Abstract
Introduction
Extracranial vascular characteristics determine the accessibility of the large vessel intracranial occlusion for endovascular treatment (EVT) in acute ischemic stroke. We developed and validated a prediction model for failure of the transfemoral approach to aid clinical decision-making regarding EVT.
Methods
A prediction model was developed from data of patients included in the Dutch multicenter MR CLEAN Registry (March 18, 2014, until June 15, 2016) with penalized logistic regression. Predictor variables were available prior to the EVT procedure and included age, hypertension, and extracranial vascular characteristics assessed on baseline CTA. The prediction model was internally validated, temporally validated within a second MR CLEAN Registry cohort (June 15, 2016, until November 1, 2017), and updated by re-estimating the coefficients using the combined cohort.
Results
Failure of the transfemoral approach occurred in 7% of patients, in both cohorts (derivation cohort: n = 887, median age 71 years, interquartile range [IQR] 60–80, 52% men; validation cohort: n = 1,111, median age 73 years, IQR: 62–81, 51% men). The prediction model had a c-statistic of 0.81 (95% CI: 0.76–0.86) in the derivation cohort, 0.69 (95% CI: 0.62–0.75) at temporal validation, and 0.75 (95% CI: 0.71–0.79) in the final prediction model, with the following penalized β-coefficients for predictor age (per decade): 0.26, hypertension: −0.16, severe aortic arch elongation: 1.45, bovine aortic arch: 0.44, elongation of the supra-aortic arteries: 0.72, cervical ICA elongation: 0.44, and high-grade stenosis of the cervical ICA: 0.78.
Conclusion
Our prediction model showed good performance for prediction of failure to reach the intracranial occlusion by the transfemoral approach.
Keywords: Endovascular treatment, Ischemic stroke, Prediction model, Transfemoral approach, Vascular elongation
Introduction
Endovascular treatment (EVT) is effective in patients with acute ischemic stroke caused by a large vessel occlusion (LVO) in the anterior cerebral circulation [1]. Rapid revascularization is important, because the chance of functional independence at 3 months’ follow-up is highly time dependent [2]. In general, access of EVT is obtained by a transfemoral approach. The time to revascularization is associated with the patients’ extracranial vascular anatomy [3]. Both anatomical variations, such as aortic variants [4], and acquired changes, such as elongation and atherosclerosis of the aortic arch, supra-aortic and internal carotid arteries (ICAs), increase technical difficulty of the procedure [4, 5]. These extracranial vascular characteristics not only increase time to revascularization [3], but may also prevent the interventionalist from reaching the intracranial occlusion site [6].
Unnecessary delay in achieving revascularization may be avoided in patients with limited probability of reaching the intracranial occlusion site via groin puncture by directly choosing an alternative approach for EVT treatment. Pre-procedural prediction of femoral approach success might also aid clinical decision-making in patients with unclear benefit of EVT, e.g., for patients with mild symptoms or a poor pre-stroke condition [7]. Extracranial vascular imaging characteristics are readily available from routine baseline imaging, and assessment of these parameters will not cause significant delay in time-sensitive treatment decisions. However, it is unknown whether extracranial vascular characteristics on CT angiography (CTA) can be used to predict failure to reach the occlusion site by the transfemoral approach. The objective of this study was to develop and validate a prediction model for failure to reach the intracranial occlusion site by the transfemoral approach for EVT, based on clinical and extracranial vascular characteristics that are available prior to the start of EVT.
Methods
Patient Population
Data were obtained from the Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke in the Netherlands (MR CLEAN) Registry. This prospective registry includes all consecutive patients in the Netherlands who presented with a proximal LVO acute ischemic stroke between March 2014 and December 2018 and in whom EVT was performed and was approved by a central Institutional Review Board (MEC-2013-235) [8].
We obtained data from patients registered from March 18, 2014, until November 1, 2017, who were treated with EVT for an anterior circulation LVO. MR CLEAN Registry data became available for research in several phases. First, patients registered from March 18, 2014, until June 15, 2016, were available and selected for model development, and second, patients registered from June 15, 2016, until November 1, 2017, were available and selected for temporal validation of the model. Data included patient history, and stroke severity assessed using the National Institutes of Health Stroke Scale scores [8]. Radiological characteristics, including Alberta stroke program early CT score and pre- and post-intervention extended thrombolysis in cerebral infarction (eTICI) scores, were assessed by an imaging core laboratory on baseline non-contrast brain CT, CTA of cervical and cerebral vessels, and pre- and post-interventional digital subtraction angiography (DSA). A detailed description of the registry procedures is published elsewhere [8]. Intervention characteristics and workflow time metrics included treatment with intravenous thrombolysis, time from symptom onset to groin puncture, duration of EVT procedure (from groin puncture to catheter removal), and post-intervention eTICI score (poor revascularization was defined as a score of 0–2A and good vascularization as a score of 2B-C) [9]. Use of a balloon guide catheter was checked in both cohorts. Patients were excluded when access was gained via the brachial artery, radial artery, or carotid artery, if recanalization had occurred on the initial DSA run, when the aortic arch was not included in the CTA field of view, when CTA imaging had insufficient quality, or when the occlusion location was missing in the database.
Predictor Variable Selection
Seven clinical and vascular predictors for failure of transfemoral approach were selected from the literature [10–12]. These were age [10], hypertension [13], aortic arch variants [11], aortic arch elongation [10], tortuosity of the supra-aortic arteries [12], and tortuosity and stenosis of the cervical ICA ipsilateral to the intracranial vessel occlusion [3, 14].
CTA Image Analysis
Baseline CTA studies of aortic arch, supra-aortic, and ICAs) were reviewed using the local Picture Archiving and Communication System (PACS, Sectra IDS7 182, Linkoping, Sweden). Aortic arch variants were defined as type A (normal variant): the innominate artery (IA), left common carotid artery (CCA), and left subclavian artery branch directly from the aortic arch; type B: IA and left CCA share a common origin; or type C (bovine arch): left CCA branches from the IA [3, 11]. Aortic arch elongation was divided into type I: no elongation; type II: mild to moderate elongation; or type III: severe elongation (online suppl. Fig. 1) [3, 10]. Tortuosity of the supra-aortic arteries ipsilateral to the side of the intracranial occlusion (IA or CCA) was defined as the presence of an angle measuring ≥90° [3, 12]. For the cervical ICA ipsilateral to the side of the intracranial occlusion, tortuosity was defined as the presence of an angle measuring ≥90° directly following the carotid artery bifurcation (online suppl. Fig. 2); this angle represents a sharp angle turn in the cervical ICA. Stenosis was assessed according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria [15] and dichotomized into <99% and ≥99% (including occlusion) [3]. Since all patients in the MR CLEAN Registry have an intracranial LVO, patients with a tandem occlusion are included in the variable ICA stenosis ≥99% (including occlusion); we did not consider tandem occlusions as a separate variable. Further, the state of the femoral arteries is not depicted on baseline imaging and is therefore not included in our prediction model. Vascular characteristics were analyzed by trained students (derivation cohort: M.S., T.D.; validation cohort: J.H., S.K.; under supervision of M.v.W., a neuroradiologist with >20 years of experience in CTA analysis) blinded to clinical information except for patients’ age, sex, and side of intracranial vessel occlusion. Agreement between observers of the validation and derivation cohort was determined with Fleiss’ kappa (κ); values 0.41–0.60 represent moderate agreement, 0.61–0.80 good agreement, and >0.80 excellent agreement [16].
Outcome Measure
The primary outcome measure was failure of the transfemoral approach, defined as failure to reach the intracranial occlusion site via groin puncture of the common femoral artery as reported by the treating interventionalist.
Statistical Analysis
Patients’ clinical and radiological characteristics were reported using descriptive statistics. The association of the 7 predictor variables with the primary outcome measure was assessed with logistic regression yielding odds ratios (ORs) and accompanying 95% confidence interval (CI). To prevent bias, missing data were multiply imputed (50 imputations, mice package [17] in R studio, version 4.0) prior to model development and validation. This has been described in more detail in the online supplement methods.
Model Development and Internal Validation
Model development on multiple imputed data was performed following previous literature [18] and described in detail in the supplemental methods. In short, we stacked the 50 imputed datasets into one large dataset; we fitted the prediction model on the development part of the stacked dataset (derivation cohort), using weighted penalized (ridge) logistic regression (with the glmnet package [19] in R). The β-coefficients were penalized to reduce potential overfitting of the model. We internally validated this model using a bootstrap procedure, with 200 bootstrap samples [20].
Temporal Validation
The model was temporally validated using the dataset with patients registered from June 15, 2016, until November 1, 2017. Additionally, we tested for differences in model coefficients between the derivation and validation cohort by specifying an “interaction by cohort” [21] for each predictor variable from the prediction model.
Final Model Development and Nomogram
For the final model, we updated the model by re-estimating the coefficients using the data from both cohorts combined; more detail can be found in the online supplement. The performance of the final model was assessed by reporting calibration (intercept and slope) and discrimination (c-statistic) and with a calibration plot. A nomogram was developed in which each predictor received a score based on the relative effect in the model. This provides a tool for calculating specific risk of failure of the transfemoral approach for each individual predictor. All statistical analyses were performed using R (version 4.0). R scripts are available online at a GitHub repository: https://github.com/edbonneville/validation-failed-EVT.
Results
Patients
Between March 18, 2014, and November 1, 2017, 3,180 patients who were treated with EVT for an anterior circulation LVO were eligible from the MR CLEAN Registry, of whom we included 1,998 patients in this study. For model development, 887 patients were used and 1,111 patients for temporal validation. Main reasons for patient exclusion were that aortic arch imaging was not available (n = 585), CTA was not retrievable (n = 227), or recanalization had occurred on the initial DSA run (n = 293) (see online suppl. Fig. 3).
Patient, radiological, intervention, and outcome characteristics for the derivation and validation cohorts are shown in Table 1. These characteristics were largely comparable, although in the derivation cohort the duration from symptom onset to groin puncture was longer (median 210 min, interquartile range [IQR]: 160–265 vs. median 180 min, IQR: 136–230), unsuccessful revascularization occurred more often (eTICI score 0–2A 45% vs. 35%), and the duration of the EVT procedure was longer (median 65 min, IQR: 45–90 vs. 57 min, IQR: 40–83). Type of guiding catheter was reported for 77% of included patients; of these patients, balloon guide catheters were used in 463/704 (66%) of the patients included in the derivation cohort and in 552/836 (66%) of the patients in the validation cohort. The number of patients with a poor functional outcome (modified Rankin Scale [mRS] score ≥3) was similar between the two cohorts (derivation cohort: n = 504 [62%] and validation cohort: n = 628 [60%]). Failure of transfemoral approach occurred in 59 (7%) EVT procedures in the derivation and 81 (7%) in the validation cohort.
Table 1.
Patient, radiological, intervention, outcome, and extracranial vascular characteristics for the derivation cohort (n = 887) and the validation cohort (n = 1,111)
| Derivation cohort (n = 887) | Validation cohort (n = 1,111) | |
|---|---|---|
| Patient demographics | ||
| Age, median (IQR), years | 71 (60–80) | 73 (62–81) |
| Men, n (%) | 461 (52) | 571 (51) |
| Previous stroke, n (%) | 159 (18) | 182 (17) |
| Diabetes mellitus, n (%) | 136 (16) | 165 (15) |
| Hypertension, n (%) | 446 (51) | 601 (56) |
| Atrial fibrillation, n (%) | 202 (23) | 287 (26) |
| Pre-mRS ≥1, n (%) | 286 (33) | 346 (32) |
| NIHSS at baseline, median (IQR) | 16 (12–20) | 16 (11–20) |
| Radiological characteristics, n (%) | ||
| Pre-eTICI ≥1 | 83 (10) | 176 (17) |
| Collateral score ≥50%a | 512 (60) | 607 (56) |
| ASPECTS ≤7b | 256 (29) | 233 (21) |
| CBS ≤7c | 548 (72) | 507 (64) |
| Occlusion location, n (%) | ||
| Intracranial ICA | 52 (6) | 52 (5) |
| ICA-T | 203 (23) | 229 (21) |
| M1 | 509 (58) | 630 (58) |
| Otherd | 110 (13) | 167 (15) |
| Intervention characteristics | ||
| IVT administered, n (%) | 683 (77) | 813 (74) |
| Symptom onset to groin puncture, min, median (IQR) | 210 (160–265) | 180 (136–230) |
| Duration EVT procedure, min, median (IQR) | 65 (45–90) | 57 (40–83) |
| Post-eTICI 0–2A, n (%) | 395 (45) | 372 (35) |
| Outcome characteristics, n (%) | ||
| Failure of transfemoral approach | 59 (7) | 81 (7) |
| Vascular characteristics | ||
| Aortic arch elongation, n (%) | ||
| Type I | 155 (18) | 191 (17) |
| Type II | 561 (63) | 699 (63) |
| Type III | 169 (19) | 213 (19) |
| Aortic variants, n (%) | ||
| Normal variant | 654 (74) | 738 (68) |
| Common origin IA-CCA | 123 (14) | 162 (15) |
| Bovine arch | 105 (12) | 191 (18) |
| Tortuosity IA and CCA, n (%)e | ||
| ≥1 angle ≥90° | 338 (39) | 472 (43) |
| ≥2 angles ≥90° | 125 (15) | 187 (17) |
| Tortuosity cervical ICA, n (%) | ||
| Angle ≥90° directly following bifurcation, n (%) | 125 (17) | 243 (27) |
| Atherosclerosis, n (%) | ||
| ICA stenosis ≥99% (including occlusion) | 86 (11) | 166 (17) |
| ICA occlusion | 53 (7) | 134 (14) |
Numbers might not add up due to missing values.
ASPECTS, Alberta stroke program early CT score; CBS, Clot Burden Score; CCA, common carotid artery; eTICI, extended thrombolysis in cerebral infarction; EVT, endovascular treatment; IA, innominate artery; ICA, internal carotid artery; ICA-T, ICA terminus; IVT, intravenous thrombolysis; IQR, interquartile range; pre-mRS, pre-stroke modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; pre-eTICI, pre-intervention eTICI.
aCollateral filling of ≥50% of the affected area, indicating moderate to good collateral circulation.
bIndicates severe early ischemic changes.
cIndicates lack of contrast in two or more anterior vessel segments.
dOther occlusion location includes M2, M3, or occlusion of the anterior cerebral artery.
eThe presence of IA and CCA angles was combined, since these vessels were measured depending on the side of the intracranial vessel occlusion (i.e., left or right).
Extracranial Vascular Characteristics
Extracranial vascular characteristics for both cohorts are shown in Table 1. Differences between the derivation and validation cohorts were present for aortic arch variant (normal variant: 74% vs. 68%, common origin of the IA-CCA: 14% vs. 15%, bovine arch: 12%, vs. 18%, respectively), angle of ≥90° directly following the carotid bifurcation (17% vs. 27%, respectively), and cervical ICA stenosis of ≥99% (including occlusion) (11% vs. 17%, respectively). Interobserver agreement was good for the measurement of aortic arch elongation (κ: 0.79), aortic arch variant (κ: 0.73), and tortuosity of the IA or CCA (κ: 0.73), and moderate for tortuosity of the cervical ICA (κ: 0.57) and ICA stenosis ≥99% (κ: 0.59).
Model Development and Internal Validation in the Derivation Cohort
The model developed in the derivation cohort for prediction of failure of transfemoral approach with the variables age, hypertension, aortic arch elongation, aortic arch variant, at least one ≥90° angle in the IA or CCA, ≥90° angle directly following the carotid bifurcation, and cervical ICA stenosis of ≥99% showed excellent discrimination with a c-statistic of 0.81 (95% CI: 0.76–0.86). The β-coefficients of the variables after internal validation are listed in Table 2. Model calibration after internal validation was good with an intercept of 0.05 (95% CI: −0.24 to 0.33) and a slope of 1.05 (95% CI: 0.84–1.29). Number and percentage of missing values for each variable used for multiple imputation are shown in online supplementary Table 1.
Table 2.
Penalized β-coefficients of predictor variables for failure of the transfemoral approach after internal validation in the derivation cohort (N = 887) and updated penalized β-coefficients of the final model based on the combined cohort (N = 1,998)
| Derivation cohort (N = 887) | Combined cohort (N = 1,998) | |
|---|---|---|
| Predictors | β-coefficients | β-coefficients |
| Intercept | −5.77 | −5.71 |
| Age (per decade) | 0.25 | 0.26 |
| Hypertension | −0.54 | −0.16 |
| Aortic arch elongation | ||
| Type IIa | −0.07 | 0.23 |
| Type IIIa | 1.33 | 1.45 |
| Aortic variants | ||
| Common origin IA-CCAb | 0.43 | 0.06 |
| Bovine archb | 0.94 | 0.44 |
| Tortuosity IA and CCAc | ||
| ≥1 angle ≥90° | 1.00 | 0.72 |
| Tortuosity cervical ICA | ||
| Angle ≥90° directly following bifurcation | 0.82 | 0.44 |
| ICA stenosis ≥99% | 1.34 | 0.78 |
CCA, common carotid artery; IA, innominate artery; ICA, internal carotid artery; LSA, left subclavian artery.
aReference category was type I (no elongation).
bReference category was “normal variant”: the IA, left CCA, and LSA branch directly from the aortic arch.
cThe presence of IA and CCA angles was combined, since these vessels were measured depending on the side of the intracranial vessel occlusion (i.e., left or right).
Temporal Validation
At temporal validation, model calibration and discrimination were moderate with an intercept of −0.11 (95% CI: −0.35 to 0.14) and slope of 0.58 (95% CI: 0.38–0.78) and a c-statistic of 0.69 (95% CI: 0.62–0.75). The following variables showed different adjusted (unpenalized) effects in the cohorts: hypertension (derivation cohort OR: 0.49, 95% CI: 0.26–0.89 vs. validation cohort OR 1.13, 95% CI: 0.68–1.87, interaction by cohort, p = 0.04) and bovine arch (derivation cohort OR 2.84, 95% CI: 1.46–5.31 vs. validation cohort OR: 0.93, 95% CI: 0.49–1.65, interaction by cohort, p = 0.03) (online suppl. Table 2).
Final Model
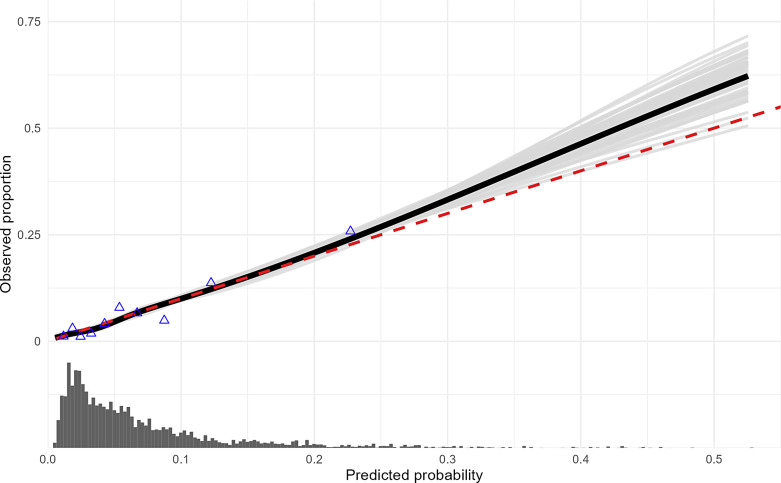
The β-coefficients of the final model are listed in Table 2. The vascular characteristics severe aortic arch (type III) elongation (β: 1.45), at least one ≥90° angle in the IA or CCA (β: 0.72), and cervical ICA stenosis of ≥99% (including occlusion) (β: 0.78) showed the largest effect on failure of the transfemoral approach. Performance of the final model was good with an intercept of 0.01 (95% CI: −0.15 to 0.20), slope of 1.02 (95% CI: 0.86–1.26), and c-statistic of 0.75 (95% CI: 0.71–0.79). The calibration plot showed good performance of the model for low predicted probability of failure of transfemoral approach, but underestimation of the actual outcome occurs for predicted probability of failure of transfemoral approach above 30% (Fig. 1). The nomogram (Fig. 2) provides the following scores given to each predictor: (1) age per decade – 1.0 point, (2) no hypertension – 0.6 points, (3) aortic variant common IA-CCA – 0.2 points, bovine arch – 1.7 points, (4) aortic arch elongation type II (mild to moderate) – 0.9 points and type III (severe) – 5.6 points, (5) at least one ≥90° angle in the IA or CCA – 2.8 points, (6) ≥90° angle directly following the carotid bifurcation – 1.7 points, and (7) degree of cervical ICA stenosis ≥99% – 3.0 points. The highest possible score for an individual patient in the combined cohort was 23 points (Fig. 2), which corresponded to a predicted probability of failure of the transfemoral approach of 60%.
Fig. 1.
Calibration plot of the final prediction model. Smooth calibration plot showing the predictive performance of the final prediction model for failure of transfemoral approach on the combined cohort (N = 1,998). The observed proportion of failure of transfemoral approach is plotted on the y-axis against the predicted probability on the x-axis. The solid black line shows the calibration plot smoothed across all 50 imputed datasets. The red line shows perfect prediction by an ideal model. The light gray lines show each smooth calibration curve of all imputed datasets separately, representing the between-imputation variance. The distribution of the predicted probabilities is shown at the bottom of the graph.
Fig. 2.
Nomogram of the final prediction model. Nomogram of the final prediction model for failure of transfemoral approach based on the penalized logistic regression model on the combined cohort (n = 1,998). This nomogram provides a relative score for each predictor and total points for individual patients with corresponding predicted probability.
Discussion
In this study, we developed a well-performing model for the prediction of failure to reach the intracranial occlusion site by the transfemoral approach for patients who presented with an anterior circulation LVO. In our cohorts, the transfemoral approach was successful in most patients (93%). In patients with unsuccessful transfemoral approach (7%), several vascular characteristics showed independent associations, with severe elongation of the aortic arch being the strongest predictor. ICA stenosis of ≥99% (including occlusion) and elongation of the supra-aortic and ICAs contributed to a lesser extent.
Our results are in line with a recent systematic review reporting that supra-aortic vessels and aortic arch together account for 92% of locations that are associated with failure of transfemoral approach, with vessel tortuosity given as the primary reason in half of the cases [6]. Previous studies proposed methods to identify patients with challenging vascular characteristics, but did not directly relate this to failure of the transfemoral approach [4, 22]. For our prediction model, we used characteristics that are readily available from routine baseline imaging with simple-to-use methods to measure elongation and atherosclerosis of extracranial vascular structures, to enhance utility in clinical practice.
Our final prediction model has good discrimination and calibration. However, the calibration plot shows that model prediction underestimates the actual outcome to a limited extent for predicted probability above 30%. The low prevalence of failure of transfemoral approach in the studied cohorts likely contributes to this, with even very low numbers in the higher range of predicted probabilities. Further, the nomogram shows that an octogenarian with all unfavorable clinical and vascular characteristics will only reach a total of 23 points with a corresponding 60% predicted probability of failure of transfemoral approach. The prediction model could therefore be useful in decision-making regarding intervention strategy, but should be interpreted together with other clinical factors such as frailty or pre-stroke modified Rankin Scale (mRS) score.
A limitation of this study is the relatively low number of patients in whom the intracranial occlusion site could not be reached via the transfemoral route. Another limitation is the exclusion of a substantial number of patients (n = 585) because aortic arch imaging is not routinely performed in the diagnostic workup in all hospitals. We expect this to be unrelated to the vascular anatomy of patients. Further, patients were excluded because recanalization had occurred on the initial DSA run (n = 293), or the baseline CTA was not retrievable (n = 227). In addition, other factors analyzed in this study probably also contributed to failure of the transfemoral approach. Cervical ICA loops (a full 360° turn of the ICA), e.g., might have influenced the chances of transfemoral approach success. This anatomical feature was present in only 2% of patients in the derivation cohort, and we therefore did not consider this a separate variable. Similarly, the number of dissections in the MR CLEAN Registry is approximately 2% and was also not taken into account. As we aimed to predict the outcome prior to the start of the procedure, interventional characteristics, such as techniques or materials used or experience of the interventionalist, were therefore not included. These factors have likely improved over the years and possibly increased the success rate of reaching the intracranial occlusion via the transfemoral approach. Also, this might have resulted in the inclusion of patients with more difficult vascular anatomy in the validation cohort, as – compared to the derivation cohort – we observe a higher percentage of patients with a bovine arch (18% vs. 12%), supra-aortic artery tortuosity (IA and CCA tortuosity: 43% vs. 39%; cervical ICA tortuosity: 27% vs. 17%), and ICA stenosis of ≥99% (including occlusion) (17% vs. 11%). This possibly explains why – despite improved techniques and materials, and increased experience of the interventionalist – failure of the transfemoral approach was similar (7% of patients) in both cohorts. Tortuosity and atherosclerosis of the common femoral artery, abdominal and thoracic aorta, influence trackability of catheters and are probably related to failure of transfemoral approach [6]. These vessels are not depicted on baseline imaging and could therefore not be included in our prediction model. Also, intracranial vessel tortuosity can interfere with access from the cervical segment to the culprit lesion and intracranial device delivery, but was not assessed for this study [23]. Finally, the prediction model should be externally validated for different stroke populations, especially in an Asian population who less often have extracranial stenosis and more often intracranial stenosis [24].
An important strength of this study is that results are likely generalizable to other LVO populations as data were derived from a large multicenter cohort of patients treated according to standard clinical practice. Further, the vascular characteristics are available from routine baseline CTA imaging. Although the prognostic value of unfavorable vascular anatomy does not directly translate to a yes or no decision regarding the transfemoral approach, they could help the intervention team make better informed treatment decisions. For example, physicians might refrain from EVT in patients for whom the benefit of EVT is unclear (e.g., frail patients with a poor pre-stroke condition). Also, interventionalists might decide to access the intracranial occlusion directly via the radial or carotid artery or more quickly decide to switch to an alternative approach after an unsuccessful first attempt. In conclusion, our prediction model with age, hypertension, and extracranial vascular characteristics derived from baseline CTA images has good performance for prediction of failure to reach the intracranial occlusion site by the transfemoral approach for EVT.
Acknowledgment
We thank the MR CLEAN Registry investigators for their support.
Statement of Ethics
Opt-out informed consent protocol was used for this study. This consent procedure was reviewed and approved by the Central Medical Ethics Committee of the Erasmus Medical Centre, Rotterdam, the Netherlands, approval number MEC-2014-235, date of decision October 6, 2014. All study procedures were conducted in accordance with institutional guidelines and the Declaration of Helsinki.
Conflict of Interest Statement
G.H., E.B., J.H., S.K., H.O., W.S., G.H., N.K., M.W., and M.v.W. declared no potential conflicts of interest with respect to research, authorship, and/or publication of this article. A.L. received grants outside of the submitted work (paid to institution) from Brain Foundation Netherlands, Cerenovus, Dutch Heart Foundation, GE HealthCare, Medtronic, Penumbra, Philips Healthcare, Siemens Healthineers, Stryker, The Netherlands Organization for Health Research and Development, Health Holland (Topsector Life Sciences & Health), and Thrombolytic Science LLC; received payment for attending meetings and lectures or other educational events (paid to institution) from Siemens Healthineers; participated in a Data Safety Monitoring Board or Advisory Board for Escape – MEVO; and is unpaid research leader of the CONTRAST consortium. B.E. received grants outside of the submitted work (paid to institution) from Health Holland (Topsector Life Sciences & Health) and Nicolab. C.M. received grants during the conduct of the study (paid to institution) from TWIN Foundation and outside of the submitted work (paid to institution) from CVON/Dutch Heart Foundation, European Commission, Healthcare Evaluation Netherlands, and Stryker, and has minority interest of Nicolab.
Funding Sources
The authors received no funding for this study. The MR CLEAN Registry was partly funded by TWIN Foundation, Erasmus MC University Medical Center, Maastricht University Medical Center, and Amsterdam University Medical Centers, University of Amsterdam.
Author Contributions
Conception and design of the study: G.H., A.L., W.S., G.L., C.M., N.K., M.W., and M.v.W. Acquisition, analysis, or interpretation of the data: all authors. Drafting of the article: G.H., E.B., J.H., and S.K. Critical revision of the article: G.H., H.O., A.L., W.S., G.L., C.M., B.E., N.K., M.W., and M.v.W. All authors approved the final version of the article. MR CLEAN Registry investigators are group authors responsible for acquisition of the data, and revising the article and final approval.
Funding Statement
The authors received no funding for this study. The MR CLEAN Registry was partly funded by TWIN Foundation, Erasmus MC University Medical Center, Maastricht University Medical Center, and Amsterdam University Medical Centers, University of Amsterdam.
Data Availability Statement
Data are not available as participants did not provide written consent for public sharing of the data. Statistical analysis codes are available online as R scripts at a GitHub repository: https://github.com/edbonneville/validation-failed-EVT. Further inquiries can be directed to the corresponding author.
Supplementary Material.
References
- 1. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–31. [DOI] [PubMed] [Google Scholar]
- 2. Mulder M, Jansen IGH, Goldhoorn RJB, Venema E, Chalos V, Compagne KCJ, et al. Time to endovascular treatment and outcome in acute ischemic stroke: MR CLEAN registry results. Circulation. 2018;138(3):232–40. [DOI] [PubMed] [Google Scholar]
- 3. Holswilder G, Stuart MP, Dompeling T, Kruyt ND, Goeman JJ, van der Lugt A, et al. The prognostic value of extracranial vascular characteristics on procedural duration and revascularization success in endovascularly treated acute ischemic stroke patients. Eur Stroke J. 2022;7(1):48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Snelling BM, Sur S, Shah SS, Chen S, Menaker SA, McCarthy DJ, et al. Unfavorable vascular anatomy is associated with increased revascularization time and worse outcome in anterior circulation thrombectomy. World Neurosurg. 2018;120:e976–e983. [DOI] [PubMed] [Google Scholar]
- 5. Kaymaz ZO, Nikoubashman O, Brockmann MA, Wiesmann M, Brockmann C. Influence of carotid tortuosity on internal carotid artery access time in the treatment of acute ischemic stroke. Interv Neuroradiol. 2017;23(6):583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Penide J, Mirza M, McCarthy R, Fiehler J, Mordasini P, Delassus P, et al. Systematic review on endovascular access to intracranial arteries for mechanical thrombectomy in acute ischemic stroke. Clin Neuroradiol. 2022;32(1):5–12. [DOI] [PubMed] [Google Scholar]
- 7. Mokin M, Ansari SA, McTaggart RA, Bulsara KR, Goyal M, Chen M, et al. Indications for thrombectomy in acute ischemic stroke from emergent large vessel occlusion (ELVO): report of the SNIS Standards and Guidelines Committee. J Neurointerv Surg. 2019;11(3):215–20. [DOI] [PubMed] [Google Scholar]
- 8. Jansen IGH, Mulder M, Goldhoorn RJB; MR CLEAN Registry investigators . Endovascular treatment for acute ischaemic stroke in routine clinical practice: prospective, observational cohort study (MR CLEAN Registry). BMJ. 2018;360:k949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goyal M, Fargen KM, Turk AS, Mocco J, Liebeskind DS, Frei D, et al. 2C or not 2C: defining an improved revascularization grading scale and the need for standardization of angiography outcomes in stroke trials. J Neurointerv Surg. 2014;6(2):83–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lam RC, Lin SC, DeRubertis B, Hynecek R, Kent KC, Faries PL. The impact of increasing age on anatomic factors affecting carotid angioplasty and stenting. J Vasc Surg. 2007;45(5):875–80. [DOI] [PubMed] [Google Scholar]
- 11. Layton KF, Kallmes DF, Cloft HJ, Lindell EP, Cox VS. Bovine aortic arch variant in humans: clarification of a common misnomer. AJNR Am J Neuroradiol. 2006;27(7):1541–2. [PMC free article] [PubMed] [Google Scholar]
- 12. Müller MD, Ahlhelm FJ, von Hessling A, Doig D, Nederkoorn PJ, Macdonald S, et al. Vascular anatomy predicts the risk of cerebral ischemia in patients randomized to carotid stenting versus endarterectomy. Stroke. 2017;48(5):1285–92. [DOI] [PubMed] [Google Scholar]
- 13. Pancera P, Ribul M, Presciuttini B, Lechi A. Prevalence of carotid artery kinking in 590 consecutive subjects evaluated by Echocolordoppler. Is there a correlation with arterial hypertension? J Intern Med. 2000;248(1):7–12. [DOI] [PubMed] [Google Scholar]
- 14. Anadani M, Spiotta A, Alawieh A, Turjman F, Piotin M, Steglich-Arnholm H, et al. Effect of extracranial lesion severity on outcome of endovascular thrombectomy in patients with anterior circulation tandem occlusion: analysis of the TITAN registry. J Neurointerv Surg. 2019;11(10):970–4. [DOI] [PubMed] [Google Scholar]
- 15. North American Symptomatic Carotid Endarterectomy Trial . Methods, patient characteristics, and progress. Stroke. 1991;22(6):711–20. [DOI] [PubMed] [Google Scholar]
- 16. Fleiss JL. Measuring nominal scale agreement among many raters. Psychol Bull. 1971;76(5):378–82. [Google Scholar]
- 17. Buuren SV, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67. [Google Scholar]
- 18. Hoogland J, van Barreveld M, Debray TPA, Reitsma JB, Verstraelen TE, Dijkgraaf MGW, et al. Handling missing predictor values when validating and applying a prediction model to new patients. Stat Med. 2020;39(25):3591–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Friedman JH, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- 20. Musoro JZ, Zwinderman AH, Puhan MA, ter Riet G, Geskus RB. Validation of prediction models based on lasso regression with multiply imputed data. BMC Med Res Methodol. 2014;14:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balmaña J, Stockwell DH, Steyerberg EW, Stoffel EM, Deffenbaugh AM, Reid JE, et al. Prediction of MLH1 and MSH2 mutations in Lynch syndrome. JAMA. 2006;296(12):1469–78. [DOI] [PubMed] [Google Scholar]
- 22. Dumont TM, Bina RW. Difficult vascular access anatomy associated with decreased success of revascularization in emergent thrombectomy. J Vasc Interv Neurol. 2018;10(2):11–4. [PMC free article] [PubMed] [Google Scholar]
- 23. Alverne FJAM, Lima FO, Rocha FD, Bandeira DD, Lucena AF, Silva HC, et al. Unfavorable vascular anatomy during endovascular treatment of stroke: challenges and bailout strategies. J Stroke. 2020;22(2):185–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leng X, Hurford R, Feng X, Chan KL, Wolters FJ, Li L, et al. Intracranial arterial stenosis in Caucasian versus Chinese patients with TIA and minor stroke: two contemporaneous cohorts and a systematic review. J Neurol Neurosurg Psychiatry. 2021;92(6):590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are not available as participants did not provide written consent for public sharing of the data. Statistical analysis codes are available online as R scripts at a GitHub repository: https://github.com/edbonneville/validation-failed-EVT. Further inquiries can be directed to the corresponding author.