inflammatory landscape of the aged brain after ethanol exposure. Brain tissue was not perfused at the time of tissue harvest in this model, and thus an additional avenue of exploration includes the contribution of infiltrating macrophages to age and ethanol related neuroinflammation. Infiltrating macrophages are thought to amplify cytokine production after chronic ethanol consumption in young mice (Lowe et al., 2020). While infiltrating macrophages are estimated to only compromise about 10% of total brain macrophages after ethanol exposure (Lowe et al., 2020), delineating their responses with lend more insight into the source of cytokine production in the aged brain from ethanol consumption. Study of these outcomes in other brain regions will also reveal if these neuroinflammatory responses are unique to CA3 or are a common consequence throughout all brain regions.
Heightened neuroinflammation is closely tied to tau hyperphosphorylation and neuronal death within the hippocampus (Chen et al., 2020; Metcalfe and Figueiredo-Pereira, 2010; Navarro et al., 2020). For example, the NLRP3 inflammasome is vital to initiate tau phosphorylation and NLRP3 blockade reduces tau aggregation in PS19 transgenic mice (Stancu et al., 2022). Here, we observed that the expression pattern of mapt, camk2a, and prkcd in the hippocampus of aged mice began to suggest early changes in tau hyperphosphorylation from ethanol exposure. We further confirmed this phenotype within CA3 of aged and ethanol treated mice using PHF-1 staining. Our findings also suggest early accumulation of apoptotic cells in the CA3 of ethanol exposed aged mice. These data demonstrate that our model of intermittent binge ethanol exposure in aged mice mimic pathological hallmarks of neurodegeneration in seen in aged adults with alcohol use disorder (Ikegami et al., 2003) and in other rodent models of advanced aging (Torres et al., 2021), Alzheimer’s disease (Hoffman et al., 2019), and tauopathies (Stancu et al., 2022). Our data are consistent with proteomic studies using chronic intermittent ethanol exposure in male rats that demonstrate changes in phosphoproteins due to advanced age and ethanol exposure in the brain (Ho et al., 2022). Together, these data provide further evidence that binge ethanol related enhancement of inflamm-aging factors may contribute to neurodegeneration. Future studies may employ anti-inflammatory drug interventions to further implicate neuroinflammation as a driver of age and ethanol-related neuronal damage.
Interestingly, young animals did not exhibit signs of neurodegeneration, as no notable differences in PHF-1 and TUNEL staining were observed between young vehicle and ethanol mice. We found modest increases in cytokine production and microglia activation in young mice after binge ethanol exposure. These patterns, while consistent with other models of binge ethanol exposure using young male mice and higher doses of ethanol (5 g/kg), are not as robust (Qin and Crews, 2012). These discrepancies may be explained by the use of C57BL/6N mice in our studies, which are thought to be less sensitive to the inflammatory effects of ethanol compared to C57BL/6J mice, due to differences in the nnt gene (Boschen et al., 2021). Additional discrepancies may be a result of the more moderate ethanol exposure employed herein. Because binge ethanol exposure models that utilize 5 g/kg ethanol binges in young male mice elicit neuron death (Liu et al., 2019), it is possible that these exposures did not reach the necessary threshold to promote cell death. Alternatively, our studies may highlight the requirement of certain advanced age-related factors that predispose the brain to ethanol-related neurodegeneration, including heightened pro- inflammatory mediators and NLRP3 inflammasome activation.
Hippocampal neurodegeneration is a leading cause for advanced age and binge ethanol related memory deficits (Moodley and Chan, 2014). Even in the absence of discrete neuronal injury, increased expression of pro-inflammatory modulators like IL-1β inhibit neurogenesis within the hippocampus (Crews, 2012; Wu et al., 2012) and negatively impacts cognition in the context of aging and disease (Kitazawa et al., 2011). Given the specific role of CA3 in the formation of spatial, contextual, and episodic memories, we utilized the RAWM and CFC to assess differences in memory due to age and ethanol exposure (Cherubini and Miles, 2015). Using our intermittent binge ethanol paradigm, no cognitive impairment was observed in young mice after ethanol binge, which is not surprising considering young mice displayed lower levels of inflammatory markers and did not exhibit neurodegeneration markers after binge ethanol exposure. Aged mice exhibited deficits in the contextual memory, which was exaggerated by binge ethanol exposure. Advanced age-related memory impairments are consistent with other aged rodent models and mimic the cognitive decline seen in otherwise healthy aged adults (Shukitt-Hale et al., 2004). However, these age-related impairments may have limited our ability to detect differences between aged vehicle and ethanol treated groups on the radial arm water maze. Given the observed decline in health and increase in frailty starting at 18 months of age (Baumann et al., 2019) in C57BL/6 mice, future studies may need to implement the use of middle-aged (12 months) and older (16–17 months) mice to detect the influence of ethanol on cognition in aged mice. Additionally, while young and aged mice did not exhibit differences in withdrawal behaviors (Supplemental Figure 1), we cannot fully rule out the possibility that mice undergo more subtle withdrawal states not detected by our measures (Metten et al., 2018). Future iterations of this study should explore cognition in animals at 3 or more days after ethanol exposure to eliminate lingering effects of withdrawal.
Other limitations of our study arose from the variability in response to ethanol in our aged mice. Phenotypic variation increases with advanced age in rodents (Anton et al., 2023), and in the future performing measures of frailty before ethanol treatment may help account for the differences in responses. In our spatial transcriptomic study, we were limited to one sample per treatment group due to the cost prohibitive nature of this assay. Trends observed using spatial transcriptomics should therefore be verified using in situ hybridization, qRT-PCR, or immunohistochemistry with powered treatment groups. The quantity of animals needed for each end point limited us to test outcomes at a single timepoint with mice that were previously naïve to ethanol. Because aged adult drinkers typically consume alcohol throughout adulthood (Breslow et al., 1997) the lasting impact of ethanol exposure on neurodegeneration throughout adulthood should be investigated. Finally, these experiments utilized female C57BL/6N mice to model the growing issue of binge drinking among aged adult women (Breslow et al., 1997). Key endpoints will need to be repeated in male mice to understand how sex influences the outcomes addressed here.
In conclusion, we provide evidence to suggest binge drinking promotes neurodegeneration and cognitive impairment during advanced age. We identified several different neuroinflammatory mediators that are associated with advanced age and ethanol related damage and our model of intermittent binge ethanol exposure can be utilized in future studies to identify potential therapeutic targets. Together these data strongly suggest binge alcohol use in aged adults should be considered a modifiable risk factor for neurodegenerative disease and dementia development.
Supplementary Material
Funding
This work was supported in part by National Institutes of Health grants F31AA030213 (PEA), T32ES029074 (PEA/LNR), R01AG018859 (EJK), R21AA026295 (EJK), R00AA025386 (RLM), R00AA025386-S1 (RLM), pilot grant funds from the CU Anschutz RNA Biosciences Initiative (RLM), and startup funds from the Department of Pharmaceutical Sciences in the School of Pharmacy (RLM).
Footnotes
CRediT authorship contribution statement
Paige E. Anton: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. Lauren N. Rutt: Investigation. Michael L. Kaufman: Formal analysis, Writing – review & editing. Nicolas Busquet: Formal analysis, Writing – review & editing. Elizabeth J. Kovacs: Funding acquisition, Project administration, Supervision, Visualization, Writing – review & editing. Rebecca L. McCullough: Conceptualization, Formal analysis, Funding acquisition, Investigation, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2023.12.034.
Data availability
Data will be made available on request.
References
- Alfonso-Loeches S, Urena-Peralta J, Morillo-Bargues MJ, Gómez-Pinedo U, Guerri C, 2016. Ethanol-induced TLR4/NLRP3 neuroinflammatory response in microglial cells promotes leukocyte infiltration across the BBB. Neurochem. Res. 41 (1–2), 193–209. [DOI] [PubMed] [Google Scholar]
- Anton PE, Rutt LN, Capper C, Orlicky DJ, McCullough RL, 2023. Profiling the oxylipidome in aged mice after chronic ethanol feeding: Identifying lipid metabolites as drivers of hepatocyte stress. Alcohol 107, 119–135. 10.1016/j.alcohol.2022.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker S, Paul BD, Pieper AA, 2023. Increased risk of aging-related neurodegenerative disease after traumatic brain injury. Biomedicines 11 (4), 1154. 10.3390/biomedicines11041154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett A, David E, Rohlman A, Nikolova VD, Moy SS, Vetreno RP, Coleman LG Jr, 2022. Adolescent binge alcohol enhances early Alzheimer’s disease pathology in adulthood through proinflammatory neuroimmune activation. Front. Pharmacol. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann CW, Kwak D, Thompson LV, 2019. Sex-specific components of frailty in C57BL/6 mice. Aging (Albany NY) 11 (14), 5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam CR, Kaneshiro C, Jang JY, Reynolds CA, Pedersen NL, Gatz M, 2018. Differences between women and men in incidence rates of dementia and Alzheimer’s disease. J. Alzheimers Dis. 64 (4), 1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Temin H, Zhang P, Chaput D, King MA, You M, Liu B, Stevens SM, 2013. Quantitative proteomic characterization of ethanol-responsive pathways in rat microglial cells. J. Proteome Res. 12 (5), 2067–2077. From NLM DOI: 10.1021/pr301038f. [DOI] [PubMed] [Google Scholar]
- Beyer MM, Lonnemann N, Remus A, Latz E, Heneka MT, Korte M, 2020. Enduring changes in neuronal function upon systemic inflammation are NLRP3 inflammasome dependent. J. Neurosci. 40 (28), 5480–5494. 10.1523/JNEUROSCI.0200-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ, 2005. Aging negatively skews macrophage TLR2-and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech. Ageing Dev. 126 (12), 1305–1313. 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Boschen KE, Ptacek TS, Berginski ME, Simon JM, Parnell SE, 2021. Transcriptomic analyses of gastrulation-stage mouse embryos with differential susceptibility to alcohol. Dis. Model. Mech. 14 (6), dmm049012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow RA, Castle IJP, Chen CM, Graubard BI, 2017. Trends in alcohol consumption among older Americans: National Health Interview Surveys, 1997 to 2014. Alcohol. Clin. Exp. Res. 41 (5), 976–986. 10.1111/acer.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow RA, Castle IP, Chen CM, Graubard BI Trends in Alcohol Consumption Among Older Americans: National Health Interview Surveys, 1997 to 2014. Alcohol Clin Exp Res 2017, 41 (5), 976–986, From NLM DOI: 10.1111/acer.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastain LG, Sarkar DK, 2014. Role of microglia in regulation of ethanol neurotoxic action. Int. Rev. Neurobiol. 118, 81–103. 10.1016/B978-0-12-801284-0.00004-X. [DOI] [PubMed] [Google Scholar]
- Chen W-T, Lu A, Craessaerts K, Pavie B, Frigerio CS, Corthout N, Qian X, Laláková J, Kühnemund M, Voytyuk I. Spatial transcriptomics and in situ sequencing to study Alzheimer’s disease. Cell 2020, 182 (4), 976–991. e919, DOI: 10.1016/j.cell.2020.06.038. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Miles R, 2015. The CA3 region of the hippocampus: how is it? What is it for? How does it do it? Frontiers Media SA 9, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG, Zou J, Crews FT, 2020. Microglial depletion and repopulation in brain slice culture normalizes sensitized proinflammatory signaling. J. Neuroinflammation 17 (1), 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG, Zou J, Crews FT, 2020. Microglial depletion and repopulation in brain slice culture normalizes sensitized proinflammatory signaling. J. Neuroinflammation 17, 1–20. 10.1186/s12974-019-1678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correas I, Díaz-Nido J, Avila J. Microtubule-associated protein tau is phosphorylated by protein kinase C on its tubulin binding domain. J Biol Chem 1992, 267 (22), 15721–15728, From NLM. [PubMed] [Google Scholar]
- Crews FT, 2008. Alcohol-related neurodegeneration and recovery: mechanisms from animal models. Alcohol Res. Health 31 (4), 377. [PMC free article] [PubMed] [Google Scholar]
- Crews F, 2012. Inflammasome-IL-1β signaling mediates ethanol inhibition of hippocampal neurogenesis. Front. Neurosci. 6, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Sarkar DK, Qin L, Zou J, Boyadjieva N, Vetreno RP, 2015. Neuroimmune function and the consequences of alcohol exposure. Alcohol Res. 37 (2), 331. [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Sarkar DK, Qin L, Zou J, Boyadjieva N, Vetreno RP, 2015. Neuroimmune function and the consequences of alcohol exposure. Alcohol Res. [PMC free article] [PubMed] [Google Scholar]
- Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, Cotman CW, 2012. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J. Neuroinflammation 9 (1), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalli P, Mitic T, Caporali A, Lauriola A, 2016. D’Arca D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxidative Medicine and Cellular Longevity 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biase D, Piegari G, Prisco F, Cimmino I, Pirozzi C, Mattace Raso G, Oriente F, Grieco E, Papparella S, Paciello O, 2020. Autophagy and NLRP3 inflammasome crosstalk in neuroinflammation in aged bovine brains. J. Cell. Physiol. 235 (6), 5394–5403. 10.1002/jcp.29426. [DOI] [PubMed] [Google Scholar]
- Di Rocco G, Baldari S, Pani G, Toietta G, 2019. Stem cells under the influence of alcohol: effects of ethanol consumption on stem/progenitor cells. Cell. Mol. Life Sci. 76 (2), 231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Gano A, Paniccia JE, Deak T, 2015. Male adolescent rats display blunted cytokine responses in the CNS after acute ethanol or lipopolysaccharide exposure. Physiol. Behav. 148, 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman N, Rotter-Maskowitz A, Okun E, 2015. DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing Res. Rev. 24, 29–39. 10.1016/j.arr.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Feng L, Wang Y, Zeng D, Wang M, Duan X, 2023. Predictors of cognitive decline in older individuals without dementia: an updated meta-analysis. Ann. Clin. Transl. Neurol. 10.1002/acn3.51740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C, 2009. Critical role of TLR4 response in the activation of microglia induced by ethanol. J. Immunol. 183 (7), 4733–4744. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Frank MG, Kitt MM, D’Angelo HM, Norden DM, Weber MD, Barrientos RM, Godbout JP, Watkins LR, Maier SF, 2016. The alarmin HMGB1 mediates age-induced neuroinflammatory priming. J. Neurosci. 36 (30), 7946–7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, 2007. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 128 (1), 92–105. 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Freeman K, Brureau A, Vadigepalli R, Staehle MM, Brureau MM, Gonye GE, Hoek JB, Hooper DC, Schwaber JS, 2012. Temporal changes in innate immune signals in a rat model of alcohol withdrawal in emotional and cardiorespiratory homeostatic nuclei. J. Neuroinflammation 9, 1–11. 10.1186/1742-2094-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman LC, Ting JPY, 2016. The pathogenic role of the inflammasome in neurodegenerative diseases. J. Neurochem. 136, 29–38. 10.1111/jnc.13217. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Brushfield AM The role of the CA3 hippocampal subregion in spatial memory: a process oriented behavioral assessment. Prog Neuropsychopharmacol Biol Psychiatry 2009, 33 (5), 774–781, From NLM DOI: 10.1016/j.pnpbp.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout J, Chen J, Abraham J, Richwine A, Berg B, Kelley K, Johnson RW, 2005. Exaggerated neuroinflammation and sickness behavior in aged mice after activation of the peripheral innate immune system. FASEB J. 19 (10), 1329–1331. [DOI] [PubMed] [Google Scholar]
- Grifasi IR; Evans WA; Rexha AD; Sako LW; Marshall SA A Comparison of hippocampal microglial responses in aged and young rodents following dependent and non-dependent binge drinking. In International Review of Neurobiology, Vol. 148; Elsevier, 2019; pp 305–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritsenko A, Green JP, Brough D, Lopez-Castejon G, 2020. Mechanisms of NLRP3 priming in inflammaging and age related diseases. Cytokine Growth Factor Rev. 55, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guergues J, Wohlfahrt J, Zhang P, Liu B, Stevens SM Jr, 2020. Deep proteome profiling reveals novel pathways associated with pro-inflammatory and alcohol- induced microglial activation phenotypes. J. Proteomics 220, 103753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri C, Pascual M. Role of neuroinflammation in ethanol neurotoxicity. In Advances in neurotoxicology, Vol. 3; Elsevier, 2019; pp 259–294. [Google Scholar]
- Hahn HK, Burch RE, 1983. Impaired ethanol metabolism with advancing age. Alcohol. Clin. Exp. Res. 7 (3), 299–301. [DOI] [PubMed] [Google Scholar]
- Han BH, Moore AA, Ferris R, Palamar JJ, 2019. Binge drinking among older adults in the United States, 2015 to 2017. J. Am. Geriatr. Soc. 67 (10), 2139–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DV, Hanson JE, Sheng M, 2018. Microglia in Alzheimer’s disease. J. Cell Biol. 217 (2), 459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, Evans DA, 2013. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80 (19), 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A-M-C, Peyton MP, Scaletty SJ, Trapp S, Schreiber A, Madden BJ, Choi DS, Matthews DB, 2022. Chronic intermittent ethanol exposure alters behavioral flexibility in aged rats compared to adult rats and modifies protein and protein pathways related to alzheimer’s disease. ACS Omega. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JL, Faccidomo S, Kim M, Taylor SM, Agoglia AE, May AM, Smith EN, Wong L, Hodge CW, 2019. Alcohol drinking exacerbates neural and behavioral pathology in the 3xTg-AD mouse model of Alzheimer’s disease. In: International Review of Neurobiology, Vol. 148;. Elsevier, pp. 169–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, Bohr VA Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol 2019, 15 (10), 565–581, From NLM DOI: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- Hu WT, Howell JC, Ozturk T, Gangishetti U, Kollhoff AL, Hatcher-Martin JM, Anderson AM, Tyor WR, 2019. CSF cytokines in aging, multiple sclerosis, and dementia. Front. Immunol. 10, 480. 10.3389/fimmu.2019.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Henry C, Dantzer R, Johnson RW, Godbout J, 2008. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol. Aging 29 (11), 1744–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Hickson LJ, Eirin A, Kirkland JL, Lerman LO, 2022. Cellular senescence: the good, the bad and the unknown. Nat. Rev. Nephrol. 18 (10), 611–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami Y, Goodenough S, Inoue Y, Dodd P, Wilce P, Matsumoto I, 2003. Increased TUNEL positive cells in human alcoholic brains. Neurosci. Lett. 349 (3), 201–205. [DOI] [PubMed] [Google Scholar]
- Järvenpää T, Rinne JO, Koskenvuo M, Räihä I. Kaprio J. Binge drinking in midlife and dementia risk. Epidemiology 2005, 16 (6), 766–771, From NLM DOI: 10.1097/01.ede.0000181307.30826.6c. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, Rolland B, Gierski F, Martinetti MP, Naassila M, 2019. Animal models of binge drinking, current challenges to improve face validity. Neurosci. Biobehav. Rev. 106, 112–121. [DOI] [PubMed] [Google Scholar]
- Jin S, Cao Q, Yang F, Zhu H, Xu S, Chen Q, Wang Z, Lin Y, Cinar R, Pawlosky RJ, 2021. Brain ethanol metabolism by astrocytic ALDH2 drives the behavioural effects of ethanol intoxication. Nature Metabolism 3 (3), 337–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Flavell RA, 2010. Molecular mechanism of NLRP3 inflammasome activation. J. Clin. Immunol. 30, 628–631. 10.1007/s10875-010-9440-3. [DOI] [PubMed] [Google Scholar]
- Joksimovic SM, Ghodsi SM, Heinsbroek JA, Orfila JE, Busquet N, Tesic V, Valdez R, Fine-Raquet B, Jevtovic-Todorovic V, Raol YH, 2023. CaV3. 1 T-type calcium channels are important for spatial memory processing in the dorsal subiculum. Neuropharmacology 226, 109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinin S, González-Prieto M, Scheiblich H, Lisi L, Kusumo H, Heneka MT, Madrigal JL, Pandey SC, Feinstein DL, 2018. Transcriptome analysis of alcohol-treated microglia reveals downregulation of beta amyloid phagocytosis. J. Neuroinflammation 15 (1), 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J, Drew PD, 2013. Effects of ethanol on immune response in the brain: region-specific changes in aged mice. J. Neuroinflammation 10 (1), 1–4. 10.1186/1742-2094-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M, Cheng D, Tsukamoto MR, Koike MA, Wes PD, Vasilevko V, Cribbs DH, LaFerla FM, 2011. Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal β-catenin pathway function in an Alzheimer’s disease model. J. Immunol. 187 (12), 6539–6549. 10.4049/jimmunol.1100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OC, Song JJ, Yang Y, Kim SH, Kim JY, Seok MJ, Hwang I, Yu JW, Karmacharya J, Maeng HJ, 2021. SGK1 inhibition in glia ameliorates pathologies and symptoms in Parkinson disease animal models. EMBO Mol. Med. 13 (4), e13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrousse VF, Nadjar A, Joffre C, Costes L, Aubert A, Gregoire S, Bretillon L, Laye S, 2012. Short-term long chain omega3 diet protects from neuroinflammatory processes and memory impairment in aged mice. PLoS One 7 (5), e36861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Duewell P. NLRP3 inflammasome activation in inflammaging. In Seminars in immunology, 2018; Elsevier: Vol. 40, pp 61–73. DOI: 10.1016/j.smim.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Li Q, Liu D, Pan F, Ho CS, Ho R, 2019. Ethanol exposure induces microglia activation and neuroinflammation through TLR4 activation and SENP6 modulation in the adolescent rat hippocampus. Neural Plast. 2019 10.1155/2019/1648736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Liu GA, Perez E, Rainer RD, Febo M, Cruz-Almeida Y, Ebner NC, 2018. Systemic inflammation mediates age-related cognitive deficits. Front. Aging Neurosci. 10, 236. 10.3389/fnagi.2018.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippai D, Bala S, Petrasek J, Csak T, Levin I, Kurt-Jones EA, Szabo G, 2013. Alcohol-induced IL-1β in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J. Leukoc. Biol. 94 (1), 171–182. 10.1189/jlb.1212659b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Sun YM, Huang H, Chen C, Wan J, Ma LH, Sun YY, Miao HH, Wu YQ Sirtuin 3 protects against anesthesia/surgery-induced cognitive decline in aged mice by suppressing hippocampal neuroinflammation. J Neuroinflammation 2021, 18 (1), 41, From NLM DOI: 10.1186/s12974-021-02089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang Y, Peng J, Wang H, Li X, Li X, Rong X, Pan J, Peng Y, 2019. Autophagy alleviates ethanol-induced memory impairment in association with anti-apoptotic and anti-inflammatory pathways. Brain Behav. Immun. 82, 63–75. [DOI] [PubMed] [Google Scholar]
- Lowe PP, Morel C, Ambade A, Iracheta-Vellve A, Kwiatkowski E, Satishchandran A, Furi I, Cho Y, Gyongyosi B, Catalano D, 2020. Chronic alcohol-induced neuroinflammation involves CCR2/5-dependent peripheral macrophage infiltration and microglia alterations. J. Neuroinflammation 17 (1), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucin KM, Wyss-Coray T, 2009. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron 64 (1), 110–122. 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson KP, de Sousa Rodrigues ME, Cintron AF, Tansey MG, 2018. Neuroinflammation in Age-Related Neurodegenerative Diseases. Elsevier, In The Molecular and Cellular Basis of Neurodegenerative Diseases, pp. 477–507. [Google Scholar]
- Majchrowicz E, 1975. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia 43, 245–254. [DOI] [PubMed] [Google Scholar]
- Malani P, Kullgren J, Solway E, Fernandez A, Singer D, Kirch M, 2021. National Poll on Healthy Aging: Alcohol Use among Older Adults. 10.7302/1328. [DOI] [Google Scholar]
- McMahan RH, Najarro KM, Mullen JE, Paul MT, Orlicky DJ, Hulsebus HJ, Kovacs EJ, 2021. A novel murine model of multi-day moderate ethanol exposure reveals increased intestinal dysfunction and liver inflammation with age. Immun. Ageing 18 (1), 1–11. 10.1186/s12979-021-00247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe MJ, Figueiredo-Pereira ME, 2010. Relationship between tau pathology and neuroinflammation in Alzheimer’s disease. Mount Sinai Journal of Medicine: A Journal of Translational and Personalized Medicine: A Journal of Translational and Personalized Medicine 77 (1), 50–58. 10.1002/msj.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Schlumbohm JP, Huang LC, Greenberg GD, Hack WR, Spence SE, Crabbe JC, 2018. An alcohol withdrawal test battery measuring multiple behavioral symptoms in mice. Alcohol 68, 19–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley K, Chan D, 2014. The hippocampus in neurodegenerative disease. In: The Hippocampus in Clinical Neuroscience, Vol. 34;. Karger Publishers, pp. 95–108. [DOI] [PubMed] [Google Scholar]
- Navarro JF, Croteau DL, Jurek A, Andrusivova Z, Yang B, Wang Y, Ogedegbe B, Riaz T, Støen M, Desler C, 2020. Spatial transcriptomics reveals genes associated with dysregulated mitochondrial functions and stress signaling in Alzheimer disease. Iscience 23 (10), 101556. 10.1016/j.isci.2020.101556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzwiedz-Massey VM, Douglas JC, Rafferty T, Johnson JW, Holloway KN, Berquist MD, Kane CJ, Drew PD, 2023. Effects of chronic and binge ethanol administration on mouse cerebellar and hippocampal neuroinflammation. Am. J. Drug Alcohol Abuse 49 (3), 345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Godbout JP, 2013. Microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. 39 (1), 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obernier JA, Bouldin T, Crews F, 2002. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol. Clin. Exp. Res. 26 (4), 547–557. 10.1111/j.1530-0277.2002.tb02573.x. [DOI] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C, 2007. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur. J. Neurosci. 25 (2), 541–550. [DOI] [PubMed] [Google Scholar]
- Pascual M, Balino P, Aragon CM, Guerri C, 2015. Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: role of TLR4 and TLR2. Neuropharmacology 89, 352–359. [DOI] [PubMed] [Google Scholar]
- Patrick ME, Azar B. High-intensity drinking. Alcohol Res 2018, 39 (1), 49–55, From NLM. [PMC free article] [PubMed] [Google Scholar]
- Phillips R, LeDoux J, 1992. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 106 (2), 274. 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pruett S, Tan W, Howell III GE, Nanduri B, 2020. Dosage scaling of alcohol in binge exposure models in mice: an empirical assessment of the relationship between dose, alcohol exposure, and peak blood concentrations in humans and mice. Alcohol 89, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Crews FT, 2012. Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. J. Neuroinflammation 9 (1), 1–18. 10.1186/1742-2094-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong J-S, Crews FT, 2008. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J. Neuroinflammation 5 (1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Zou J, Barnett A, Vetreno RP, Crews FT, Coleman LG Jr, 2021. TRAIL mediates neuronal death in AUD: a link between neuroinflammation and neurodegeneration. Int. J. Mol. Sci. 22 (5), 2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero A, Dongil P, Valencia I, Vallejo S, San Hipólito-Luengo Á, Díaz-Araya G, Bartha JL, González-Arlanzón MM, Rivilla F, De La Cuesta F, 2022. ´ Pharmacological blockade of NLRP3 inflammasome/IL-1β-positive loop mitigates endothelial cell senescence and dysfunction. Aging Dis. 13 (1), 284. 10.14336/AD.2021.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvioli S, Monti D, Lanzarini C, Conte M, Pirazzini C, Giulia Bacalini M, Garagnani P, Giuliani C, Fontanesi E, Ostan R, 2013. Immune system, cell senescence, aging and longevity-inflamm-aging reappraised. Curr. Pharm. Des. 19 (9), 1675–1679. [PubMed] [Google Scholar]
- Santa-Maria I, Varghese M, 2012. Paired helical filaments from Alzheimer disease brain induce intracellular accumulation of Tau protein in aggresomes. J. Biol. Chem. 287 (24), 20522–20533. 10.1074/jbc.M111.323279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saponaro F, Kim JH, Chiellini G. Transthyretin stabilization: an emerging strategy for the treatment of alzheimer’s disease? Int J Mol Sci 2020, 21 (22), From NLM DOI: 10.3390/ijms21228672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R, Bandiera S, Souza DG, Bellaver B, Caletti G, Quincozes-Santos A, Elisabetsky E, Gomez R, 2017. N-acetylcysteine prevents alcohol related neuroinflammation in rats. Neurochem. Res. 42, 2135–2141. 10.1007/s11064-017-2218-8. [DOI] [PubMed] [Google Scholar]
- Sebastian-Valverde M, Pasinetti GM, 2020. The NLRP3 inflammasome as a critical actor in the inflammaging process. Cells 9 (6), 1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang D, Hong Y, Xie W, Tu Z, Xu J, 2020. Interleukin-1β drives cellular senescence of rat astrocytes induced by oligomerized amyloid β peptide and oxidative stress. Front. Neurol. 11, 929. 10.3389/fneur.2020.00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukitt-Hale B, McEwen JJ, Szprengiel A, Joseph JA, 2004. Effect of age on the radial arm water maze—a test of spatial learning and memory. Neurobiol. Aging 25 (2), 223–229. 10.1016/s0197-4580(03)00041-1. [DOI] [PubMed] [Google Scholar]
- Sikora E, Bielak-Zmijewska A, Dudkowska M, Krzystyniak A, Mosieniak G, Wesierska M, Wlodarczyk J, 2021. Cellular senescence in brain aging. Front. Aging Neurosci. 13, 646924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancu IC, Lodder C, Botella Lucena P, Vanherle S, Gutiérrez de Ravé M, Terwel D, Bottelbergs A, Dewachter I, 2022. The NLRP3 inflammasome modulates tau pathology and neurodegeneration in a tauopathy model. Glia 70 (6), 1117–1132. 10.1002/glia.24160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipa R, 2019. Proinflammatory and anti-inflammatory cytokines in the CSF of patients with Alzheimer’s disease and their correlation with cognitive decline. Neurobiol. Aging 76, 125–132. 10.3389/fimmu.2019.00480. [DOI] [PubMed] [Google Scholar]
- Tajuddin N, Moon K-H, Marshall SA, Nixon K, Neafsey EJ, Kim H-Y, Collins MA, 2014. Neuroinflammation and neurodegeneration in adult rat brain from binge ethanol exposure: abrogation by docosahexaenoic acid. PLoS One 9 (7), e101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres AK, Jara C, Olesen MA, Tapia-Rojas C, 2021. Pathologically phosphorylated tau at S396/404 (PHF-1) is accumulated inside of hippocampal synaptic mitochondria of aged Wild-type mice. Sci. Rep. 11 (1), 1–17. 10.1097/PPO.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallés SL, Blanco AM, Pascual M, Guerri C, 2004. Chronic ethanol treatment ´ enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathol. 14 (4), 365–371. 10.1111/j.1750-3639.2004.tb00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter TJ, Crews FT, 2017. Microglial depletion alters the brain neuroimmune response to acute binge ethanol withdrawal. J. Neuroinflammation 14 (1), 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, McDaniel K, Wu N, Ramos-Lorenzo S, Glaser T, Venter J, Francis H, Kennedy L, Sato K, Zhou T, 2017. Regulation of cellular senescence by miR-34a in alcoholic liver injury. Am. J. Pathol. 187 (12), 2788–2798. 10.1016/j.ajpath.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang K, Yang F, Ren Z, Xu M, Frank JA, Ke Z-J, Luo J, 2018. Minocycline protects developing brain against ethanol-induced damage. Neuropharmacology 129, 84–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendimu MY, Hooks SB Microglia Phenotypes in Aging and Neurodegenerative Diseases. Cells 2022, 11 (13), From NLM DOI: 10.3390/cells11132091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MD, Hein AM, Moravan MJ, Shaftel SS, Olschowka JA, O’Banion MK, 2012. Adult murine hippocampal neurogenesis is inhibited by sustained IL-1β and not rescued by voluntary running. Brain Behav. Immun. 26 (2), 292–300. 10.1016/j.bbi.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J-Y, Xue X, Tian H, Wang X-X, Dong Y-X, Wang F, Zhao Y-N, Yao X-C, Cui W, Wu C-F, 2014. Role of microglia in ethanol-induced neurodegenerative disease: Pathological and behavioral dysfunction at different developmental stages. Pharmacol. Ther. 144 (3), 321–337. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Ichinose T, Yamauchi T. Phosphorylation of tau protein to sites found in Alzheimer’s disease brain is catalyzed by Ca2+/calmodulin-dependent protein kinase II as demonstrated tandem mass spectrometry. Neurosci Lett 2003, 353 (3), 185–188, From NLM DOI: 10.1016/j.neulet.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Youm Y-H, Grant RW, McCabe LR, Albarado DC, Nguyen KY, Ravussin A, Pistell P, Newman S, Carter R, Laque A, 2013. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 18 (4), 519–532. 10.1016/j.cmet.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y-N, Wang F, Fan Y-X, Ping G-F, Yang J-Y, Wu C-F, 2013. Activated microglia are implicated in cognitive deficits, neuronal death, and successful recovery following intermittent ethanol exposure. Behav. Brain Res. 236, 270–282. [DOI] [PubMed] [Google Scholar]
- Zou J, Crews F, 2010. Induction of innate immune gene expression cascades in brain slice cultures by ethanol: key role of NF-κB and proinflammatory cytokines. Alcohol. Clin. Exp. Res. 34 (5), 777–789. [DOI] [PubMed] [Google Scholar]
- Zou JY, Crews FT, 2014. Release of neuronal HMGB1 by ethanol through decreased HDAC activity activates brain neuroimmune signaling. PLoS One 9 (2), e87915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.