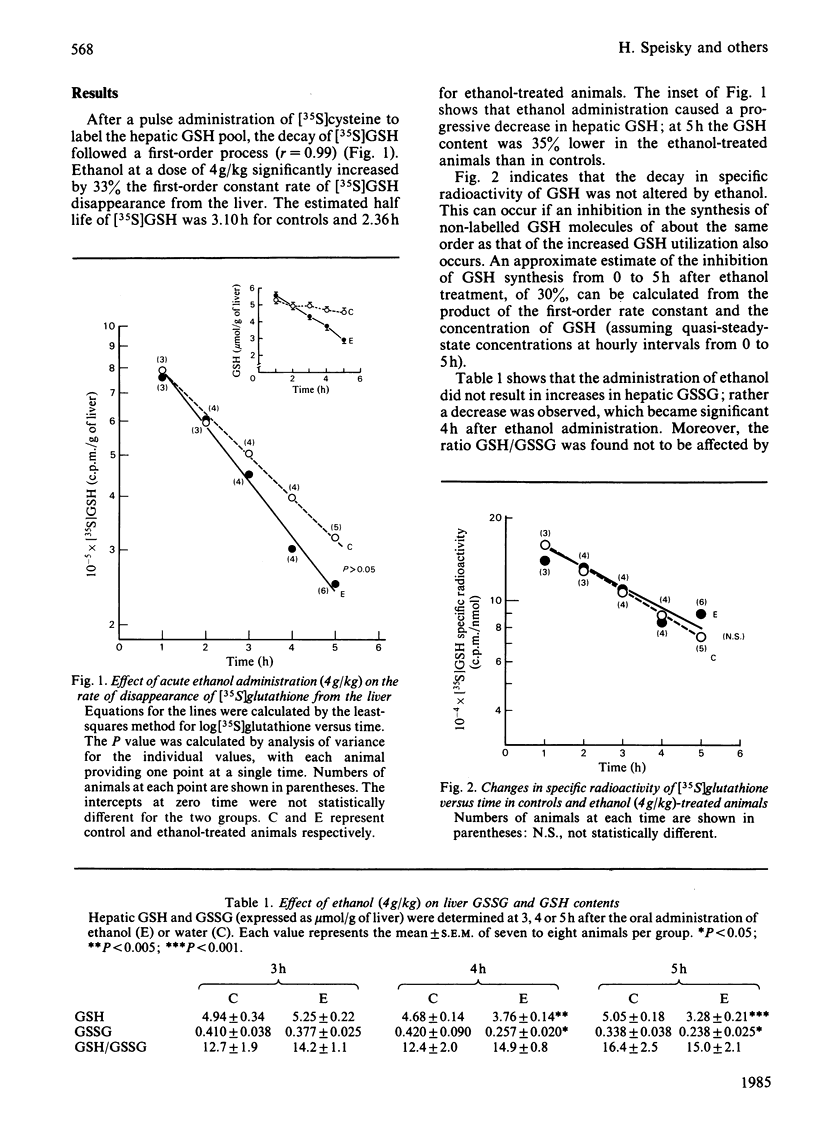
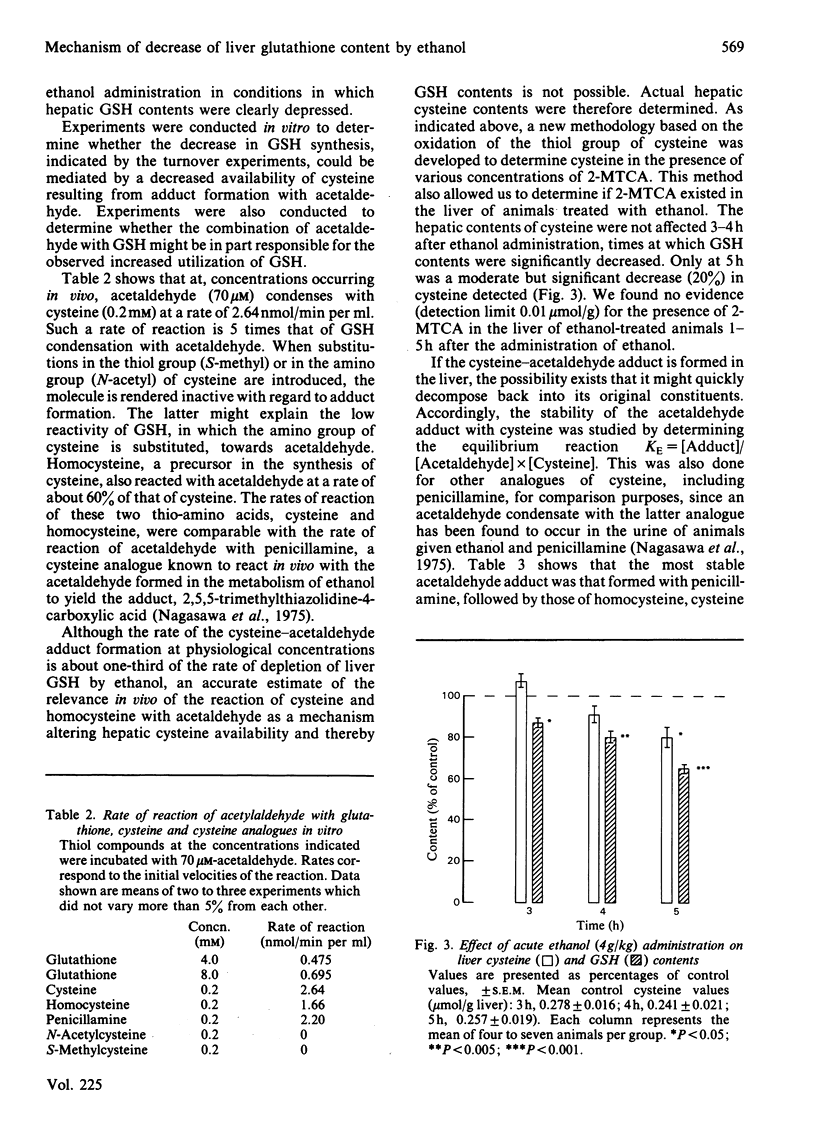
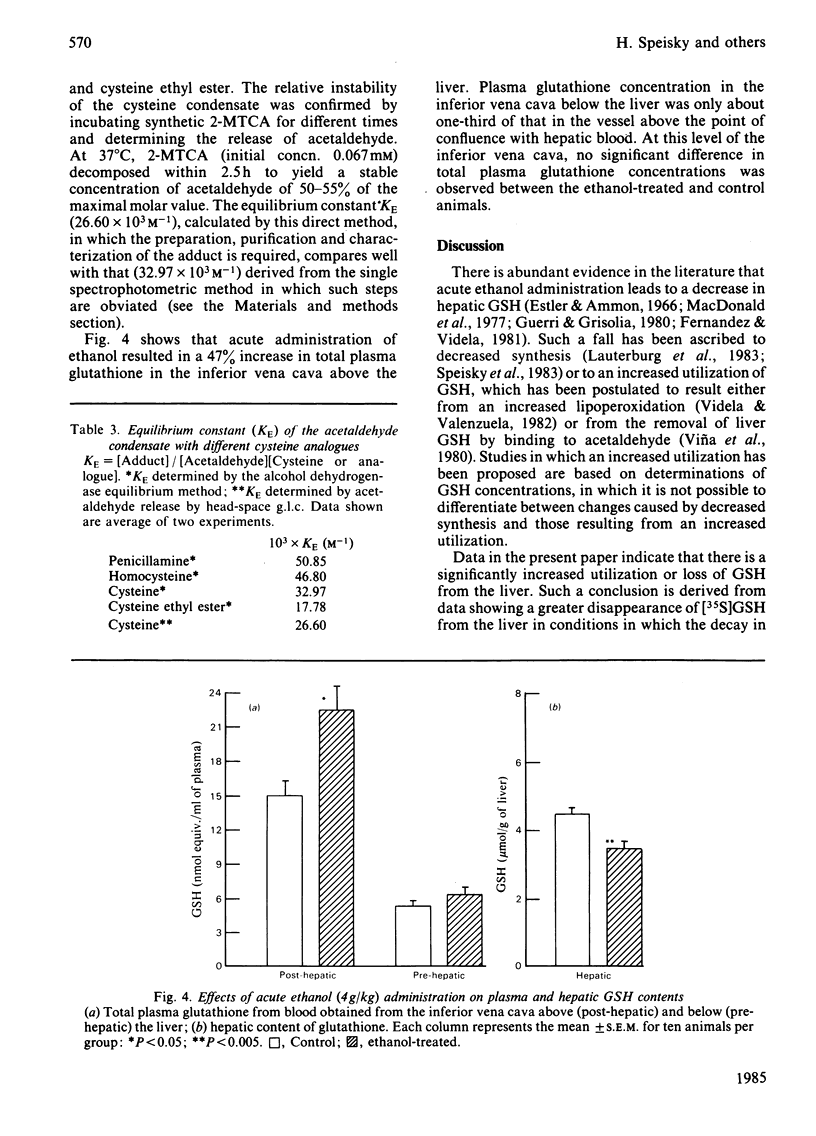
Abstract
The effect of acute ethanol administration on rates of synthesis and utilization of hepatic glutathione (GSH) was studied in rats after a pulse of [35S]cysteine. A 35% decrease in hepatic GSH content 5h after administration of 4 g of ethanol/kg body wt. was accompanied by a 33% increase in the rate of GSH utilization. The decrease occurred without increases in hepatic oxidized glutathione (GSSG) or in the GSH/GSSG ratio. The rate of non-enzymic condensation of GSH with acetaldehyde could account for only 6% of the rate of hepatic GSH disappearance. The increased loss of [35S]GSH induced by ethanol was not accompanied by an increased turnover; rather, a 30% inhibition of GSH synthesis balanced the increased rate of loss, leaving the turnover rate unchanged. The rate of acetaldehyde condensation with cysteine in vitro occurred at about one-third of the rate of GSH loss in ethanol-treated animals. However, ethanol induced only a minor decrease in liver cysteine content, which did not precede, but followed, the decrease in GSH. The characteristics of 2-methylthiazolidine-4-carboxylic acid, the condensation product between acetaldehyde and cysteine, were studied and methodologies were developed to determine its presence in tissues. It was not found in the liver of ethanol-treated animals. Ethanol administration led to a marked increase (47%) in plasma GSH in the post-hepatic inferior vena cava, but not in its pre-hepatic segment. Data suggest that an increased loss of GSH from the liver constitutes an important mechanism for the decrease in GSH induced by ethanol. In addition, an inhibition of GSH synthesis is observed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. D., Jr, Lauterburg B. H., Mitchell J. R. Plasma glutathione and glutathione disulfide in the rat: regulation and response to oxidative stress. J Pharmacol Exp Ther. 1983 Dec;227(3):749–754. [PubMed] [Google Scholar]
- Cederbaum A. I., Rubin E. Protective effect of cysteine on the inhibition of mitochondrial functions by acetaldehyde. Biochem Pharmacol. 1976 Apr 15;25(8):963–973. doi: 10.1016/0006-2952(76)90323-3. [DOI] [PubMed] [Google Scholar]
- DEBEY H. J., MACKENZIE J. B., MACKENZIE C. G. The replacement by thiazolidinecarboxylic acid of exogenous cystine and cysteine. J Nutr. 1958 Dec 10;66(4):607–619. doi: 10.1093/jn/66.4.607. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fernández V., Fernández N., Valenzuela A., Videla L. A. Time course study of the changes in blood glutathione induced by acute ethanol intoxication in the rat. Experientia. 1983 Aug 15;39(8):880–882. doi: 10.1007/BF01990416. [DOI] [PubMed] [Google Scholar]
- Fernández V., Videla L. A. Effect of acute and chronic ethanol ingestion on the content of reduced glutathione of various tissues of the rat. Experientia. 1981 Apr 15;37(4):392–394. doi: 10.1007/BF01959881. [DOI] [PubMed] [Google Scholar]
- Gaitonde M. K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J. 1967 Aug;104(2):627–633. doi: 10.1042/bj1040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles H. G., Meggiorini S. Artifactual production and recovery of acetaldehyde from ethanol in urine. Can J Physiol Pharmacol. 1983 Jul;61(7):717–721. doi: 10.1139/y83-111. [DOI] [PubMed] [Google Scholar]
- Higashi T., Tateishi N., Naruse A., Sakamoto Y. A novel physiological role of liver glutathione as a reservoir of L-cysteine. J Biochem. 1977 Jul;82(1):117–124. doi: 10.1093/oxfordjournals.jbchem.a131659. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N. The importance and regulation of hepatic glutathione. Yale J Biol Med. 1981 Nov-Dec;54(6):497–502. [PMC free article] [PubMed] [Google Scholar]
- Lauterburg B. H., Vaishnav Y., Stillwell W. G., Mitchell J. R. The effects of age and glutathione depletion on hepatic glutathione turnover in vivo determined by acetaminophen probe analysis. J Pharmacol Exp Ther. 1980 Apr;213(1):54–58. [PubMed] [Google Scholar]
- Lieber C. S. Alcohol, liver injury and protein metabolism. Pharmacol Biochem Behav. 1980;13 (Suppl 1):17–30. doi: 10.1016/s0091-3057(80)80004-9. [DOI] [PubMed] [Google Scholar]
- Macdonald C. M., Dow J., Moore M. R. A possible protective role for sulphydryl compounds in acute alcoholic liver injury. Biochem Pharmacol. 1977 Aug 15;26(16):1529–1531. doi: 10.1016/0006-2952(77)90428-2. [DOI] [PubMed] [Google Scholar]
- Nagasawa H. T., Elberling J. A., DeMaster E. G. Structural requirements for the sequestration of metabolically generated acetaldehyde. J Med Chem. 1980 Feb;23(2):140–143. doi: 10.1021/jm00176a007. [DOI] [PubMed] [Google Scholar]
- Nagasawa H. T., Goon D. J., Constantino N. V., Alexander C. S. Diversion of ethanol metabolism by sulfhydryl amino acids. D-penicillamine-directed excretion of 2,5,5-trimethyl-D-thiazolidine-4-carboxylic acid in the urine of rats after ethanol administration. Life Sci. 1975 Sep 1;17(5):707–713. doi: 10.1016/0024-3205(75)90525-1. [DOI] [PubMed] [Google Scholar]
- Richman P. G., Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem. 1975 Feb 25;250(4):1422–1426. [PubMed] [Google Scholar]
- Sies H., Summer K. H. Hydroperoxide-metabolizing systems in rat liver. Eur J Biochem. 1975 Sep 15;57(2):503–512. doi: 10.1111/j.1432-1033.1975.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Videla L. A., Fernández V., Valenzuela A., Ugarte G. Effect of (+)-cyanidanol-3 on the changes in liver glutathione content and lipoperoxidation induced by acute ethanol administration in the rat. Pharmacology. 1981;22(6):343–348. doi: 10.1159/000137514. [DOI] [PubMed] [Google Scholar]
- Viña J., Estrela J. M., Guerri C., Romero F. J. Effect of ethanol on glutathione concentration in isolated hepatocytes. Biochem J. 1980 May 15;188(2):549–552. doi: 10.1042/bj1880549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M. D., Wilcox R. E., Riffee W. H., Abraham L. D. Strain differences in dopamine receptor function and the initiation of movement. Pharmacol Biochem Behav. 1980 Jul;13(1):5–7. doi: 10.1016/0091-3057(80)90111-2. [DOI] [PubMed] [Google Scholar]


