Abstract
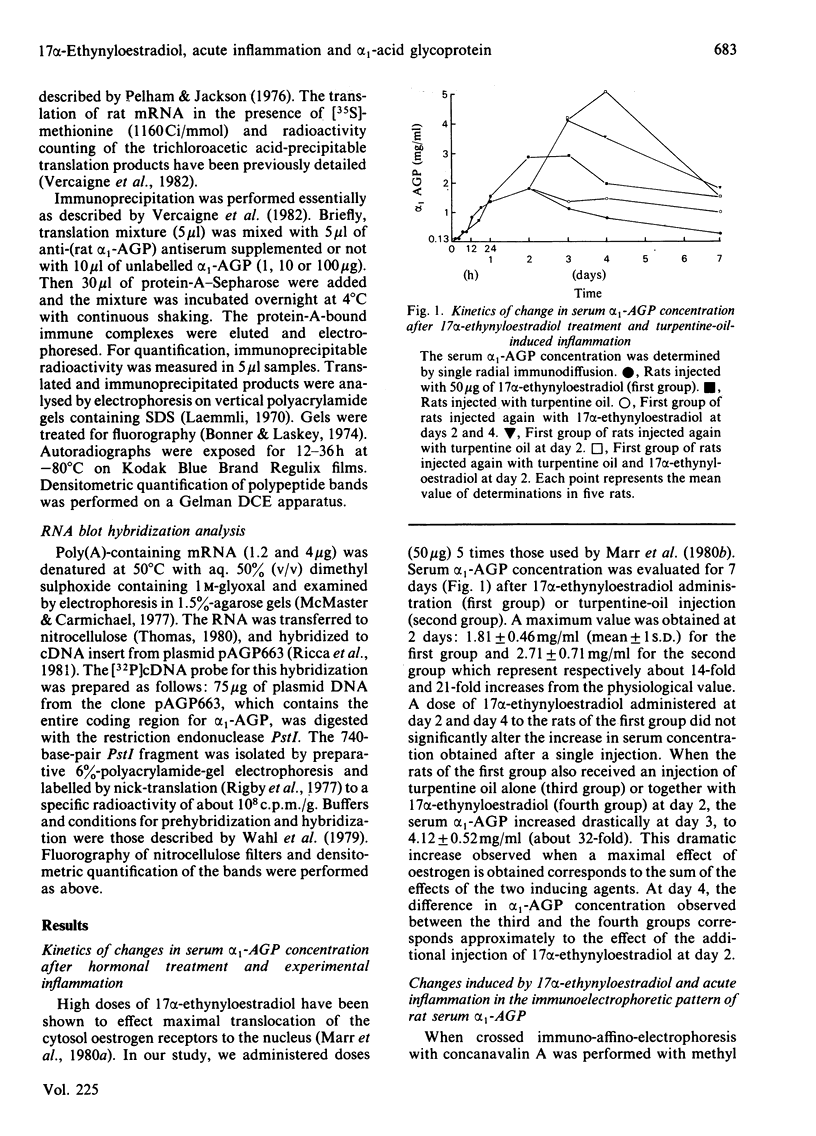
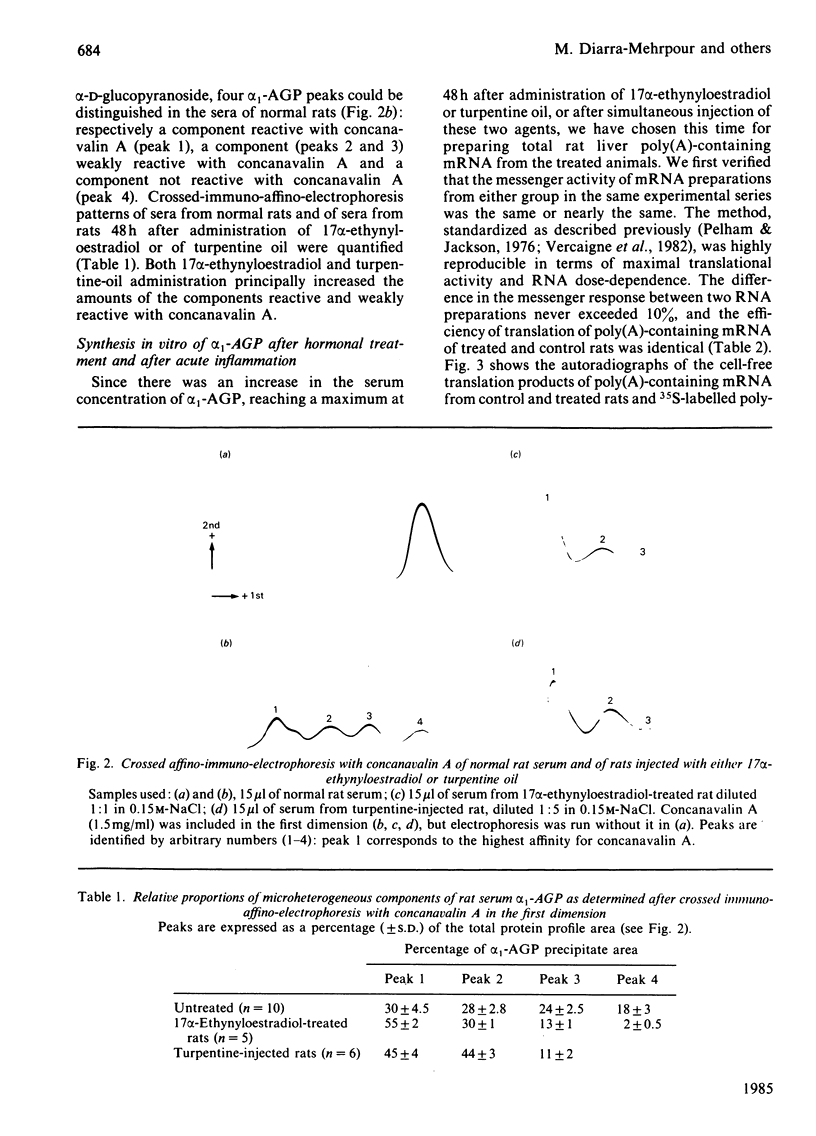
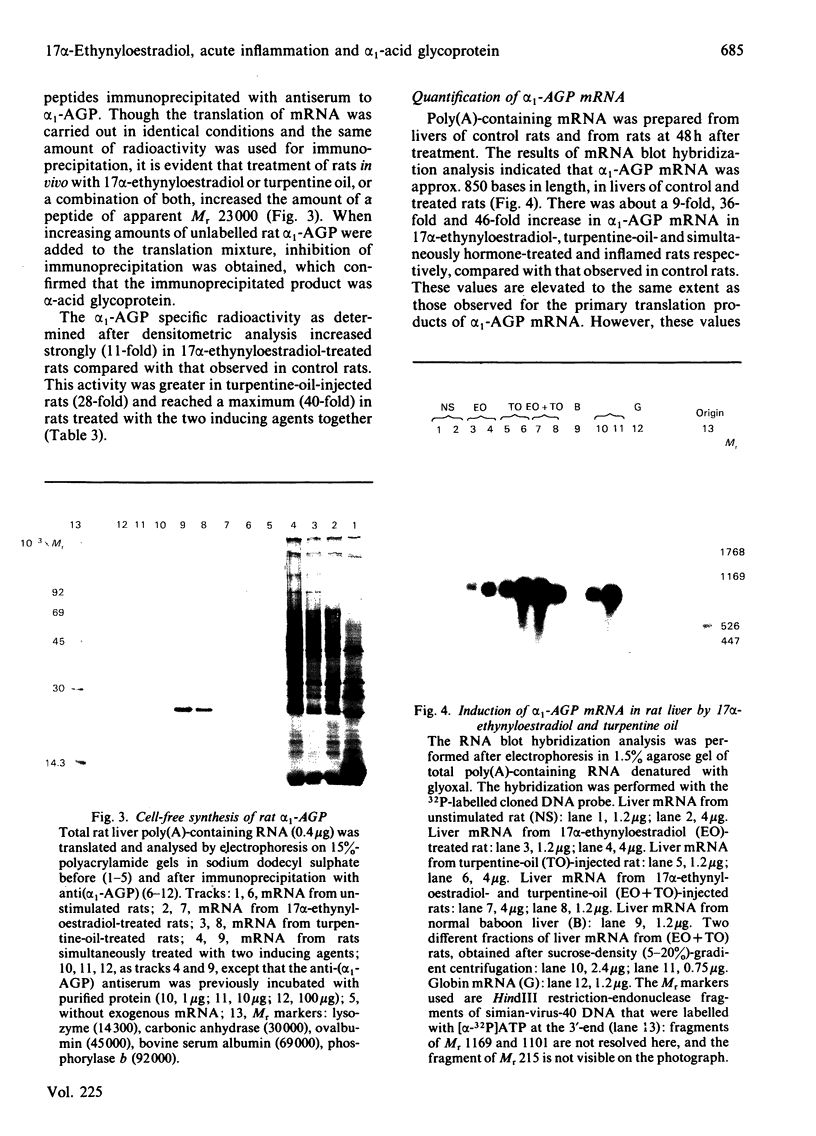
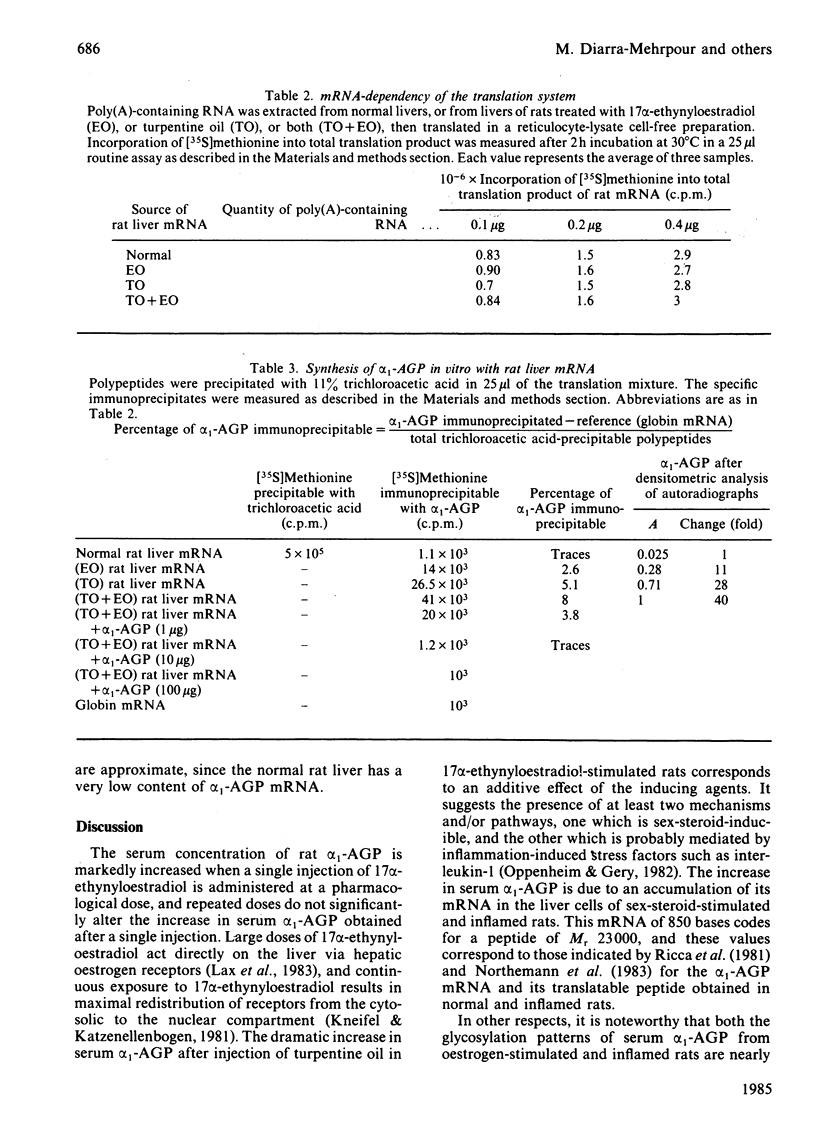
We measured the serum concentration of alpha 1-acid glycoprotein (alpha 1-AGP) and we evaluated the content of its hepatic mRNA in rats after 17 alpha-ethynyloestradiol treatment or after turpentine-induced acute inflammation, or after both treatments performed simultaneously. We have also studied the affinity of serum alpha 1-AGP for concanavalin A under these conditions. Both types of stimuli induce a marked retention of the glycoprotein on free concanavalin A. The serum concentration of alpha 1-AGP is increased about 14-fold compared with that in control rats when a single pharmacological dose (50 micrograms) or multiple injections of 17 alpha-ethynyloestradiol are administered. This increase is greater in turpentine-oil-injected rats (about 21-fold) and reaches a maximum (about 32-fold) in rats injected with 17 alpha-ethynyloestradiol plus turpentine oil; this increase in alpha 1-AGP corresponds to the addition of the effects of the two inducing agents. Similar changes are also observed either in the alpha 1-AGP mRNA content as estimated by using an alpha 1-AGP-specific cDNA probe, or in the amount of translatable alpha 1-AGP mRNA. The results indicate that: after a high dose of 17 alpha-ethynyloestradiol and after acute inflammation, the increase of the alpha 1-AGP serum concentration is due to an accumulation of the alpha 1-AGP mRNA; different mechanisms and/or pathways are probably involved in regulating the synthesis of alpha 1-AGP under various stimuli; 17 alpha-ethynyloestradiol as well as acute inflammation seem to control the glycosylation process of alpha 1-AGP in an identical manner.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bantle J. A., Hahn W. E. Complexity and characterization of polyadenylated RNA in the mouse brain. Cell. 1976 May;8(1):139–150. doi: 10.1016/0092-8674(76)90195-1. [DOI] [PubMed] [Google Scholar]
- Barbosa J., Seal U. S., Doe R. P. Anti-estrogens and plasma proteins. II. Contraceptive drugs and gestagens. J Clin Endocrinol Metab. 1973 Apr;36(4):706–714. doi: 10.1210/jcem-36-4-706. [DOI] [PubMed] [Google Scholar]
- Baumann H., Firestone G. L., Burgess T. L., Gross K. W., Yamamoto K. R., Held W. A. Dexamethasone regulation of alpha 1-acid glycoprotein and other acute phase reactants in rat liver and hepatoma cells. J Biol Chem. 1983 Jan 10;258(1):563–570. [PubMed] [Google Scholar]
- Baumann H., Held W. A. Biosynthesis and hormone-regulated expression of secretory glycoproteins in rat liver and hepatoma cells. Effect of glucocorticoids and inflammation. J Biol Chem. 1981 Oct 10;256(19):10145–10155. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Eisenfeld A. J., Krakoff L. R., Aten R. F. Developmental correlation of higher levels of estrogen binding by macromolecules in rat liver supernatant and of increases in plasma renin substrate levels after estrogen administration. Biochem Pharmacol. 1977 May 15;26(10):923–927. doi: 10.1016/0006-2952(77)90467-1. [DOI] [PubMed] [Google Scholar]
- Feigelson P., Kurtz D. T. Hormonal modulation of specific messenger RNA species in normal and neoplastic rat liver. Adv Enzymol Relat Areas Mol Biol. 1978;47:275–312. doi: 10.1002/9780470122921.ch4. [DOI] [PubMed] [Google Scholar]
- Gordon A. H., Koj A. Changes in the rates of synthesis of certain plasma proteins following tissue damage due to talc injection. A study using the perfused rat liver. Br J Exp Pathol. 1968 Oct;49(5):436–447. [PMC free article] [PubMed] [Google Scholar]
- Hager L. J., McKnight G. S., Palmiter R. D. Glucocorticoid induction of egg white mRNAs in chick oviduct. J Biol Chem. 1980 Aug 25;255(16):7796–7800. [PubMed] [Google Scholar]
- Houdebine L. M., Devinoy E., Delouis C. Stabilization of casein mRNA by prolactin and glucocorticoids. Biochimie. 1978;60(1):57–63. doi: 10.1016/s0300-9084(78)80198-9. [DOI] [PubMed] [Google Scholar]
- Kneifel M. A., Katzenellenbogen B. S. Comparative effects of estrogen and antiestrogen on plasma renin substrate levels and hepatic estrogen receptors in the rat. Endocrinology. 1981 Feb;108(2):545–552. doi: 10.1210/endo-108-2-545. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurell C. B., Rannevik G. A comparison of plasma protein changes induced by danazol, pregnancy, and estrogens. J Clin Endocrinol Metab. 1979 Nov;49(5):719–725. doi: 10.1210/jcem-49-5-719. [DOI] [PubMed] [Google Scholar]
- Lax E. R., Rumstadt F., Plasczyk H., Peetz A., Schriefers H. Antagonistic action of estrogens, flutamide, and human growth hormone on androgen-induced changes in the activities of some enzymes of hepatic steroid metabolism in the rat. Endocrinology. 1983 Sep;113(3):1043–1055. doi: 10.1210/endo-113-3-1043. [DOI] [PubMed] [Google Scholar]
- Marr W., Elder M. G., Lim L. The effects of oestrogens and progesterone on oestrogen receptors in female rat liver. Biochem J. 1980 Sep 15;190(3):563–570. doi: 10.1042/bj1900563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr W., White J. O., Elder M. G., Lim L. Nucleo-cytoplasmic relationships of oestrogen receptors in rat liver during the oestrous cycle and in response to administered natural and synthetic oestrogen. Biochem J. 1980 Jul 15;190(1):17–25. doi: 10.1042/bj1900017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicollet I., Lebreton J. P., Fontaine M., Hiron M. Evidence for alpha-1-acid glycoprotein populations of different pI values after concanavalin A affinity chromatography. Study of their evolution during inflammation in man. Biochim Biophys Acta. 1981 Apr 28;668(2):235–245. doi: 10.1016/0005-2795(81)90031-3. [DOI] [PubMed] [Google Scholar]
- Northemann W., Andus T., Gross V., Nagashima M., Schreiber G., Heinrich P. C. Messenger RNA activities of four acute phase proteins during inflammation. FEBS Lett. 1983 Sep 19;161(2):319–322. doi: 10.1016/0014-5793(83)81033-3. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Raynes J. Variations in the relative proportions of microheterogeneous forms of plasma glycoproteins in pregnancy and disease. Biomed Pharmacother. 1982 Mar;36(2):77–86. [PubMed] [Google Scholar]
- Ricca G. A., Hamilton R. W., McLean J. W., Conn A., Kalinyak J. E., Taylor J. M. Rat alpha 1-acid glycoprotein mRNA. Cloning of double-stranded cDNA and kinetics of induction of mRNA levels following acute inflammation. J Biol Chem. 1981 Oct 25;256(20):10362–10368. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Song C. S., Merkatz I. R., Rifkind A. B., Gillette P. N., Kappas A. The influence of pregnancy and oral contraceptive steroids on the concentration of plasma proteins. Studies with a quantitative immunodiffusion method. Am J Obstet Gynecol. 1970 Sep 15;108(2):227–231. doi: 10.1016/0002-9378(70)90301-7. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercaigne D., Bourguignon J., Lefebvre F., Martin J. P. Identification of the translation products of alpha -1-antitrypsin mRNA from baboon liver polysomes. FEBS Lett. 1982 Jan 25;137(2):231–235. doi: 10.1016/0014-5793(82)80356-6. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]