Abstract
Despite significant immune recovery with potent highly active antiretroviral therapy (HAART), eradication of human immunodeficiency virus (HIV) from the bodies of infected individuals represents a challenge. We hypothesized that an inadequate or inappropriate signal in virus-specific antigen presentation might contribute to the persistent failure to mount efficient anti-HIV immunity in most HIV-infected individuals. Here, we conducted an in vitro study with untreated (n = 10) and HAART-treated (n = 20) HIV type 1 (HIV-1) patients which showed that pulsing of monocyte-derived dendritic cells (DC) with aldrithiol-2-inactivated autologous virus resulted in the expansion of virus-specific CD8+ T cells which were capable of killing HIV-1-infected cells and eradicating the virus from cultured patient peripheral blood mononuclear cells independently of the disease stages and HAART response statuses of the patients. This in vitro anti-HIV effect was further enhanced by the HIV protease inhibitor indinavir (at a nonantiviral concentration), which has been shown previously to be able to up-regulate directly patient T-cell proliferation following immune stimulation. However, following a 2-day treatment with culture supernatant derived from immune-activated T cells (which mimics an in vivo environment of HIV-disseminated and immune-activated lymphoid tissues), DC lost their capacity to present de novo inactivated-virus-derived antigens. These findings provide important information for understanding the establishment of chronic HIV infection and indicate a perspective for clinical use of DC-based therapeutic vaccines against HIV.
Although the introduction of highly active antiretroviral therapy (HAART) including at least one human immunodeficiency virus (HIV) protease inhibitor (PI) allows dramatic decreases in plasma HIV RNA loads and significant recovery of the T-cell compartment in the majority of patients (2, 5), HIV eradication by prolonged HAART treatment appears to be unlikely due to the persistence of a cellular reservoir of infectious HIV (4, 7, 13). On the other hand, HAART-treated patients fail to mount anti-HIV immunity, as evidenced by the rapid viral rebound observed in almost all patients after discontinuation of HAART (6, 10, 11) or by a maintained high viral load in patients experiencing a virologic failure despite significant T-cell recovery (8, 15–19). We hypothesized that the persistent failure in mounting anti-HIV immunity in untreated or HAART-treated patients might be caused by an inadequate or inappropriate signal in virus-specific antigen presentation, possibly resulting from a disturbance in the generation and/or function of antigen-presenting cells (APCs) in chronically immune-activated lymphoid organs or tissues of HIV-infected patients.
We report here that monocyte-derived dendritic cells (DC) (the most potent APCs capable of priming major histocompatibility complex class I- and II-restricted antigen-specific T-cell responses) pulsed with inactivated autologous virus can result in the expansion of virus-specific CD8+ T cells capable of killing HIV-infected cells and suppressing HIV type 1 (HIV-1) replication. Since we previously observed that HIV PIs even at nonantiviral doses could restore proliferative responses of patient T cells (19), we hypothesized that PIs might be helpful in enhancing the generation of virus-specific effector cells. Indeed, a combination of inactivated-virus-pulsed DC and the HIV PI indinavir (at a nonantiviral concentration) resulted in an ample expansion of virus-specific CD8+ T cells which was sufficient to eradicate HIV-1 in peripheral blood mononuclear cells (PBMC) taken from HIV-infected patients.
MATERIALS AND METHODS
Study populations.
A total of 30 HIV-1-infected adults were selected from our outpatient clinic. Ten of them had never received antiretroviral drugs, had a CD4 cell count of between 200 and 600 cells/μl, and had a plasma viral load of between 4 and 6 log10 HIV RNA equivalent (eq) copies/ml (Quantiplex HIV-1 3.0; Bayer, Emeryville, Calif.). Ten other patients had received prolonged HAART (>3 years), had a CD4 cell count of between 300 and 700 cells/μl, and had a sustained undetectable plasma HIV-1 RNA level (<50 eq copies/ml; Quantiplex HIV-1 3.0) for at least 30 months. The last 10 patients had received prolonged HAART and had a CD4 cell count of between 300 and 700 cells/μl but had maintained a plasma viral load of between 4 and 6 log10 HIV RNA eq copies/ml (Table 1). Informed consent was obtained from all participants.
TABLE 1.
Characteristics of 30 HIV-1-infected adults (10 who were naive for antiviral treatment, 10 HAART-treated virologic responders, and 10 HAART-treated virologic nonresponders)
| Patient no. | Sexa | Antiretroviral
regimenb
|
Mo of HAART | CD4 cell count (cells/μl) | Plasma viral load (log10 eq copies/ml) | |
|---|---|---|---|---|---|---|
| Initial | Modification (moc) | |||||
| 1 | M | Naive | 247 | 4.85 | ||
| 2 | M | Naive | 262 | 4.62 | ||
| 3 | F | Naive | 302 | 4.24 | ||
| 4 | M | Naive | 374 | 5.14 | ||
| 5 | M | Naive | 395 | 4.88 | ||
| 6 | M | Naive | 415 | 4.21 | ||
| 7 | F | Naive | 421 | 4.38 | ||
| 8 | M | Naive | 467 | 5.22 | ||
| 9 | F | Naive | 541 | 4.39 | ||
| 10 | M | Naive | 557 | 5.01 | ||
| 11 | M | 3TC-d4T-IDV | None | 40 | 332 | <1.70 |
| 12 | M | 3TC-d4T-IDV | None | 42 | 426 | <1.70 |
| 13 | F | 3TC-d4T-IDV | None | 40 | 441 | <1.70 |
| 14 | M | 3TC-d4T-IDV | None | 38 | 449 | <1.70 |
| 15 | M | 3TC-d4T-IDV | None | 41 | 483 | <1.70 |
| 16 | F | 3TC-d4T-IDV | 3TC-ZDV-IDV (1) | 38 | 559 | <1.70 |
| 17 | M | 3TC-d4T-IDV | 3TC-ZDV-IDV (5) | 43 | 566 | <1.70 |
| 18 | F | 3TC-d4T-IDV | None | 38 | 605 | <1.70 |
| 19 | M | 3TC-d4T-IDV | None | 40 | 612 | <1.70 |
| 20 | M | 3TC-d4T-IDV | None | 40 | 696 | <1.70 |
| 21 | M | 3TC-d4T-IDV | None | 39 | 311 | 5.44 |
| 22 | M | 3TC-d4T-IDV | 3TC-ZDV-IDV (4) | 41 | 385 | 4.26 |
| 23 | M | 3TC-d4T-IDV | 3TC-d4T-RTV-SQV (6) | 43 | 397 | 4.79 |
| 24 | M | 3TC-d4T-IDV | None | 45 | 401 | 4.28 |
| 25 | M | 3TC-d4T-IDV | 3TC-d4T-RTV-SQV (8) | 42 | 409 | 5.87 |
| 26 | M | 3TC-d4T-IDV | 3TC-d4T-RTV-SQV (8) | 42 | 523 | 4.67 |
| 27 | M | 3TC-d4T-IDV | None | 41 | 569 | 5.63 |
| 28 | F | 3TC-d4T-IDV | 3TC-d4T-RTV-SQV (7) | 44 | 646 | 5.18 |
| 29 | M | 3TC-d4T-IDV | 3TC-ZDV-IDV (5) | 42 | 654 | 4.47 |
| 30 | M | 3TC-d4T-IDV | 3TC-ZDV-IDV (4) | 41 | 672 | 5.15 |
M, male; F, female.
ZDV, zidovudine; 3TC, lamivudine; d4T, stavudine; IDV, indinavir; RTV, ritonavir; SQV, saquinavir.
Indicates the time after which the initial antiretroviral regimen was modified because of side effects of IDV or d4T.
DC generation.
PBMC were isolated from fresh whole citrate-treated blood obtained by venipuncture from HIV-1-infected patients using Ficoll-Hypaque (Eurobio, Les Ulis, France) density gradient centrifugation. After four washes with Hanks' balanced salt solution (Hanks' buffer), monocytes were enriched by negative immunoselection using a commercial kit (Dynal, Great Neck, N.Y.). Isolated monocytes which were 90% pure (CD14+ cells) were plated at 106/ml of serum-free AIM-V medium (Life Technologies, Grand Island, N.Y.) in flasks (Nunc, Roskilde, Denmark). Cells were then cultured for 5 days in AIM-V medium supplemented with 250 ng of granulocyte-macrophage colony-stimulating factor (GM-CSF) (R&D Systems, Minneapolis, Minn.) per ml and 100 ng of interleukin-4 (IL-4) (R&D Systems) per ml. Following this culture period, nonadherent cells were determined to be >90% immature DC based on their morphology and their expression of CD1a, CD11c, CD40, CD80, CD86, CD4, and HLA-DR assessed by direct immunofluorescence flow cytometry (see below).
Induction of virus-specific T-cell response.
Viruses were isolated by coculture of phytohemagglutinin (Sigma, St. Louis, Mo.)-stimulated HIV-negative donor PBMC with patient CD4+ T cells (3). Viral isolates were inactivated with 250 μM aldrithiol-2 (AT-2) (Sigma) for 1 h at 37°C in order to preserve the intact native conformation and fusogenic activity of HIV Env protein gp120 (24). Immature DC were pulsed with AT-2-inactivated autologous isolate (50 ng of p24) for 2 h at 37°C. After three washes with Hanks' buffer, virus-pulsed DC were cultured in AIM-V medium supplemented with 100 ng of GM-CSF per ml, 5 ng of IL-4 per ml, 50 ng of tumor necrosis factor alpha (TNF-α) (R&D Systems) per ml, and 1,000 U of alpha interferon 2b (IFN-α-2b) (Schering-Plough, Brinny, Ireland) per ml for 3 days. In certain experiments, immature DC were treated for 2 h before or after pulsing with inactivated autologous virus with the culture supernatant collected from anti-CD3/CD28 antibody-stimulated normal donor T cells. Peripheral blood lymphocytes (PBL) were freshly prepared from nonadherent PBMC. PBL (3 × 106) were stimulated on day 0 and restimulated on day 7 with virus-pulsed autologous DC (at a stimulator/responder ratio of 1:3) in the absence or the presence of a nonantiviral concentration of PI (10 nM indinavir; Merck Research Laboratories, West Point, Pa.). PBL (106/ml) were cultured in RPMI 1640 containing 10% heat-inactivated fetal calf serum (Eurobio), nonessential amino acids, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 2 mM l-glutamine, 1 mM sodium pyruvate, and 10 mM HEPES buffer (referred as complete medium). Exogenous IL-2 (25 IU/ml; Roche Molecular Biomedicals, Mannheim, Germany) was added every 3 to 4 days.
Proliferation assay.
At day 14 of culture, 105 PBL were added to AT-2-inactivated virus-pulsed DC at stimulator/responder ratios of 1:3, 1:10, 1:30, and 1:100 in quadruplicate wells in 96-well round-bottomed microtiter plates (Nunc). After 24 h, cells were pulsed with 0.037 MBq (1 μCi) of [methyl-3H]thymidine (Amersham Pharmacia Biotech, Aylesbury, United Kingdom) per well, and incubation was continued for an additional 18 h. Cells were harvested on glass fiber filters by an automated multisample harvester; filters were then put in tubes with liquid scintillation fluid, and radioactivity was counted on a beta-scintillation counter. Net counts per minute were calculated by subtracting the counts per minute of control PBL which were cultured with unloaded autologous DC for 14 days and then exposed to unloaded autologous DC for an additional 24 h prior to addition of [methyl-3H]thymidine.
Cytotoxicity assay.
Autologous Epstein-Barr virus-transformed B-lymphoblastoid cell lines (B-LCL) were infected with recombinant vaccinia virus containing a gag gene of HIV-1 (25). Vaccinia virus-infected B-LCL were incubated with 100 μCi of 51Cr in a total volume of 200 μl for 2 h at 37°C before use as targets. The effector cells were derived from HIV-infected patient PBL cultured with inactivated-virus-pulsed autologous DC for 14 days as described above. Targets were washed and seeded at 5 × 103 cells/well at an effector/target ratio of 10:1 in quadruplicate and assayed for cytotoxicity in a standard chromium release assay. The percentage of specific lysis was calculated by subtracting the specific 51Cr release of wild-type vaccinia virus-infected targets (controls) (25). Anti-CD4 and anti-CD8 monoclonal antibodies (clones RPA-T4 and RPA-T8; PharMingen) were used for blocking analysis.
Anti-HIV activity assay.
The direct anti-HIV activity of PBL expanded with virus-pulsed DC with or without PI was evaluated with autologous patient PBMC using our recently described assay system (26) with slight modifications. Briefly, patient PBMC were superinfected with 100 50% tissue culture infective doses of autologous viral isolate. After three washes with Hanks' buffer, cells were stimulated with 0.5 μg of anti-CD3 monoclonal antibodies (Becton Dickinson France, Le Pont-de-Claix, France) per ml plus 100 ng of anti-CD28 monoclonal antibodies (PharMingen, Los Angeles, Calif.) per ml for 24 h. After additional washes, PBL expanded with virus-pulsed DC with or without PI were added to superinfected patient PBMC at an effector/target ratio of 1:1 (the lowest ratio for demonstrating a significant antiviral activity in most patient T cells). The coculture was maintained in complete medium supplemented with 20 IU of IL-2 per ml. Half of the culture supernatant was replaced with fresh medium every 2 to 3 days. At day 15, cells were collected for measuring the proviral HIV DNA load and supernatants were harvested for measuring the cell-free HIV RNA concentration. Anti-CD4 and anti-CD8 monoclonal antibodies (PharMingen) were used for blocking analysis.
Viral quantitation assay.
Culture supernatant viral concentrations were determined by measuring cell-free HIV RNA by multiple-primer-induced overlapping amplification assay with a detection threshold of 10 eq copies/ml (21). Proviral DNA was determined by a quantitative PCR assay (22) with several recent modifications. Briefly, 2 × 106 cells were used for cellular DNA purification with a commercial kit (QIAamp; Qiagen, Hilden, Germany). Purified DNA equivalent to that of 106 cells was used for HIV-specific amplification as described previously (22). Four standard dilutions (10, 100, 1,000, and 10,000 copies in HIV-negative donor PBMC DNA equivalent to that of 106 cells) of HIV-1 DNA plasmid (pBH10-R3) were amplified in parallel as external standards. At the same time, each standard and sample DNA (amount equivalent to that of 104 cells) was amplified in parallel as calibrators (DNA input control) using the primer pair 5′-GCGGGAAATCGTGCGTGACATT-3′ (sense; nucleotides [nt] 2280 to 2301 of the β-actin genomic sequence HUMACCYBB; GenBank accession number M10277) and 5′-GATGGAGTTGAAGGTAGTTTCGTG-3′ (antisense; nt 2606 to 2583 of HUMACCYBB). After amplification, 10 μl of HIV or β-actin reaction product of each sample was distributed into streptavidin-coated 96-well microtiter plates (Roche Molecular Biochemicals) preincubated with biotin-labeled probe specific for HIV-1 (21) or β-actin (5′-TGTGCTGTGGAAGCTAAGTCCTGCCCTCATTT-5′; nt 2522 to 2553 of HUMACCYBB). Quantitation was performed by a hybridization-linked enzyme-linked immunosorbent assay as previously described (21). The sensitivity of the assay reached 5 HIV copies per 106 cells, with a quantitative range of 5 to 105 HIV DNA copies per 106 cells and intra- and interassay variabilities of less than 0.05 and 0.08 log10 copies, respectively.
Flow cytometry.
Counts of CD4+ and CD8+ T cells (CD3+ CD4+ and CD3+ CD8+), monocytes (CD14+), and DC (CD1a+, CD11c+, CD40+, CD80+, CD83+, CD86+, CD4+, and HLA-DR+) were assessed by flow cytometry analysis (FACScan; Becton Dickinson, San Jose, Calif.) using a panel of direct fluorescence-labeled monoclonal antibodies: CD3peridinin chloropy II protein (PerCP), CD4-fluorescein isothiocyanate (CD4-FITC), CD8-phycoerythrin (CD8-PE), and CD14-PE (Becton Dickinson) and CD1a-FITC, CD11c-PE, CD40-FITC, CD80-PE, CD83-FITC, CD86-FITC, and HLA-DR–PE (PharMingen).
Statistical analysis.
Unpaired data for different groups of patients or paired data for different in vitro treatments were compared by the Mann-Whitney test or the Wilcoxon test, respectively.
RESULTS
Proliferation of patient T cells following stimulation with virus-pulsed autologous DC.
Viruses were isolated from 10 untreated asymptomatic HIV-seropositive patients (CD4 cell count, 200 to 600 cells/μl; plasma HIV RNA load, 4 to 6 log10 eq copies/ml) and 20 patients treated by prolonged HAART (>3 years) (CD4 cell count, 300 to 700 cells/μl; 10 patients with virologic response [plasma HIV RNA load, <50 log10 eq copies/ml] and 10 patients with virologic resistance [plasma HIV RNA load, 4 to 6 log10 eq copies/ml]) (Table 1). To mimic the antigen capture by DC in peripheral tissues, immature DC (i.e., competent in antigen capture) generated by culturing patient blood monocytes with GM-CSF and IL-4 for 5 days were pulsed with autologous viral isolates inactivated by AT-2, which preserves the intact native conformation and fusogenic activity of HIV Env protein (24). To model the presentation of antigens in lymphoid tissue, virus-pulsed DC were matured in the presence of TNF-α and IFN-α for an additional 3 days to maximize their T-cell-stimulatory activity (27, 29). Matured virus-pulsed DC were then used to stimulate autologous PBL at a stimulator/responder ratio of 1:3 in the absence or presence of PI at a nonantiviral concentration (10 nM indinavir). By day 7 of coculture, PBL were restimulated with the same virus-pulsed DC for an additional 7 days. At day 14, proliferation was measured by incubating PBL with virus-pulsed DC at stimulator/responder ratios of 1:3 to 1:100.
Inactivated virus-pulsed DC stimulated [methyl-3H]thymidine incorporation by autologous PBL from both untreated and HAART-treated patients independently of their blood CD4 cell counts and plasma viral loads (P > 0.5), while this DC-mediated T-cell proliferation was significantly enhanced by the presence of PI (at a nonantiviral dose) (P < 0.001) (Fig. 1A). Phenotype analysis by flow cytometry showed that both CD4+ and CD8+ T cells were equally stimulated by virus-pulsed DC in the presence or absence of PI (Fig. 1B).
FIG. 1.
Proliferation of patient T cells following stimulation with inactivated-virus-pulsed autologous DC in the absence or presence of HIV PI (indinavir, 10 nM). (A) Mean (± SD) [3H]thymidine incorporation in T cells from untreated patients in the absence (□) or presence (▪) of PI, in T cells from HAART-treated plasma viral load responders in the absence (▵) or presence (▴) of PI, and in T cells from plasma viral load nonresponders in the absence (○) or presence (●) of PI. (B) Mean (± SD) relative CD4/CD8 ratio in T cells from untreated patients (□), plasma viral load responders (▨), and plasma viral load nonresponders (▩). The baseline CD4/CD8 ratio in the absence of stimulation was normalized to 1. The baseline ranges of the CD3+ CD4+ phenotype in untreated patients, plasma viral load responders, and plasma viral load nonresponders were 15 to 32%, 24 to 53%, and 21 to 41%, respectively.
HIV-1 gag-specific CTL activity following stimulation with virus-pulsed autologous DC.
We next evaluated HIV-specific cytotoxic-T-lymphocyte (CTL) activity of autologous PBL expanded with inactivated-virus-pulsed DC using as target cells autologous B-LCL infected by recombinant vaccinia virus containing a gag gene of HIV-1 as described previously (25). HIV gag-specific B-LCL killing was up-regulated by autologous PBL stimulated with virus-pulsed DC (at an effector/target ratio of 10:1) in both untreated and HAART-treated patients independently of their blood CD4 cell counts and plasma viral loads (P > 0.4). Such a gag-specific CTL activity was significantly enhanced (P < 0.01) by the presence of indinavir (10 nM) (Fig. 2A). Similar enhancement by PI was also observed with PBL stimulated with virus-pulsed DC alone when the effector/target ratio was increased to 50:1 (data not shown). This CTL-mediated B-LCL killing was executed exclusively by CD8+ T cells, since cell killing was blocked by the addition of anti-CD8 antibodies whereas it was unaffected by the addition of anti-CD4 antibodies (Fig. 2B).
FIG. 2.
HIV-1 gag-specific CTL activity in patient T cells expanded by inactivated-virus-pulsed autologous DC in the absence or presence of indinavir. (A) Mean (± SD) percent specific lysis (at an effector/target ratio of 10:1) of autologous B-LCL targets (infected with recombinant vaccinia virus containing a HIV-1 gag gene) by T cells stimulated with virus-pulsed DC with or without PI. T cells were from untreated patients (□), plasma viral load responders to HAART (▨), and plasma viral load nonresponders to HAART (▩). (B) Mean (± SD) percent specific lysis from all patients in the absence of antibodies (▤) or in the presence of blocking antibodies against CD4 (░⃞) or CD8 (▪). The background percent gag-specific lysis using unloaded DC-treated T cells was <10%.
Anti-HIV activity of patient T cells following stimulation with virus-pulsed autologous DC.
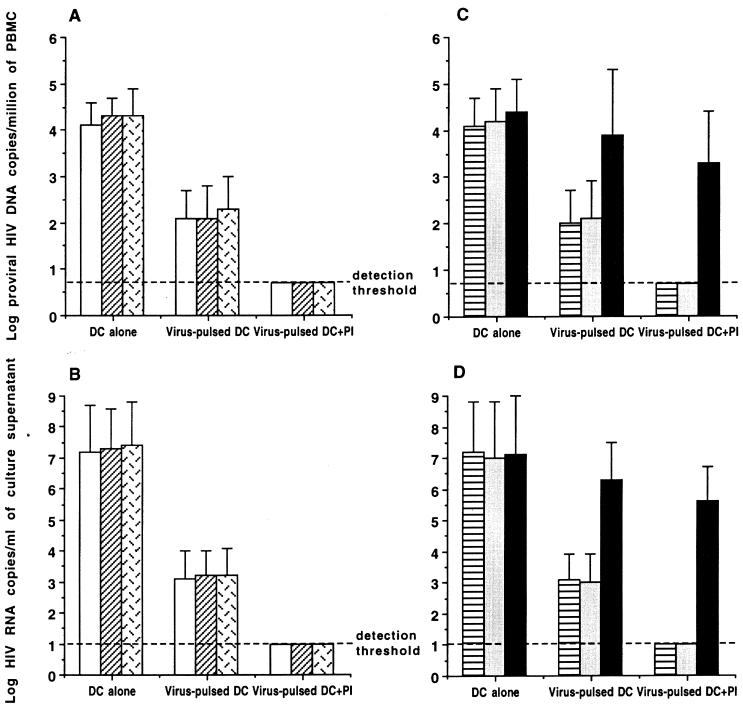
Having observed that virus-pulsed DC were capable of stimulating the proliferation and CTL activity of autologous T cells from both untreated and HAART-treated patients regardless of their CD4 cell count and level of HIV viremia, we then examined the direct antiviral activity of autologous T cells expanded by virus-pulsed DC in the presence or the absence of PI. To minimize the variation in the frequency of PBMC harboring infectious HIV among untreated and HAART-treated patients, all patient PBMC were superinfected with the same dose (100 50% tissue culture infective doses) of autologous isolates as described previously (26). The total HIV proviral DNA concentrations (means ± standard deviations [SDs]) measured before and after 12 h of superinfection were 2.9 ± 0.5 (range, 2.1 to 3.8) and 4.1 ± 0.2 (range, 3.7 to 4.5) log10 copies per million PBMC, respectively. To mimic immune activation in lymphoid organs, superinfected patient PBMC were stimulated with anti-CD3 and anti-CD28 antibodies and then cocultured with autologous PBL expanded with virus-pulsed DC with or without PI at an effector/target ratio of 1:1. Unloaded DC-treated T cells were used in parallel as a control. Cell-associated proviral DNA and supernatant viral RNA concentrations were measured by previously described quantitative assays (21, 22). The proviral DNA load (copies per 106 cells) was decreased by 2 log10 (P < 0.001) in autologous T cells expanded with virus-pulsed DC without PI, whereas it was decreased by >3 log10 (P < 0.001) (i.e., below the detection threshold of 5 copies/106 cells) in T cells expanded with virus-pulsed DC with PI. On the other hand, HIV RNA in the supernatants of the same cultures was decreased by 4 log10 (P < 0.001) and >6 log10 (i.e., below the detection threshold of 10 copies/ml) in these two situations (Fig. 3A and B). Optimum suppressions of proviral DNA and supernatant RNA to levels below the detection threshold were also obtained by patient T cells stimulated with virus-pulsed DC alone when the effector/target ratio was increased to 5:1 (data not shown). Addition of anti-CD8 antibodies abolished these antiviral activities, while addition of anti-CD4 antibodies did not have any effect on the clearance of proviral HIV DNA or supernatant HIV RNA (Fig. 3C and D). Again, the antiviral activity of autologous CD8+ T cells expanded by virus-pulsed DC or by virus-pulsed DC plus PI (indinavir, 10 nM) was achieved equally in untreated and HAART-treated patients whatever their CD4 cell counts and viral load levels (P > 0.3). The cultures showing undetectable proviral DNA and supernatant HIV-1 RNA were further cocultured with phytohemagglutinin-stimulated normal donor PBMC for 30 days. No infectious virus was recovered from any of these cultured patient T cells that had demonstrated undetectable proviral DNA and supernatant viral RNA.
FIG. 3.
Quantitative analysis of anti-HIV activity of patient T cells stimulated with inactivated-virus-pulsed autologous DC in the absence or presence of PI. Each result is the mean (± SD) number of proviral HIV DNA copies/106 cells (A and C) or the mean (± SD) number of supernatant HIV RNA copies per milliliter (B and D) in the coculture of autologous virus-pulsed-DC-stimulated T cells and superinfected T cells from untreated patients (□), plasma viral load responders to HAART (▨), and plasma viral load nonresponders to HAART (▩) or from all patients in the absence of antibodies (▤) or the presence of blocking antibodies against CD4 (░⃞) or CD8 (▪).
DC functions following treatment with activated-T-cell-derived supernatant.
Since the immune-activated lymphoid organs and tissues are the major sites for HIV replication and dissemination, we questioned whether DC could uptake and process HIV and/or present HIV antigens to effector T cells in such an immune-activated environment. Immature DC were pretreated for 2 days with the culture supernatant derived from T cells stimulated with anti-CD3/CD28 antibodies for 7 days, and then proliferation, CTL, and antiviral activities were analyzed as described above. When pretreated with activated-T-cell supernatant before the virus pulse, patient DC lost their capacity to stimulate proliferation, gag-specific CTL response, and HIV-expressing cell killing of autologous T cells. However, these DC functions were preserved when the activated-T-cell supernatant was added to DC after pulsing with inactivated virus (Fig. 4). Flow cytometric analysis showed a supermaturation phenotype (up-regulated expression of CD40, CD80, CD83, CD86, and major histocompatibility complex class II) of DC following exposure to activated-T-cell supernatant.
FIG. 4.
Functions of DC following treatment with activated-T-cell supernatant. (A) Mean (± SD) [3H]thymidine incorporation in patient T cells stimulated with virus-pulsed DC (○) or DC pretreated with activated-T-cell supernatant before (▴) or after (▪) pulsing with inactivated autologous virus. (B) Mean (± SD) percent HIV gag-specific lysis (at an effector/target ratio of 10:1) of autologous B-LCL targets by patient T cells expanded with virus-pulsed DC (□) or DC pretreated with activated-T-cell supernatant before (▨) or after (▩) pulsing with inactivated autologous virus. (C) Mean (± SD) number of proviral HIV DNA copies/106 cells or supernatant HIV RNA copies per milliliter in the coculture of superinfected T cells with autologous T cells expanded with virus-pulsed DC (□) or DC pretreated with activated-T-cell supernatant before (▨) or after (▩) pulsing with inactivated autologous virus.
DISCUSSION
Our data provide the first evidence that a high frequency of PBMC harboring HIV can be eradicated in vitro by cultured patient T cells expanded with inactivated-virus-pulsed autologous DC. This potent antiviral activity of patient T cells stimulated with virus-pulsed DC is CD8 dependent and independent of the patient's disease stage and treatment status. Treatment of patient DC with activated-T-cell supernatant results in the loss of their integrated APC functions to present de novo viral antigens. These findings indicate that a disturbance in the presentation of viral antigens is most likely the cause of failure in mounting an efficient anti-HIV immunity in untreated HIV-seropositive individuals as well as in HAART-treated patients despite a significant improvement of T-cell reactivity (9, 14). The viral clearance obtained in vitro with autologous T cells expanded by inactivated-virus-pulsed DC opens the possibility of an in vivo restoration of anti-HIV immunity, which is readily developed in most cases shortly after infection (probably before virus dissemination into lymph notes) (12) but is progressively lost during the course of the infection (20, 26).
APC functions (including up-regulation of T-cell proliferation, CTL response, and anti-HIV activities) of patient DC are enhanced by a nonantiviral concentration of PI (indinavir). This is no longer surprising, since recent in vivo and in vitro studies by our group (18, 19) and others (1, 28) show that PIs exhibit direct up-regulatory effects on proliferation and down-regulatory effects on apoptosis of patient T cells following immune stimulation. Thus, a PI (at both antiviral and nonantiviral concentrations) could be used as a potent adjuvant for optimizing the virus-specific CTL response in individuals following either preventive or therapeutic vaccination.
Although the in vivo evolving HIV-1 variants that evade the antiviral immunity developed during early infection have been known for many years (23), the reason that the infected host fails to mount de novo mutant-virus-specific immunity remains unknown. In a chronically HIV-infected individual (i.e., one in whom the virus has already been disseminated into lymph notes), viral replication is directly linked to local activation of lymphoid tissues characterized by huge in situ expression and release of cytokines. Certain components of these lymphoid cytokines (such as IL-10 and IFN-β) are known to interfere with generation of immature DC, and others (such as IL-1β, IL-6, TNF-α, IFN-α, and IFN-γ, etc.) provoke DC maturation. Since supermatured DC lose their ability to process and present viral antigens (Fig. 4), it is conceivable that supermatured DC in immune-activated lymphoid tissues could not exert their APC function to process and present the evolving mutant antigens of viral variants. Such paralyzed DC in situ, in fact, could thus provide the prerequisite for establishing chronic HIV infection. However, our data demonstrate that such a defect in the generation of functional DC in HIV-infected patients can be overcome by DC-based vaccines generated in vitro from peripheral blood monocytes taken from infected patients.
It is interesting to observe that proviral DNA of patient PBMC can disappear (or become undetectable) when cocultured with autologous T cells pretreated with virus-pulsed DC with PI, suggesting that latent forms of HIV provirus might be rare in immune-activated lymphoid tissues. Our data suggest the possibility of eradicating the virus in vivo with a repeated vaccination regimen. Although HIV provirus might reside in quiescent T cells as a temporary viral reservoir escaping from recognition or killing by virus-specific effector cells (30), immune stimulation strategies such as IL-2-based therapy could help to activate quiescent T cells harboring HIV provirus, thereby exhausting such a temporary reservoir (3).
Taking these results together, it is clear that the complete eradication of HIV from the body of an infected individual represents a challenge. Reconstitution of anti-HIV immunity by potent DC-based therapeutic vaccines could be one of the keys to eventually curing HIV diseases. Controlled clinical trials are required to prove the utility of such an exciting and encouraging perspective.
ACKNOWLEDGMENTS
We thank M. Arlie, L. Cao, H. Gozard, J. Yuan, and Y. J. Zhao for excellent technical assistance; M. Gozard and G. Nasso for secretarial assistance; Merck Research Laboratories for providing the HIV PI (indinavir) used in this study; and all of our patients for their encouraging collaboration.
This work was supported by the Agence Nationale de Recherche sur le SIDA (ANRS), the Fondation pour la Recherche Médicale (SIDACTION), and the Association de Recherche sur les Maladies Tumorales et Virales (AREMAS).
REFERENCES
- 1.Bohler T, Debatin K M, Wintergerst U. T-cell apoptosis in HIV-1-infected individuals receiving highly active antiretroviral therapy. Blood. 2001;97:1898–1901. doi: 10.1182/blood.v97.6.1898a. [DOI] [PubMed] [Google Scholar]
- 2.Cavert W, Notermans D W, Staskus K, Wietgrefe S W, Zupancic M, Gebhard K, Henry K, Zhang Z Q, Mills R, McDade H, Schuwirth C M, Goudsmit J, Danner S A, Haase A T. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science. 1997;276:960–964. doi: 10.1126/science.276.5314.960. [DOI] [PubMed] [Google Scholar]
- 3.Chun T W, Engel D, Mizell S B, Hallahan C W, Fischette M, Park S, Davey R T, Jr, Dybul M, Kovacs J A, Metcalf J A, Mican J M, Berrey M M, Corey L, Lane H C, Fauci A S. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5:651–655. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 4.Chun T W, Stuyver L, Mizell S B, Ehler L A, Mican J A, Baseler M, Lloyd A L, Nowak M A, Fauci A S. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collier A C, Coombs R W, Schoenfeld D A, Bassett R L, Timpone J, Baruch A, Jones M, Facey K, Whitacre C, McAuliffe V J, Friedman H M, Merigan T C, Reichman R C, Hooper C, Corey L. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. AIDS Clinical Trials Group. N Engl J Med. 1996;334:1011–1017. doi: 10.1056/NEJM199604183341602. [DOI] [PubMed] [Google Scholar]
- 6.Davey R T, Jr, Bhat N, Yoder C, Chun T W, Metcalf J A, Dewar R, Natarajan V, Lempicki R A, Adelsberger J W, Miller K D, Kovacs J A, Polis M A, Walker R E, Falloon J, Masur H, Gee D, Baseler M, Dimitrov D S, Fauci A S, Lane H C. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA. 1999;96:15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finzi D, Blankson J, Siliciano J D, Margolick J B, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn T C, Chaisson R E, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano R F. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 8.Grabar S, Moing V L, Goujard C, Leport C, Kazatchkine M D, Costagliola D, Weiss L. Clinical outcome of patients with HIV-1 infection according to immunologic and virologic response after 6 months of highly active antiretroviral therapy. Ann Intern Med. 2000;133:401–410. doi: 10.7326/0003-4819-133-6-200009190-00007. [DOI] [PubMed] [Google Scholar]
- 9.Gray C M, Lawrence J, Schapiro J M, Altman J D, Winters M A, Crompton M, Loi M, Kundu S K, Davis M M, Merigan T C. Frequency of class I HLA-restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy (HAART) J Immunol. 1999;162:1780–1788. [PubMed] [Google Scholar]
- 10.Harrigan P R, Whaley M, Montaner J S. Rate of HIV-1 RNA rebound upon stopping antiretroviral therapy. AIDS. 1999;13:F59–F62. doi: 10.1097/00002030-199905280-00001. [DOI] [PubMed] [Google Scholar]
- 11.Hatano H, Vogel S, Yoder C, Metcalf J A, Dewar R, Davey R T, Jr, Polis M A. Pre-HAART HIV burden approximates post-HAART viral levels following interruption of therapy in patients with sustained viral suppression. AIDS. 2000;14:1357–1363. doi: 10.1097/00002030-200007070-00008. [DOI] [PubMed] [Google Scholar]
- 12.Haynes B F, Pantaleo G, Fauci A S. Toward an understanding of the correlates of protective immunity to HIV infection. Science. 1996;271:324–328. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 13.Ibanez A, Puig T, Elias J, Clotet B, Ruiz L, Martinez M A. Quantification of integrated and total HIV-1 DNA after long-term highly active antiretroviral therapy in HIV-1-infected patients. AIDS. 1999;13:1045–1049. doi: 10.1097/00002030-199906180-00007. [DOI] [PubMed] [Google Scholar]
- 14.Kalams S A, Goulder P J, Shea A K, Jones N G, Trocha A K, Ogg G S, Walker B D. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufmann D, Pantaleo G, Sudre P, Telenti A. CD4-cell count in HIV-1-infected individuals remaining viraemic with highly active antiretroviral therapy (HAART). Swiss HIV Cohort Study. Lancet. 1998;351:723–724. doi: 10.1016/s0140-6736(98)24010-4. [DOI] [PubMed] [Google Scholar]
- 16.Ledergerber B, Egger M, Opravil M, Telenti A, Hirschel B, Battegay M, Vernazza P, Sudre P, Flepp M, Furrer H, Francioli P, Weber R. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Swiss HIV Cohort Study. Lancet. 1999;353:863–868. doi: 10.1016/s0140-6736(99)01122-8. [DOI] [PubMed] [Google Scholar]
- 17.Levitz S M. Improvement in CD4+ cell counts despite persistently detectable HIV load. N Engl J Med. 1998;338:1074–1075. doi: 10.1056/NEJM199804093381517. [DOI] [PubMed] [Google Scholar]
- 18.Lu W, Andrieu J M. T-cell recovery in HIV-infected patients experiencing virologic failure under highly active antiretroviral therapy. Blood. 2001;97:1900–1901. [Google Scholar]
- 19.Lu W, Andrieu J M. HIV protease inhibitors restore impaired T-cell proliferative response in vivo and in vitro: a viral-suppression-independent mechanism. Blood. 2000;96:250–258. [PubMed] [Google Scholar]
- 20.Lu W, Andrieu J M. Prospective views of HIV pathology. Clues for therapeutic strategies. Adv Exp Med Biol. 1995;374:235–242. doi: 10.1007/978-1-4615-1995-9_21. [DOI] [PubMed] [Google Scholar]
- 21.Lu W, Cao L, Ty L, Arlie M, Andrieu J M. Equivalent amplification of intrinsically variable nucleic acid sequences by multiple-primer-induced overlapping amplification assay: applications for universal detection and quantitation. Nat Med. 1999;5:1081–1085. doi: 10.1038/12520. [DOI] [PubMed] [Google Scholar]
- 22.Lu W, Han D S, Yuan J, Andrieu J M. Multi-target PCR analysis by capillary electrophoresis and laser-induced fluorescence. Nature. 1994;368:269–271. doi: 10.1038/368269a0. [DOI] [PubMed] [Google Scholar]
- 23.McMichael A J, Phillips R E. Escape of human immunodeficiency virus from immune control. Annu Rev Immunol. 1997;15:271–296. doi: 10.1146/annurev.immunol.15.1.271. [DOI] [PubMed] [Google Scholar]
- 24.Rossio J L, Esser M T, Suryanarayana K, Schneider D K, Bess J W, Jr, Vasquez G M, Wiltrout T A, Chertova E, Grimes M K, Sattentau Q, Arthur L O, Henderson L E, Lifson J D. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salerno-Goncalves R, Lu W, Achour A, Andrieu J M. Lysis of CD4+ T cells expressing HIV-1 gag peptides by gag-specific CD8+ cytotoxic T cells. Immunol Lett. 1998;64:71–77. doi: 10.1016/s0165-2478(98)00084-4. [DOI] [PubMed] [Google Scholar]
- 26.Salerno-Goncalves R, Lu W, Andrieu J M. Quantitative analysis of the antiviral activity of CD8+ T cells from human immunodeficiency virus-positive asymptomatic patients with different rates of CD4+T-cell decrease. J Virol. 2000;74:6648–6651. doi: 10.1128/jvi.74.14.6648-6651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santini S M, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, Belardelli F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–1788. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sloand E M, Kumar P N, Kim S, Chaudhuri A, Weichold F F, Young N S. Human immunodeficiency virus type 1 protease inhibitor modulates activation of peripheral blood CD4(+) T cells and decreases their susceptibility to apoptosis in vitro and in vivo. Blood. 1999;94:1021–1027. [PubMed] [Google Scholar]
- 29.Wilson C C, Olson W C, Tuting T, Rinaldo C R, Lotze M T, Storkus W J. HIV-1-specific CTL responses primed in vitro by blood-derived dendritic cells and Th1-biasing cytokines. J Immunol. 1999;162:3070–3078. [PubMed] [Google Scholar]
- 30.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus K A, Reimann K A, Reinhart T A, Rogan M, Cavert W, Miller C J, Veazey R S, Notermans D, Little S, Danner S A, Richman D D, Havlir D, Wong J, Jordan H L, Schacker T W, Racz P, Tenner-Racz K, Letvin N L, Wolinsky S, Haase A T. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]