Abstract
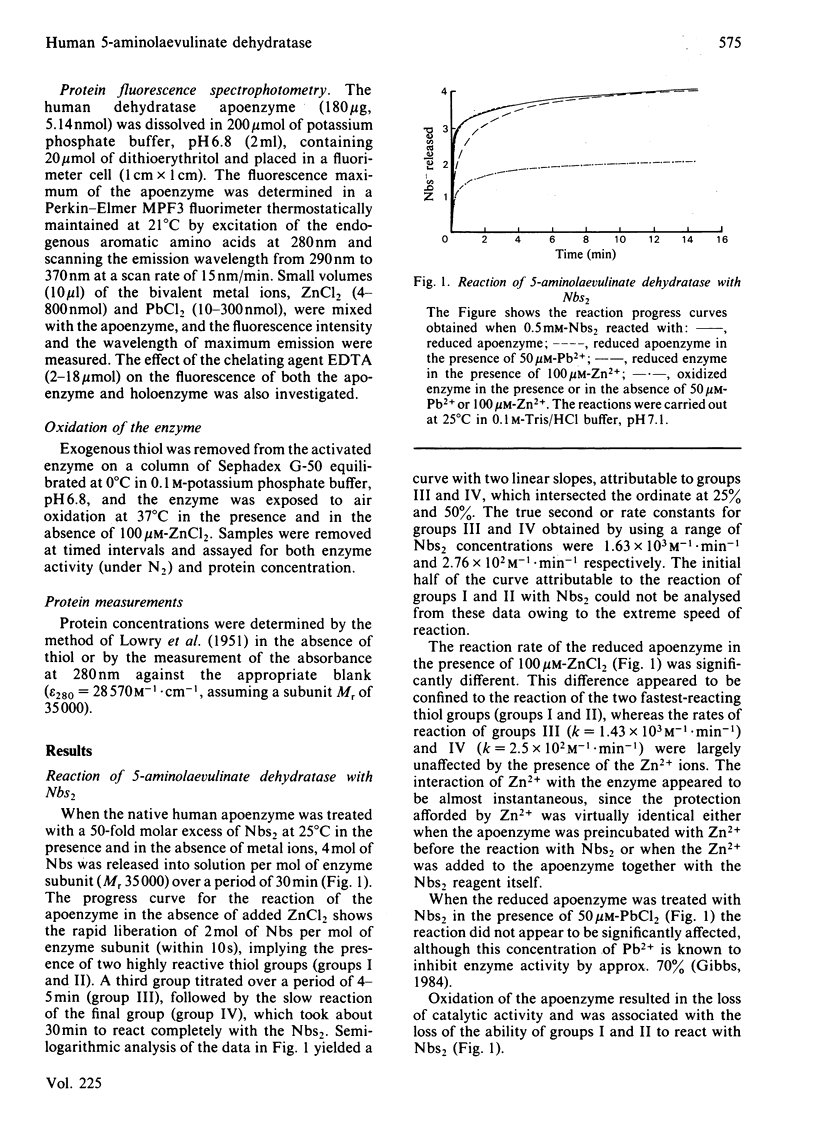
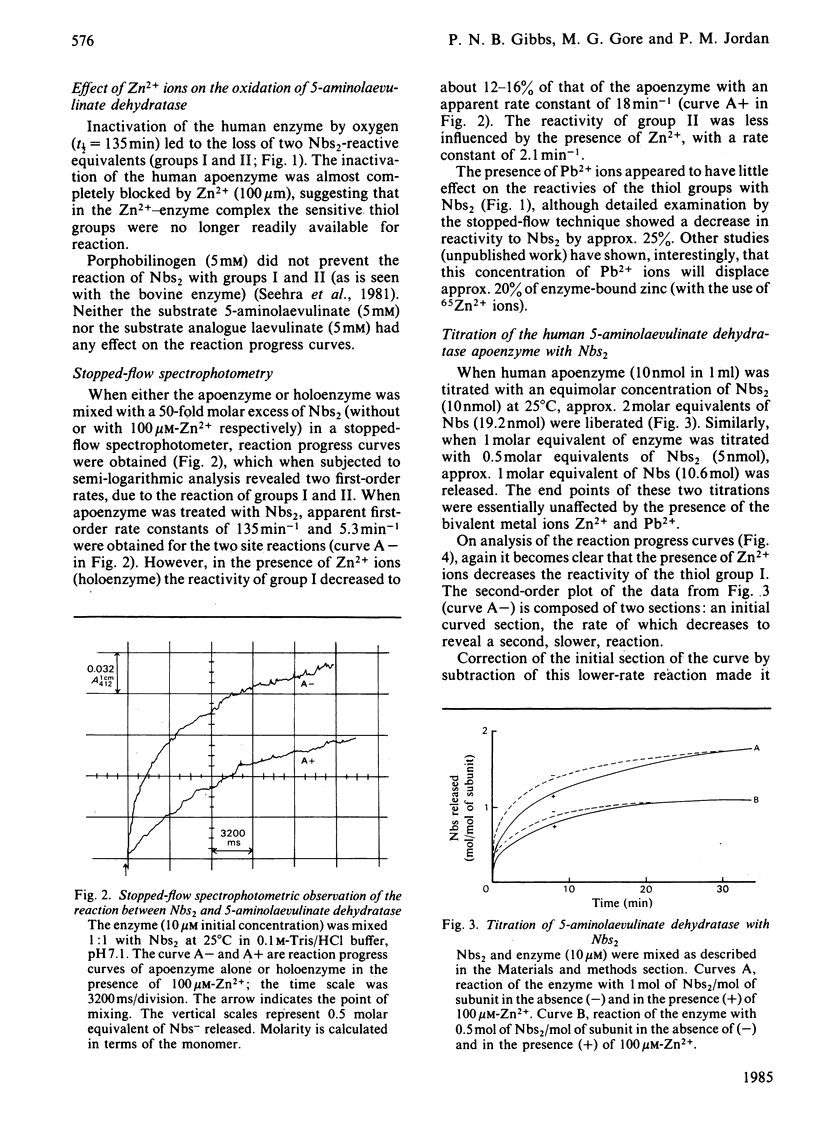
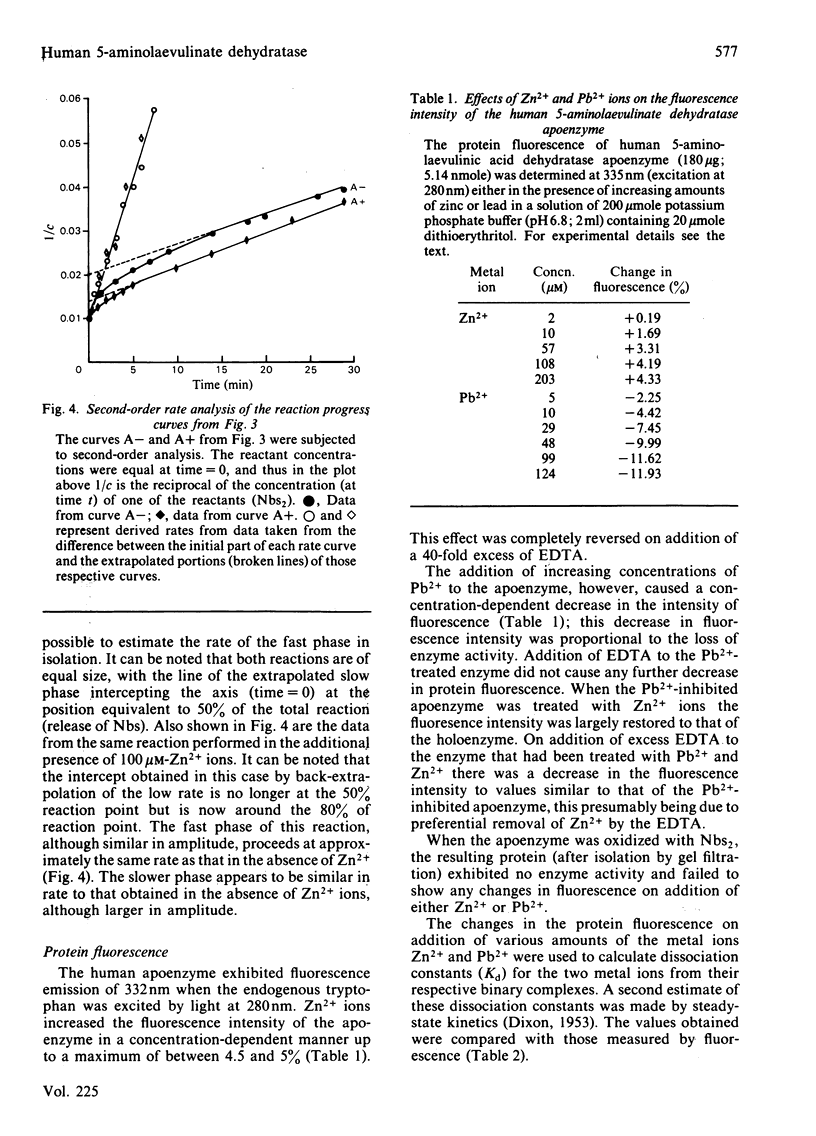
The reaction of human 5-aminolaevulinate dehydratase with 5,5'-dithiobis-(2-nitrobenzoic acid) (Nbs2) results in the release of 4 molar equivalents of 5-mercapto-2-nitrobenzoic acid (Nbs) per subunit. Two of the thiol groups reacted very rapidly (groups I and II), and their rate constants were determined by stopped-flow spectrophotometry; the other two thiol groups (groups III and IV) were observed by conventional spectroscopy. Titration of the enzyme with a 1 molar equivalent concentration of Nbs2 resulted in the release of 2 molar equivalents of Nbs and the concomitant formation of an intramolecular disulphide bond between groups I and II. Removal of zinc from the holoenzyme increased the reactivity of groups I and II without significantly affecting the rate of reaction of the other groups. The reactions of the thiol groups in both the holoenzyme and apoenzyme were little affected by the presence of Pb2+ ions at concentrations that strongly inhibit the enzyme, suggesting that Zn2+ and Pb2+ ions may have independent binding sites. Protein fluorescence studies with Pb2+ and Zn2+ have shown that the binding of both metal ions results in perturbation of the protein fluorescence.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Desnick R. J. Purification and properties of delta-aminolevulinate dehydrase from human erythrocytes. J Biol Chem. 1979 Aug 10;254(15):6924–6930. [PubMed] [Google Scholar]
- Barnard G. F., Itoh R., Hohberger L. H., Shemin D. Mechanism of porphobilinogen synthase. Possible role of essential thiol groups. J Biol Chem. 1977 Dec 25;252(24):8965–8974. [PubMed] [Google Scholar]
- Carlson G. M., Colombo G., Lardy H. A. A vicinal dithiol containing an essential cysteine in phosphoenolpyruvate carboxykinase (guanosine triphosphate) from cytosol of rat liver. Biochemistry. 1978 Dec 12;17(25):5329–5338. doi: 10.1021/bi00618a002. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finelli V. N., Klauder D. S., Karaffa M. A., Petering H. G. Interaction of zinc and lead on delta-aminolevulinate dehydratase. Biochem Biophys Res Commun. 1975 Jul 8;65(1):303–312. doi: 10.1016/s0006-291x(75)80093-3. [DOI] [PubMed] [Google Scholar]
- Flashner M., Hollenberg P. F., Coon M. J. Mechanism of action of pyruvate kinase. Role of sulfhydryl groups in catalytic activity as determined by disulfide interchange. J Biol Chem. 1972 Dec 25;247(24):8114–8121. [PubMed] [Google Scholar]
- Gomes B., Kloepfer H. G., Oi S., Yasunobu K. T. The reaction of sulfhydryl reagents with bovine hepatic monoamine oxidase. Evidence for the presence of two cysteine residues essential for activity. Biochim Biophys Acta. 1976 Jul 8;438(2):347–357. doi: 10.1016/0005-2744(76)90252-7. [DOI] [PubMed] [Google Scholar]
- Haeger-Aronsen B., Abdulla M., Fristedt B. I. Effect of lead on -aminolevulinic acid dehydrase activity in red blood cells. Arch Environ Health. 1971 Dec;23(6):440–445. doi: 10.1080/00039896.1971.10666033. [DOI] [PubMed] [Google Scholar]
- Haeger-Aronsen B., Schütz A. Antagonistic effect in vivo of zinc on inhibition of delta-aminolevulinic acid dehydratase by lead. Arch Environ Health. 1976 Jul-Aug;31(4):215–220. doi: 10.1080/00039896.1976.10667222. [DOI] [PubMed] [Google Scholar]
- Hernberg S., Nikkanen J. Enzyme inhibition by lead under normal urban conditions. Lancet. 1970 Jan 10;1(7637):63–64. doi: 10.1016/s0140-6736(70)91846-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Seehra J. S., Gore M. G., Chaudhry A. G., Jordan P. M. 5-Aminolevulinic acid dehydratase. The role of sulphydryl groups in 5-aminolevulinic acid dehydratase from bovine liver. Eur J Biochem. 1981 Feb;114(2):263–269. doi: 10.1111/j.1432-1033.1981.tb05145.x. [DOI] [PubMed] [Google Scholar]
- THIERS R. E. Contamination in trace element analysis and its control. Methods Biochem Anal. 1957;5:273–335. doi: 10.1002/9780470110218.ch6. [DOI] [PubMed] [Google Scholar]
- Tsukamoto I., Yoshinaga T., Sano S. The role of zinc with special reference to the essential thiol groups in delta-aminolevulinic acid dehydratase of bovine liver. Biochim Biophys Acta. 1979 Sep 12;570(1):167–178. doi: 10.1016/0005-2744(79)90211-0. [DOI] [PubMed] [Google Scholar]


