Abstract
Urticarial vasculitis is characterized by persistent urticarial lesions lasting over 24 h. Urticarial vasculitis is often triggered by medications, infections, and autoimmune disorders. However, vaccinations against viral and bacterial pathogens have recently been documented to induce urticarial vasculitis. We describe the case of a 67-year-old woman who was presented with an extensive erythematous and purpuric rash without systemic symptoms 3 days after an influenza vaccination. She was diagnosed with normocomplementemic urticarial vasculitis based on clinical findings, normal complement levels, and histopathological findings of leukocytoclastic vasculitis. After receiving oral histamines, she showed complete resolution 3 months after receiving the influenza vaccination. Although vaccination-associated vasculitis is common, urticarial vasculitis following vaccinations is rare. We reviewed 13 cases of urticarial vasculitis following a wide range of vaccines, including those against Bacillus Calmette–Guérin, serogroup B meningococcus, influenza, and coronavirus disease. We conducted a comprehensive review of various aspects, including age, sex, past medical history, type of vaccination, number of vaccinations, onset time, cutaneous symptoms, place of eruption, systemic symptoms, laboratory disorders, treatment period, and treatment of urticarial vasculitis. Two patients developed hypocomplementemic urticarial vasculitis after vaccination, and both experienced systemic symptoms such as arthralgia and fever. In this review, no significant differences were found in the data, which may be attributed to the small number of cases. The mechanisms underlying the induction of urticarial vasculitis by vaccines remain unknown; however, in addition to immune complex deposition and complement activation due to vaccine components, molecular mimicry may trigger urticarial vasculitis by producing vaccine-derived pathogenic antigen antibodies. This case study emphasizes the need for heightened awareness and further investigation of urticarial vasculitis as a rare adverse effect of vaccination.
Keywords: vaccination, vasculitis, anticardiolipin antibody, molecular mimicry
Introduction
Urticarial vasculitis (UV) is distinguished by urticarial lesions that persist for more than 24 hours.1 Histopathological examination of UV reveals leukocytoclastic vasculitis, which is characterized by disruption of vascular walls, extravasation of erythrocytes, and perivascular infiltration of neutrophils and lymphocytes.1 Diagnosis of UV is commonly based on the presence of wheal formation and histological evidence of leukocytoclastic vasculitis.1 Unlike typical urticaria, UV often presents with distinctive skin manifestations, such as purpura or rashes, that regress with pigmentation.1 UV can be triggered by medications, cancer, autoimmune or auto-inflammatory diseases, or infections.1 Recently, vaccinations against viral or bacterial pathogens have also been found to induce UV.2–13 Cases of UV after vaccination against Bacillus Calmette–Guérin (BCG), serogroup B meningococcal, influenza, and coronavirus disease 2019 (COVID-19) have been reported.2–13 Many cases of vasculitis induced by vaccinations, such as those against influenza or COVID-19, have been reported, but cases of UV following viral vaccinations are limited.14,15 Herein, we report the second case of UV after influenza vaccination4 and review 13 reported cases of UV attributed to vaccinations against viral or bacterial infections.2–13
Case Report
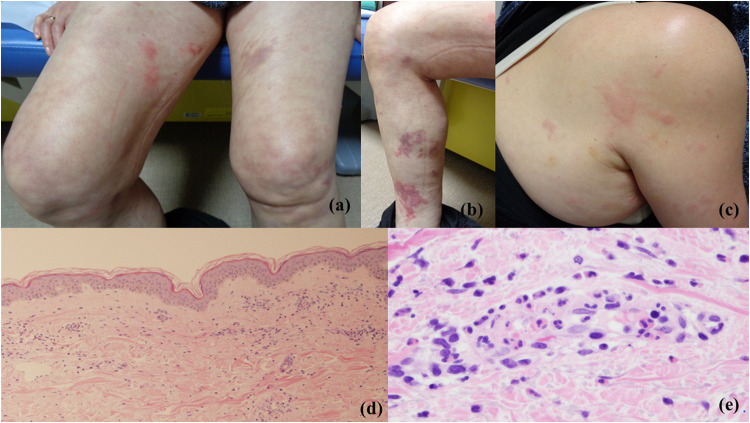
A 67-year-old Japanese woman with no significant medical history or regular medication intake developed long-lasting edematous erythema and a purpuric rash 3 days after receiving the influenza vaccination. The eruptions gradually worsened and spread to her entire body; she was subsequently referred to our department. Physical examination revealed a variety of rash manifestations across the trunk and extremities, including wheals, edematous erythema, purpura, and livedo reticularis (Figure 1a–c). However, other systemic symptoms indicative of systemic vasculitis or autoimmune disease, such as fever or arthralgia, were not observed. Laboratory examination revealed a slightly elevated C-reactive protein level (0.66 mg/dL), elevated D-dimer level (9.82 μg/mL), mildly elevated antinuclear antibody level (titer of 40), and elevated anticardiolipin antibody (aCL) level (19.8 U/mL). Eosinophil counts and immunoglobulin (Ig)E, C3, C4, myeloperoxidase-anti-neutrophil cytoplasmic antibody, proteinase 3-anti-neutrophil cytoplasmic antibody, and double-stranded deoxyribonucleic acid antibody levels were within the reference ranges. Histopathological examination of the edematous erythema revealed capillary dilation and perivascular cellular infiltration of the upper dermis (Figure 1d). Additionally, perivascular neutrophil and eosinophil infiltration, vessel wall destruction, and fibrin deposition in the upper dermis were observed in a high-power view (Figure 1e). Direct immunofluorescence revealed no immune cell deposition. Contrast-enhanced computed tomography revealed no clinical symptoms, organ dysfunction, or thrombotic symptoms. Therefore, systemic vasculitis and antiphospholipid syndrome were not suspected. The combination of long-lasting urticaria-like eruptions, normal complement levels, absence of systemic symptoms, and histopathological findings of urticaria and leukocytoclastic vasculitis led to a diagnosis of normocomplementemic UV (NUV). In light of the report by Hughes et al4 and because our patient had no significant medical history and denied any medication use or infectious incidents in the preceding 6 months, we speculated that the influenza vaccination had triggered UV. Based on the first report of UV after influenza vaccination,4 treatment was initiated with oral bilastine (20 mg/day) without oral prednisone. The cutaneous conditions fluctuated over a span of 3 months but ultimately resolved despite no treatment with oral prednisone. In due course, the aCL level almost normalized. No recurrence was noted over a 2-year observation period following the avoidance of influenza vaccination.
Figure 1.
Clinical and histopathological features. (a–c) Physical examination revealed wheals, edematous erythema, purpura, and livedo reticularis. (d) Histopathological examination of the edematous erythema showing capillary dilation and perivascular cellular infiltration. (HE staining, x40) (e) Histopathological examination of the edematous erythema showing perivascular neutrophil and eosinophil infiltration, nuclear dust, vessel wall destruction, and fibrin deposition in the upper dermis (HE staining, x400).
Literature Review
The onset of UV due to vaccination against viral or bacterial pathogens is a rare occurrence.2–13 To our knowledge, one case of BCG vaccination-associated UV, one case of serogroup B meningococcal vaccination-associated UV, one case of influenza vaccination-associated UV,4 and nine cases of COVID-19 vaccination-associated UV5–13 have been documented thus far (Table 1). Hughes et al reported that a 21-year-old woman with no relevant medical history developed UV 6 days after influenza vaccination.4 To our knowledge, this is the second reported case of influenza vaccination-induced UV.
Table 1.
13 Cases of Vaccination-Associated Urticarial Vasculitis
| Case | Age | Sex | Past medical history | Type of vaccination | Number of vaccination | Onset time | Cutaneous symptoms | Place of eruptions | Systemic symptoms | Laboratory data | Treatment period | Treatment for urticarial vasculitis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Misely L, et al2 | 21 | Male | None | Bacillus Calmette-Guérin | First | 30 days after vaccination | Urticarial eruption | Upper limbs | Fever and polyarthralgia | Hyperleukocytosis and lowered C4 level | 24 weeks | None |
| Velasco-Tamariz V, et al3 | 6 | Female | None | Serogroup B meningococcal | First | 7 days after vaccination | Annular and arciform urticarial wheals | Lower limbs | None | None | 2 weeks | Antihistamines |
| Hughes R, et al4 | 21 | Female | None | Influenza | Unknown | 6 days after vaccination | Urticarial plaques | Trunk | None | None | 2 weeks | Antihistamines |
| Ono H, et al5 | 68 | Male | Diabetes mellitus, and hyperlipidemia | COVID-19 | Third | 4 days after vaccination | Edematous erythema | Trunk and extremities | Fever and polyarthralgia | Hyperleukocytosis and elevated C-reactive protein | 1 week | Oral prednisone and antihistamines |
| Baraldi C, et al6 | 78 | Female | None | COVID-19 | First | 7 days after vaccination | Urticarial rash | Extremities | None | None | 4 weeks | Oral prednisone |
| Nazzaro G, et al7 | 27 | Female | None | COVID-19 | First | 10 days after vaccination | Erythematous wheals | Trunk and extremities | None | None | 8 weeks | Oral prednisone and antihistamines |
| Dash S, et al8 | 27 | Male | None | COVID-19 | Second | 1 day after vaccination | Urticarial plaques | Trunk and extremities | None | Elevated C-reactive protein level | 1 week | Oral indomethacin and antihistamines |
| Daldoul M, et al9 | 73 | Male | Chronic urticaria | COVID-19 | Second | 2 days after vaccination | Purpuric and reticulated plaques | Trunk and extremities | None | Hyperleukocytosis and elevated C-reactive protein level | 1 week | None |
| Larson V, et al10 | 35 | Female | None | COVID-19 | First | 1 day after vaccination | Erythematous and edematous plaques | Trunk and extremities | None | Unknown | Unknown | Oral prednisone, dapsone, and antihistamines |
| Tihy H, et al11 | 73 | Male | None | COVID-19 | Second | 21 days after vaccination | Papules and urticarial plaques | Trunk | None | Unknown | Unknown | Unknown |
| Holmes GA, et al12 | 86 | Female | None | COVID-19 | Second | 5 days after vaccination | Purpuric rash | Face, trunk and extremities | None | None | 2 weeks | Oral prednisone and antihistamines |
| Imamura N, et al13 | 61 | Male | None | COVID-19 | First | 14 days after vaccination | Purpura and urticaria-like rash | Extremities | Fever, polyarthralgia, and swollen lymph nodes | Pancytopenia, increase in ferritin and C-reactive protein level, and decrease in CH50, C3, and C4 levels | More than 40 weeks | Steroid pulse with tapered oral prednisone, and oral cyclosporine |
| Our case | 67 | Female | None | Influenza | More than five times | 3 days after vaccination | Purpura and urticaria-like rash | Trunk and extremities | None | Elevated C-reactive protein, D-dimer, and anticardiolipin antibody level | 12 weeks | Antihistamines |
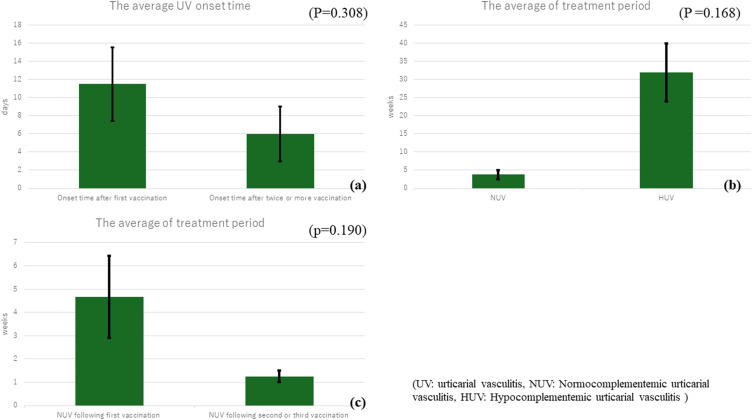
Among the 13 patients examined,2–13 six (46%) were men and seven (54%) were women. The age range was 6–86 years, with an average age of 49.5 years and a median of 61 years. Six (46%) were over 60 years and four (31%) were in their twenties, highlighting notable occurrences in both older and young adults; 11 (85%) had no relevant medical history, one (7.7%) had chronic urticaria, and one (7.7%) had diabetes mellitus and hyperlipidemia. Six (46%) patients developed UV after the first vaccination, with five among them developing UV more than 1 week after vaccination. Contrarily, six (46%) developed UV after two or more vaccinations, with five of them developing UV within 5 days. The average UV onset time in patients after the first vaccination was 11.5 ± 9 days, while the average time after the second or later vaccination dose was 6 ± 6.8 days. However, a statistically significant difference was not observed between the two groups (P = 0.308) (Figure 2a). A purpuric rash was prominently observed in four patients (31%).
Figure 2.
Graph data features (a) Graph of the data regarding onset time of urticarial vasculitis following the first vaccination versus that following second or later vaccination, showing no statistical difference between the two groups. (b) Graph of the data regarding treatment periods of normocomplementemic urticarial vasculitis versus hypocomplementemic urticarial vasculitis, showing no statistical difference between the two groups. (c) Graph of the data regarding treatment periods of normocomplementemic urticarial vasculitis following the first vaccination versus that following the second/third vaccination, showing no statistical difference between the two groups.
While most patients presented with urticaria-like or purpuric eruptions on the trunk and extremities, in some patients, the rashes appeared only on the lower limbs, only on the upper limbs, or on the face. Ten (77%) patients had no systemic symptoms, whereas three (23%) had fever and arthralgia. Eleven (85%) patients had normal complement levels, while two (15%) had lowered complement levels. Two patients with hypocomplementemic UV (HUV) experienced systemic symptoms such as arthralgia and fever and were treated for more than 6 months; one of them developed hemophagocytic lymphohistiocytosis (HLH) during the course of HUV treatment and required steroid pulse therapy with tapered oral prednisone and cyclosporine.
The condition of 11 patients with NUV improved within 2 weeks to 3 months. The average treatment period for HUV was 32 ± 11.3 weeks, while that for NUV was 3.78 ± 3.77 weeks. However, the difference between the two groups was not statistically significant (P= 0.168) (Figure 2b). Moreover, the average treatment period of NUV after the first vaccination was 4.67 ± 3.06 weeks, while that of NUV after the second or third vaccination was 1.25 ± 0.50 weeks. However, the difference between the two groups was not statistically significant (p = 0.190) (Figure 2c). In our patients, the treatment period for NUV might have been longer because oral prednisone was not initiated.
Among the patients with NUV, two (15%) showed improvements without any treatment, four (31%) showed improvements primarily with antihistamines without oral prednisone, and six (46%) required oral prednisone. Other treatments included oral administration of indomethacin or dapsone.
Discussion
UV is classified into idiopathic cases with no known cause and secondary cases that occur due to triggers such as autoimmune diseases, medications, infections, or malignancies.1
Both UV and systemic lupus erythematosus (SLE) are believed to primarily involve inflammation and tissue damage due to immune complex deposition associated with complement activation, including C1q activation. Consequently, reports suggest that UV can coexist with autoimmune diseases such as SLE and Sjögren’s syndrome.16 Additionally, drugs, such as diltiazem, cimetidine, antibiotics, interferon, NSAIDs, and potassium iodide, have been implicated in approximately 10% of UV cases.17 Viral infections, such as hepatitis C and COVID-19, also trigger UV by activating the complement system.18,19 Furthermore, the onset of UV sometimes precedes the development of malignancies, and it may appear as an initial symptom of malignancy.20 Cases where UV improved after surgical removal of malignancy have been reported, suggesting a potential link between UV and malignancies.21 Malignancy-associated UV tends to be resistant to various treatments, including oral corticosteroid therapy.20
Recently, vaccinations against viral or bacterial pathogens have also been found to induce UV.2–13 For example, both influenza and COVID-19 vaccinations can cause a wide variety of vasculitis, including UV.14,22 Maronese et al reported that between 2021, when COVID-19 vaccination was introduced, and 2022, a total of 63 cases of vasculitis following COVID-19 vaccination were documented.22 This included 42 cutaneous leukocytoclastic vasculitis, 12 IgA vasculitis, 4 lymphocytic vasculitis, 4 UV, and 2 anti-neutrophil cytoplasmic antibody-associated vasculitis cases.22 In contrast, during the 40 years from 1966 to 2016, only 65 cases of vasculitis were reported following influenza vaccination.14 Comparing the two virus vaccinations, it appears that COVID-19 vaccination is more likely to induce vasculitis than influenza vaccination.14,22
The exact mechanism underlying the development of UV following vaccination remains unclear.1 The development of vasculitis following vaccinations may be related to immune complex deposition and subsequent complement activation.23,24 Vaccine components that share structural similarities with host proteins could trigger an inflammatory response, leading to the activation of autoreactive B/T cells and antibody formation, which in turn results in the deposition of immune complexes in cutaneous blood vessels and complement activation, ultimately causing vasculitis.23,24
Recently, molecular mimicry has also been proposed as a factor. Segal et al considered that molecular mimicry might be the factor that induces post-vaccination autoimmune phenomena.25 This theory suggests that the notable similarities between the pathogenic elements of vaccines and specific human proteins lead to immune cross-reactivity.14 As a result, the immune system response to vaccine-derived pathogenic antigens can inadvertently target analogous human proteins, ultimately leading to the onset of various autoimmune conditions.25 UV is considered a consequence of a type III hypersensitivity reaction, in which antigen–antibody complexes are deposited in the vascular lumina, triggering complement activation and neutrophil infiltration.1 Therefore, in the present case, considering the elevated aCL levels, molecular mimicry might have produced vaccine-derived pathogenic antigen antibodies, leading to the development of UV, manifested by vascular destruction and inflammatory cellular infiltration. Differences in the degree of immune complex deposition, complement activation, and molecular mimicry among vaccines may contribute to variations in the susceptibility to vasculitis following vaccination, as seen with influenza and COVID-19 vaccines.
In this review, no significant differences were observed in the data of P-values, which may be attributed to the small number of cases. Although cases of UV following vaccination are rare, one case was severe enough to require treatment with steroid pulses due to HLH; thus, recognizing the potential for developing UV following vaccination is crucial. Accumulation of further cases and research is required to investigate the clinical course and underlying mechanisms of vaccine-induced UV.
Acknowledgments
We thank Editage for providing English language editing services for our manuscript.
Funding Statement
There is no funding to report.
Data Sharing Statement
All statistical data are presented in Table 1, showing 13 examined cases. The P-values were calculated using the independent two-sample t-test. Additional data concerning this article may be requested from the corresponding author for reasonable reasons.
Informed Consent Statement
Written informed consent was obtained from the patient for the publication of this paper. Approval to publish the case details was not required in our institution.
Disclosure
The authors declare no conflicts of interest related to this article.
References
- 1.Kolkhir P, Grakhova M, Bonnekoh H, Krause K, Maurer M. Treatment of urticarial vasculitis: a systematic review. J Allergy Clin Immunol. 2019;143(2):458–466. doi: 10.1016/j.jaci.2018.09.007 [DOI] [PubMed] [Google Scholar]
- 2.Misery L, Combemale P. BCG-vaccine-induced lupus vulgaris and urticarial vasculitis. Dermatology. 1993;186(4):274. doi: 10.1159/000247372 [DOI] [PubMed] [Google Scholar]
- 3.Velasco-Tamariz V, Prieto-Barrios M, Tous-Romero F, Palencia-Pérez SI, Postigo-Llorente C. Urticarial vasculitis after meningococcal serogroup B vaccine in a 6-year-old girl. Pediatr Dermatol. 2018;35(1):e64–e65. doi: 10.1111/pde.13339 [DOI] [PubMed] [Google Scholar]
- 4.Hughes R, Lacour JP, Baldin B, Reverte M, Ortonne JP, Passeron T. Urticarial vasculitis secondary to H1N1 vaccination. Acta Derm Venereol. 2010;90(6):651–652. doi: 10.2340/00015555-0950 [DOI] [PubMed] [Google Scholar]
- 5.Ono H, Yamaguchi R, Shimizu A. Urticarial vasculitis after COVID-19 vaccination: a case report and literature review. Dermatol Ther. 2022;35(8):e15613. doi: 10.1111/dth.15613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baraldi C, Boling LB, Patrizi A, et al. Unique cases of urticarial skin eruptions after COVID-19 vaccination. Am J Dermatopathol. 2022;44(3):198–200. doi: 10.1097/DAD.0000000000002036 [DOI] [PubMed] [Google Scholar]
- 7.Nazzaro G, Maronese CA. Urticarial vasculitis following mRNA anti-COVID-19 vaccine. Dermatol Ther. 2022;35(3):e15282. doi: 10.1111/dth.15282 [DOI] [PubMed] [Google Scholar]
- 8.Dash S, Behera B, Sethy M, Mishra J, Garg S. COVID-19 vaccine-induced urticarial vasculitis. Dermatol Ther. 2021;34(5):e15093. doi: 10.1111/dth.15093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daldoul M, Korbi M, Bellalah A, Ben Fadhel N, Belhadjali H, Zili J. Urticarial vasculitis triggered by SARS-CoV-2 vaccine (mRNA vaccine). J Eur Acad Dermatol Venereol. 2022;36(10):e743–e744. doi: 10.1111/jdv.18253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larson V, Seidenberg R, Caplan A, Brinster NK, Meehan SA, Kim RH. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID-19 vaccination. J Cutan Pathol. 2022;49(1):34–41. doi: 10.1111/cup.14104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tihy M, Menzinger S, André R, Laffitte E, Toutous-Trellu L, Kaya G. Clinicopathological features of cutaneous reactions after mRNA-based COVID-19 vaccines. J Eur Acad Dermatol Venereol. 2021;35(12):2456–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmes GA, Desai M, Limone B, et al. A case series of cutaneous COVID-19 vaccine reactions at Loma Linda University Department of Dermatology. JAAD Case Rep. 2021;16:53–57. doi: 10.1016/j.jdcr.2021.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwamura N, Eguchi K, Koga T, et al. Hypocomplementemic urticarial vasculitis case with hemophagocytic lymphohistiocytosis following SARS-CoV-2 mRNA vaccination. Immunol Med. 2023;46(2):97–107. doi: 10.1080/25785826.2023.2193286 [DOI] [PubMed] [Google Scholar]
- 14.Watanabe T. Vasculitis following influenza vaccination: a review of the literature. Curr Rheumatol Rev. 2017;13(3):188–196. doi: 10.2174/1573397113666170517155443 [DOI] [PubMed] [Google Scholar]
- 15.Sanker V, Mylavarapu M, Gupta P, Syed N, Shah M, Dondapati VVK. Post COVID-19 vaccination medium vessel vasculitis: a systematic review of case reports. Infection. 2024;52(4):1207–1213. doi: 10.1007/s15010-024-02217-w [DOI] [PubMed] [Google Scholar]
- 16.Bulva J, Simon R. Hypocomplementemic urticarial vasculitis (HUV) and hypocomplementemic urticarial vasculitis syndrome (HUVS); Background, pathogenesis, diagnosis, laboratory testing, management and treatment. J Autoimmun Res. 2017;4(3):1028. [Google Scholar]
- 17.Kulthanan K, Cheepsomsong M, Jiamton S. Urticarial vasculitis: etiologies and clinical course. Asian Pac J Allergy Immunol. 2009;27(2–3):95–102. [PubMed] [Google Scholar]
- 18.El-Shamy A, Branch AD, Schiano TD, Gorevic PD. The complement system and C1q in chronic hepatitis C virus infection and mixed cryoglobulinemia. Front Immunol. 2018;9:1001. doi: 10.3389/fimmu.2018.01001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nasiri S, Dadkhahfar S, Abasifar H, Mortazavi N, Gheisari M. Urticarial vasculitis in a COVID-19 recovered patient. Int J Dermatol. 2020;59(10):1285–1286. doi: 10.1111/ijd.15112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greer JM, Longley S, Edwards NL, Elfenbein GJ, Panush RS. Vasculitis associated with malignancy. Experience with 13 patients and literature review. Medicine. 1988;67(4):220–230. doi: 10.1097/00005792-198807000-00003 [DOI] [PubMed] [Google Scholar]
- 21.Younis AA. Urticarial vasculitis as an initial manifestation of colonic carcinoma: a case report and review of the literature. Reumatismo. 2018;70(4):259–263. doi: 10.4081/reumatismo.2018.1052 [DOI] [PubMed] [Google Scholar]
- 22.Maronese CA, Zelin E, Avallone G, et al. Cutaneous vasculitis and vasculopathy in the era of COVID-19 pandemic. Front Med Lausanne. 2022;9:996288. doi: 10.3389/fmed.2022.996288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y, Kang J, Lee SG, Kim GT. COVID-19 vaccination-related small vessel vasculitis with multiorgan involvement. Z Rheumatol. 2022;81(6):509–512. doi: 10.1007/s00393-022-01159-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agmon-Levin N, Paz Z, Israeli E, Shoenfeld Y. Vaccines and autoimmunity. Nat Rev Rheumatol. 2009;5(11):648–652. doi: 10.1038/nrrheum.2009.196 [DOI] [PubMed] [Google Scholar]
- 25.Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15(6):586–594. doi: 10.1038/cmi.2017.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All statistical data are presented in Table 1, showing 13 examined cases. The P-values were calculated using the independent two-sample t-test. Additional data concerning this article may be requested from the corresponding author for reasonable reasons.