Abstract
Advanced microfabrication technologies and biocompatible hydrogel materials facilitate the modeling of 3D tissue microenvironment. Encapsulation of cells in hydrogel microparticles offers an excellent high-throughput platform for investigating multicellular interaction with their surrounding microenvironment. Compartmentalized microparticles support formation of various unique cellular structures. Alginate has emerged as one of the most dominant hydrogel materials for cell encapsulation owing to its cytocompatibility, ease of gelation, and biocompatibility. Alginate hydrogel provides a permeable physical boundary to the encapsulated cells and develops an easily manageable 3D cellular structure. The interior structure of alginate hydrogel can further regulate the spatio-temporal distribution of the embedded cells. This review provides a specific overview of the representative engineering approaches to generate various structures of cell-laden alginate microparticles in a uniform and reproducible manner. Capillary nozzle systems, microfluidic droplet systems, and non-chip based high-throughput microfluidic systems are highlighted for developing well-regulated cellular structure in alginate microparticles to realize potential drug screening platform and cell-based therapy. We conclude with the discussion of current limitations and future directions for realizing the translation of this technology to the clinic.
Introduction
Tissue consists of cellular components and extracellular matrix (ECM). ECM provides physical support to the cells constituting three-dimensional (3D) structure. Tissue at organ-level has a unique spatial organization of cellular components and cellular microenvironment. Cell-to-cell communication and microenvironmental factors involve in regulating cell viability, proliferation, behavior and functions [1–4]. Although the protocol of two-dimensional (2D) cell culture has been well established for a long period of time, the 2D platform often fails to predict and represent in vivo physiology [5,6]. In 3D, cells are subjected to different chemical gradients including oxygen and nutrient due to their spatial distribution in tissue [7]. The discrepancy between 2D and 3D culture leads to the misinterpretation of cellular drug response, which often hinders the drug development [8,9]. Accordingly, the development of in vitro 3D models reconstituting in vivo physiology is essential for better understanding cell biology and predicting clinical outcomes from appropriate therapeutic strategies.
In recent years, cell encapsulation in hydrogel microparticles has gathered great attention as a high-throughput platform for studying 3D cell culture. In this platform, cells are encapsulated in small hydrogel particles (generally, diameter < 1000 μm). Each microparticle provides a micro-cell culture environment with a controlled volume. It also provides physical boundary to the cells to isolate them from other cellular components. Well established droplet generation technologies with advanced hydrogel material science facilitates the utilization of the cell-laden hydrogel microparticle for various biomedical applications [10].
Various techniques have been introduced to generate cell-laden hydrogel microparticles, for example, microcapillary nozzle devices, coaxial microcapillary devices, and droplet microfluidic devices [10–12]. The fundamental aspects of forming cell-laden hydrogel microparticles are the hydrogel particle formation and cell encapsulation in the particle. Before the active use of hydrogels, aqueous droplets in oil was widely used to encapsulate cells. The droplets are maintained in liquid phase and thus required storage in oil (immiscible liquid) to maintain their shape. Typical oil has a limited gas diffusion and does not allow the diffusion of culture media due to phase separation. The volume of culture media was therefore radically limited by the volume of droplet in this system. This made it unsuitable for high cell density culture. The exchange of culture media is difficult, limiting a long-term cell culture in such aqueous droplets. As an alternative solution, hydrogel-based microparticle formation gathers great attention.
Hydrogel microparticles provide a highly permeable elastic scaffold and tissue-like structure to support the long-term culture of encapsulated cells, which can also be treated with chemical substances (e.g. drugs) via external loading. Various hydrogel materials have been proposed for cell encapsulation, including Matrigel [13], collagen [14], polyethylene glycol diacrylate (PEGDA) [15], gelatin methacryloyl (GelMa) [16], and alginate [17]. Among these materials, alginate has particularly gained attention as the cell encapsulation material owing to its abundance as a natural polymer, proven biocompatibility, excellent permeability, and convenient gelation process that is easy to control and rapid [17,18]. Alginate is generally recognized as a safe (GRAS) material by the Food and Drug Administration (FDA) [19], and is in commercial use or under clinical evaluation in various pharmaceutical and biomedical applications. Based on the outstanding capacity of alginate gel to immobilize living cells in it, recent studies have focused on potential clinical applications of alginate as an injectable cell-laden microparticles [20,21].
In this review, we provide a specific overview of engineered alginate hydrogel-based cell-laden microparticles for recapitulating 3D tissue. We discuss major engineering platforms that are actively used for generating cell-laden microparticles in the laboratory. In addition, recently developed technologies enabling high-throughput generation of the microparticle are introduced. Lastly, we discuss current technical limitations and potential strategies to translate this technology into the clinic.
Alginate as an excellent hydrogel material for cell-laden microparticles
Extraction and synthesis of alginate
Alginate is an anionic block copolymer composed of α-l-guluronic acid (G unit) and β-d-mannuronic acid (M unit), which has been extensively used for biomedical applications based on its advantages of availability from natural sources, low cost, ease of gelation, and biocompatibility. The most common source to extract alginate is brown algae seaweed (kelp). For example, sodium alginate can be extracted from kelp after treating with an alkali such as sodium carbonate solution, which can be further converted into insoluble calcium alginate by treating with calcium chloride solution [17]. This insoluble alginate salt can transform into alginic acid by treating it with an acidic solution. After purification, the salts of alginic acid with metals, such as sodium and calcium, can be dried, resulting in water-soluble alginate powders in various ionic forms [17,22]. Although mass production of alginate is available from harvesting algae, the composition and material properties of algal alginate can vary depending on their sources (species, age) and conditions (natural environment, harvest season.) Alternatively, alginate can also be produced by bacterial biosynthesis, which could provide alginate with more regulated chemical structures and properties compared with algal alginate [17,23]. Bacterial alginate can be obtained from the extracellular polymer of bacteria such as Azotobacter vinelandii and Pseudomonas spp. [24,25] By manipulating the culture conditions of the bacteria and genetically modifying the bacteria [26], different properties of alginate can be produced, although the scale of production is limited due to the lack of massive scale of bioreactor.
Properties of alginate
Alginate can be gelled to form a hydrogel by chemically and/or physically crosslinking its chains. Ionic crosslinking of alginate chains is one of the most widely used methods to gel alginate by combining aqueous alginate solution with various ionic crosslinking agents such as divalent cationic ions (e.g. Ca2+). One divalent cation can bind to four G units of two different alginate chains, resulting in a crosslinked gel structure. Mechanical properties of alginate hydrogel can be tuned by regulating the gelation method, crosslinking density, and viscosity of alginate polymer, while polymer composition (ratio between M and G), monomer unit sequence, and molecular weight are the critical factors that affect the viscosity of alginate [17]. Alginate hydrogel can be optimized to exhibit similar mechanical stiffness to tissue extracellular matrix, which enables the encapsulation of living cells. The highly permeable and hydrophilic nature of alginate hydrogel allows rapid diffusion of oxygen and nutrients to encapsulated cells and can support their growth continuously. In general, alginate is nondegradable in mammals, which lack the enzyme to cleave the alginate chains (alginase) [17]. Ionically crosslinked alginate gel can be dissolved based on ion exchange between divalent cations in the crosslinked alginate network and monovalent cations from surrounding media that disintegrate the alginate gel structure. However, it is difficult to completely remove high MW alginate from the body as it cannot be cleared through the kidney [27]. To overcome this limitation, the polymeric backbone of alginate can be partially oxidized to increase its vulnerability to hydrolysis and induces faster degradation [28].
Spatial organization of cells in alginate hydrogel microparticles
Based on these unique features of alginate, various spatial organizations of cells in alginate hydrogel microparticles are reported previously. In general, the spatial cellular organization in alginate beads can be grouped into two, unstructured and structured microparticles (Figure 1A). Unstructured cell-laden microparticles are easily obtained by simply mixing cellular component with alginate solution at a desired concentration. A simple droplet technique is required to obtain these microparticles. Thus, it is one of the most simple and easiest ways to study the interaction between cells and 3D microenvironment by providing a tissue-like structure. However, this unstructured cell-laden microparticle is not suitable for investigating the cellular interaction requiring a specific spatial cellular organization, where different types of cellular aggregates are forming distinct functional compartments. Core-shell structure has been of favor for providing isolated cellular microenvironment (Figure 1B). Core-shell microparticles typically consist of alginate shell to provide spatial isolation of cells in the core or spatial organization of different types of cells in the core and shell regions of the microparticles. In addition, anisotropic and multicompartmental microparticles enable various unique spatial cellular organization (Figure 1C). We list the selective applications in Table 1 and discussed them in the later section of this review. In the following section, we will discuss the methods for generating cell-laden microparticle and their specific applications.
Figure 1. Spatial organization of cells in alginate hydrogel microparticles.
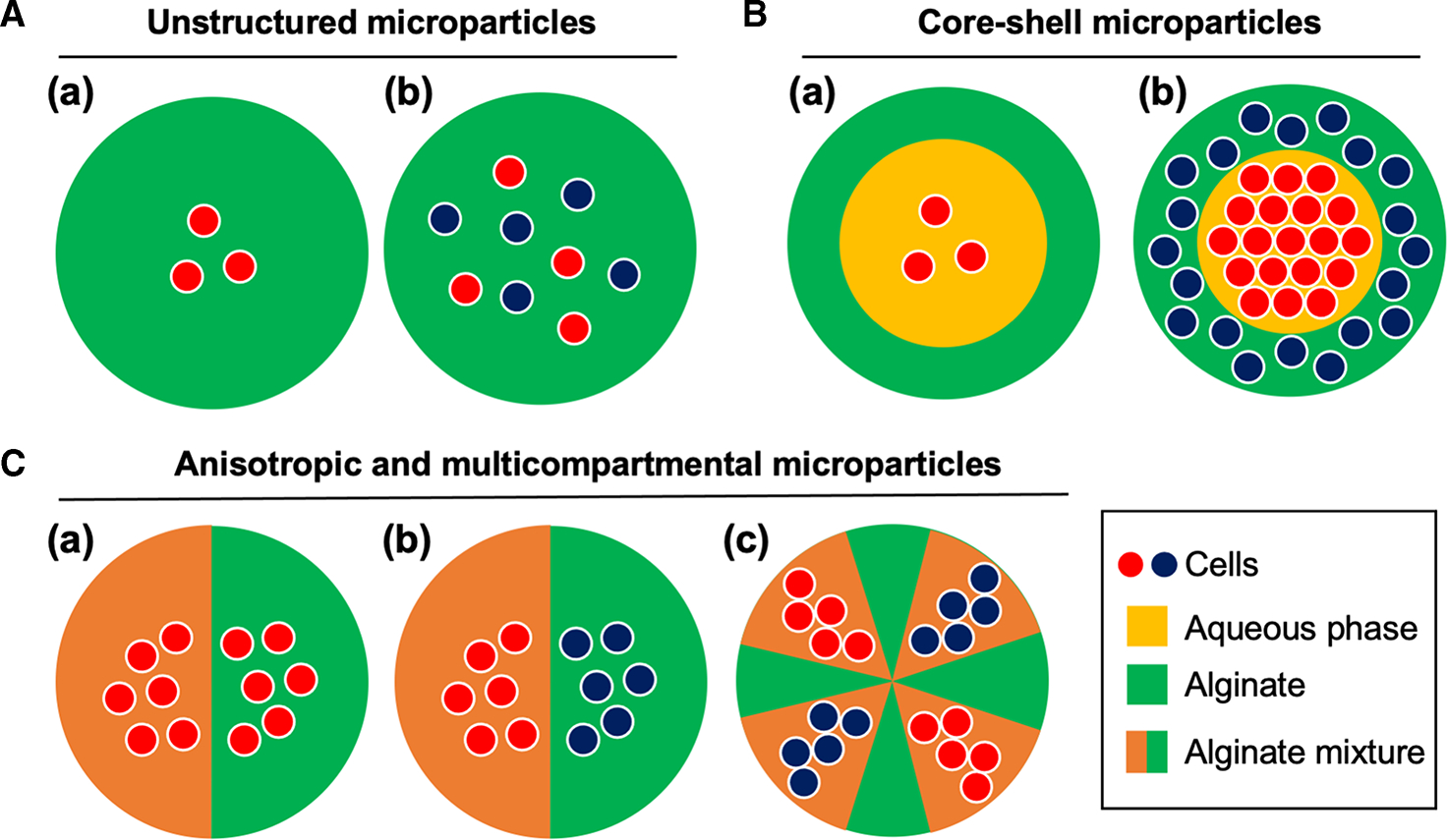
(A) Unstructured microparticles with (a) mono- and (b) multicellular encapsulation. (B) Core-shell microparticles with (a) mono- and (b) multicellular encapsulation. (C) (a) Mono- and (b) multicellular encapsulation of anisotropic microparticles and (c) multicompartmental microparticles.
Table 1.
Summary of material and methods of alginate-based cell-laden microparticles discussed in this review
| Materials |
Cellular components |
|||||
|---|---|---|---|---|---|---|
| Platform | Core | Shell | Core | Shell | Applications | Ref. |
|
| ||||||
| Microcapillary nozzle and coaxial microcapillary devices | SC cellulose (1%) & Alginate (1%) | Alginate (2%) | pADSCs | - | Cryopreservation of stem cell | [30] |
| Culture media | Alginate (2.5%) | CT26 cells | - | Modeling 3D tumor microenvironment | [29] | |
| SC cellulose (1%) | Alginate (1%) | PSCs | - | All-aqueous phase core-shell microparticle generation | [35] | |
| HE cellulose (0.5%) | Alginate (1.7%) | CT26 cells | - | Parametric investigation of forming core-shell microparticles | [31] | |
| Microfluidic device | Culture media | Alginate (1.7%) | HepG2 cells | NIH/3T3 cells | 3D liver microenvironment modeling | [52] |
| Type 1 collagen | Alginate (2%) | MCF-7 cells | - | Vascularized 3D tumor modeling | [38] | |
| SC-cellulose (1%) | Alginate (2%) | Mouse ESCs | - | Embryo-like core-shell microcapsules | [55] | |
| In-air microfluidics | Multiple materials | Alginate (1%) | MSCs | - | High-throughput fabrication of 3D multiscale biomaterials | [57,59] |
| Centrifuge-based microfluidics | Multiple hydrogel materials with alginate (3%) | NIH/3T3 cells | Compartmentalized microparticles for 3D cell culture | [56] | ||
| Alginate (4%) | NIH/3T3 cells | Massive production of cell-laden particles | [58] | |||
Engineering approaches for cell-laden microparticles
Microcapillary nozzle and coaxial microcapillary devices
Polymer-based microfluidic devices (e.g. Polydimethylsiloxane, PDMS) or glass microcapillary-based devices are widely used for generating cell-laden microparticles as an oil-free method. General approach to form the microparticle relies on alginate droplet generation followed by crosslinking. Different device designs and methods are used depending on the structure of microparticle. Microcapillary nozzle devices were commonly used to generate cell-laden alginate microparticles due to their simplicity (Figure 2A). A pulled glass microcapillary nozzle can be vertically positioned, and cell-alginate mixture is extruded through the nozzle at a desired flow rate to form microparticles. The droplet generation occurs due to the perturbations in the fluid stream (Rayleigh instability) and eventually pinch-off from the fluid column into droplets mainly due to surface tension. The alginate droplets fall into the gelling bath with CaCl2 solution and are cross-linked (gelation). The microcapillary nozzle system is an oil-free method minimizing the cell damage and has a simple configuration and thus, it is easy to fabricate and cost-effective.
Figure 2. Capillary-based devices for cell-laden microparticle generation.
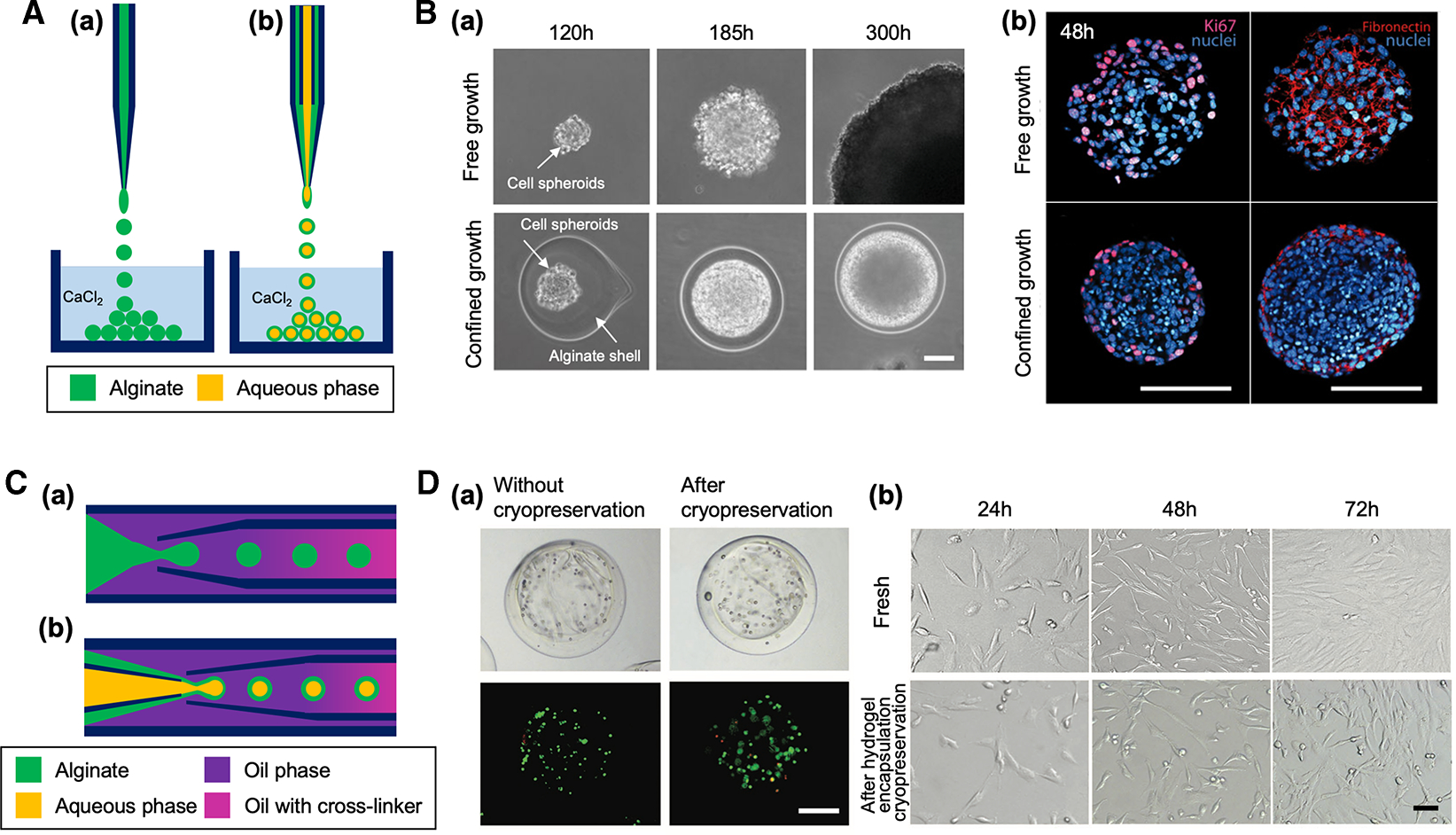
(A) Schematic of capillary nozzle devices for generating unstructured (a) and core-shell microparticles (b). (B) Confined growth of CT26 cellular spheroid in alginate shell for studying the mechanics of tumor progression [29]. (a) Phase contrast micrographs showing the growth of cell spheroid in confined alginate shell compared with free growth. Scale bar: 50 μm. (b) Confocal images showing a higher cell density of confined cell spheroid compared with free cell spheroid. Expression of the proliferation KI67 markers (magenta) and fibronectin (red) was restricted to the outermost cellular layers of the confined cell spheroid. Scale bars: 100 μm. Reproduced with permission from ref. [29]. Copyright © 2013 National Academy of Sciences. (C) Schematic of coaxial capillary devices for engineering unstructured (a) and core-shell microparticles (b). (D) Alginate hydrogel encapsulation to prevent cellular damage from ice formation during cryopreservation. (a) Brightfield and fluorescent images of porcine adipose-derived stem cells (pADSCs) in alginate hydrogel microcapsules after live/dead staining (live: green, dead: red), showing cell viability was not significantly changed after cryopreservation. Cells were encapsulated in the core-shell microparticle after treated with a cryopreservation agent. Scale bar: 200 μm. (b) Similar morphology of fresh pADSCs and the cells after cryopreserved under the protection of alginate hydrogel microcapsule. Scale bar: 50 μm. Reproduced with permission from ref. [30]. Copyright © 2017 John Wiley and Sons.
Alessandri et al. [29] previously demonstrated a massive production of size-controlled multicellular spheroids with microcapillary nozzle system for tumor study. Tumor cells were encapsulated in alginate core-shell microparticles to evaluate the tumor growth under environmental pressure generated by tumor expansion in confined tissue-mimetic structure (Figure 2B). They massively produced the size-controlled multicellular tumor spheroids with their device. The alginate shell provides a physical boundary for the tumor cells in the core until they became confluent. After tumor spheroid formed in the controlled volume of the microparticle, further expansion of the tumor spheroid is subjected to the compressing pressure due to the alginate boundary. This compressive stress contributes to the regulation of tumor growth, which potentially suppress the tumor evolution while triggering tumor cell invasion and metastasis.
Sub-millimeter liquid core hydrogel microparticles with controlled particle size monodispersity were reported using membrane and piezoelectric actuator [31]. In their system, capillary instability is controlled by harmonic vibration of the membrane via piezoelectric actuator to regulate microparticle generation. This study provided in-depth evaluation of experimental conditions ensuring core-shell formation in capillary nozzle system. The formation of core-shell particle is governed by a critical alginate shell thickness, which ensuring the gelation of alginate shell and preventing the mixing between aqueous core and alginate shell. The shell thickness is determined by the flow rate ratio between core and shell or by the correlation with the viscoelastic shear instability of the alginate flow. Detailed experimental investigation on the formation of core-shell microparticles with thin outer alginate layer in capillary nozzle system is also available from the study by Bredmond et al. [32].
Coaxial microcapillary device is another type of microcapillary-based device for the generation of microparticles, which often utilizes immiscible two-phase flow (oil and aqueous phase) (Figure 2C). A generation of monodispersed double immersions was demonstrated with the coaxial microcapillary device by Weitz’s group previously [33]. In this device geometry, the core and shell fluids (e.g. aqueous phase) are hydrodynamically focused (coaxial flow), and then this coaxial flow is cut into drops by immiscible carrier fluid (e.g. oil phase). The double immersions fabricated by this device can have a core-shell structure, in which the shell can be a solid phase by incorporating polymers, including those which are crosslinkable into hydrogels. Parametric investigation of droplet generation with coaxial microcapillary device is available from the study by Nabavi et al. [34]. In this study, they utilized a three-phase axisymmetric numerical model and validated the simulation with a set of experimental data to evaluate the effect of variations in flow rates, fluid properties, and geometry on droplet morphology and production rate and identify the experimental condition that can successfully generate core-shell droplets.
Long-term storage of such cell-laden core-shell particles can boost their therapeutic applications by enabling off-the-shelf availability. Cryopreservation of large volume core-shell structured hydrogel cell encapsulation approach was demonstrated by Zhao et al. [30]. In the study, they utilized tube-in-tube flow-focusing microcapillary device to encapsulate stem cells in alginate core-shell microparticles. The stem-cell laden core-shell microparticles showed high cell viability (>70%) even when ice formation was observed outside the particles, indicating that the hydrogel layer inhibited ice propagation inside the particles and minimized cryoinjury of cells in the core (Figure 2D).
Oil-free microfluidic devices
Unlike microcapillary nozzle, one technical concern in the generation of cell-laden microparticle with coaxial microcapillary devices is the use of oil as a continuous phase. After forming cell-laden particles, oil needs to be cleared. The washing process of oil that includes surfactant may cause microbubble formation due to vigorous stirring, which is difficult to remove once formed. Eliminating the washing step can significantly simplify the overall engineering process for generating core-shell microparticles. In a recent study by Zhu et al. [35], they demonstrated an oil-free method, utilizing all-aqueous-phase, for generating cell-laden core-shell microparticle. The water-in-water (W/W) system (Figure 3A(a)) was utilized by controlling the concentrations of two immiscible aqueous phases above a critical value that the interaction energy of the system exceeds Gibbs free energy of mixing [36]. The alginate-based core-shell microparticles were formed by applying oscillation of the immiscible aqueous fluid with a solenoid valve (pulsed modulation). They demonstrated 3D culture of pADSCs with high viability using this W/W system (Figure 3A(b)).
Figure 3. Oil-free microfluidic methods for cell-laden microparticle generation.
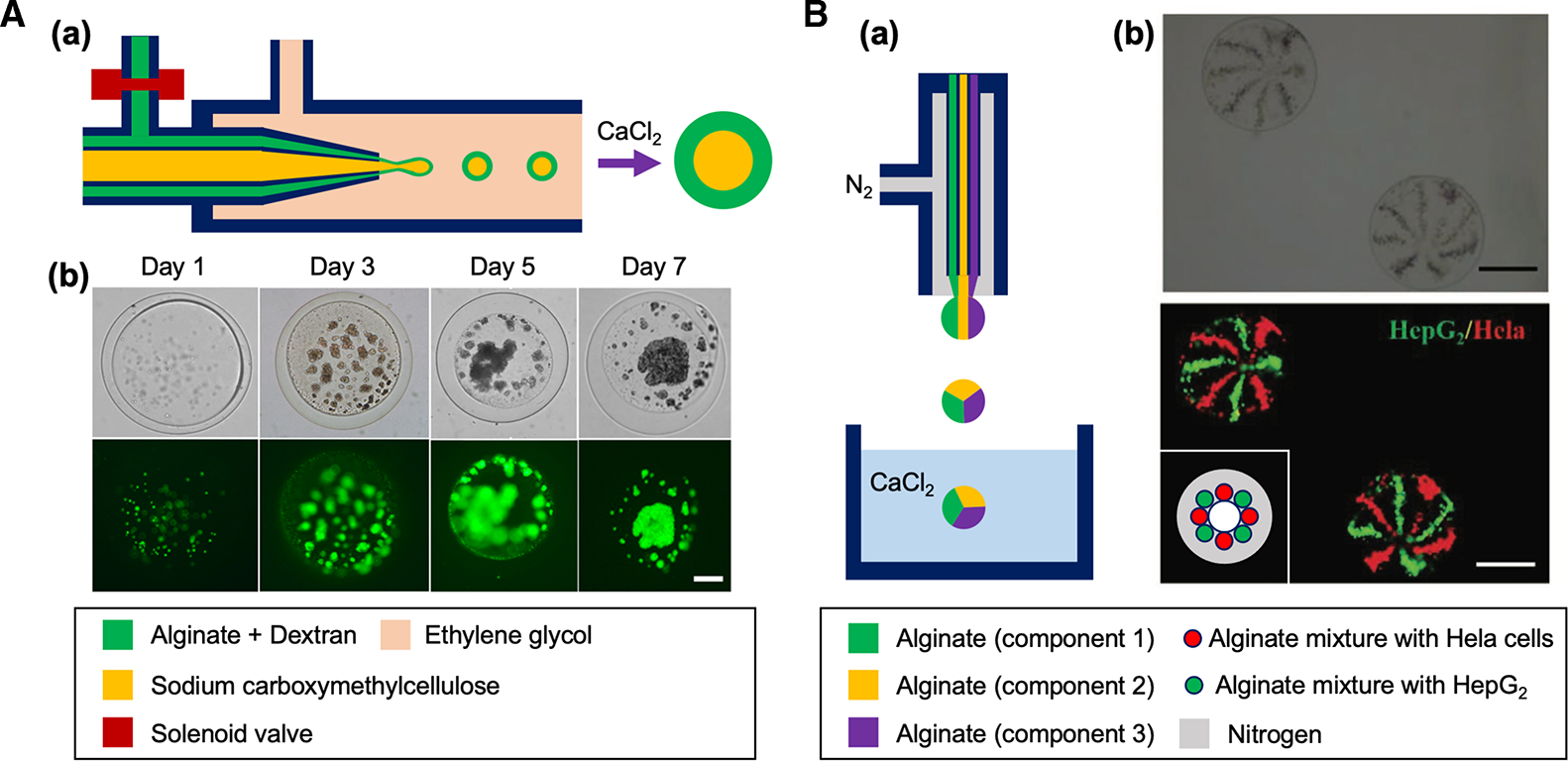
(A) (a) Schematic of a water-in-water microfluidic system for generating cell-laden core-shell microparticles. (b) Brightfield and fluorescent images showing the proliferation and aggregation of pADSCs in alginate microparticle during a week of culture (green: live cells). Scale bar: 50 μm. Reproduced with permission from ref. [35]. Copyright © 2019 American Chemical Society. (B) (a) Schematic of a gas-shearing microfluidic system for engineering multifaced alginate microspheres. (b) Brightfield and fluorescent images of the eight-faced microsphere with HepG2 and Hela cells, respectively. Inset schematic illustrates the cross-sectional view of the spray ejector of the device in (a) that can fabricate eight-faced microsphere in (b). Scale bars: 400 μm. Reproduced under the terms of the Creative Commons CC BY license from ref. [37]. Copyright © 2019 John Wiley and Sons.
Similarly, Tang et al. [37] proposed an oil-free gas-shearing method (Figure 3B(a)) to generate multicompartmental cell-laden alginate microparticles. Various types of multifaced microparticles were formed by injecting the solution with multicellular types (HepG2 and Hela cells) through the assembly of different numbers of microneedles (Figure 3B(b)). In their method, nitrogen gas was used to apply shear (‘gas-shearing’) to the aqueous flow to form the particles. The shear stress generated by the nitrogen gas flow overcomes the surface tension of the aqueous flow to break the flow into droplets. This system demonstrated the generation of broad range of particle sizes (55 to 1400 μm) by simply increasing the nitrogen gas flow from 0.1 to 1 L/min without the device modification. However, the device system requires a compressed nitrogen gas source at high flow rate to generate substantial shear, which consumes a large amount of the gas source and may not be practical to apply for highly viscous fluids.
Microfluidic droplet devices
Microfluidic platform has been widely used as a versatile tool for advanced biological applications such as single cell sequencing [39,40], single cell mechanical phenotyping [41,42], and reconstitution of organs [43,44]. In particular, droplet microfluidic device is a powerful platform for producing monodispersed droplets with controlled physical and chemical properties (Figure 4A). This device allows to generate hydrogel microparticles with various shapes including hollow and solid cylindrical fibers with different aspect ratio, symmetric and asymmetric spheres, core-shell structures, etc. [45,46]. The size of hydrogel microparticles can be manipulated by adjusting the flow rate of the working fluids, fluid properties (e.g. viscosity and interfacial tension) and the device geometry [47–49]. These parameters also affect the number of cells encapsulated in each hydrogel microparticle and overall cell encapsulation efficiency.
Figure 4. Microfluidic droplet devices for cell-laden microparticle generation.
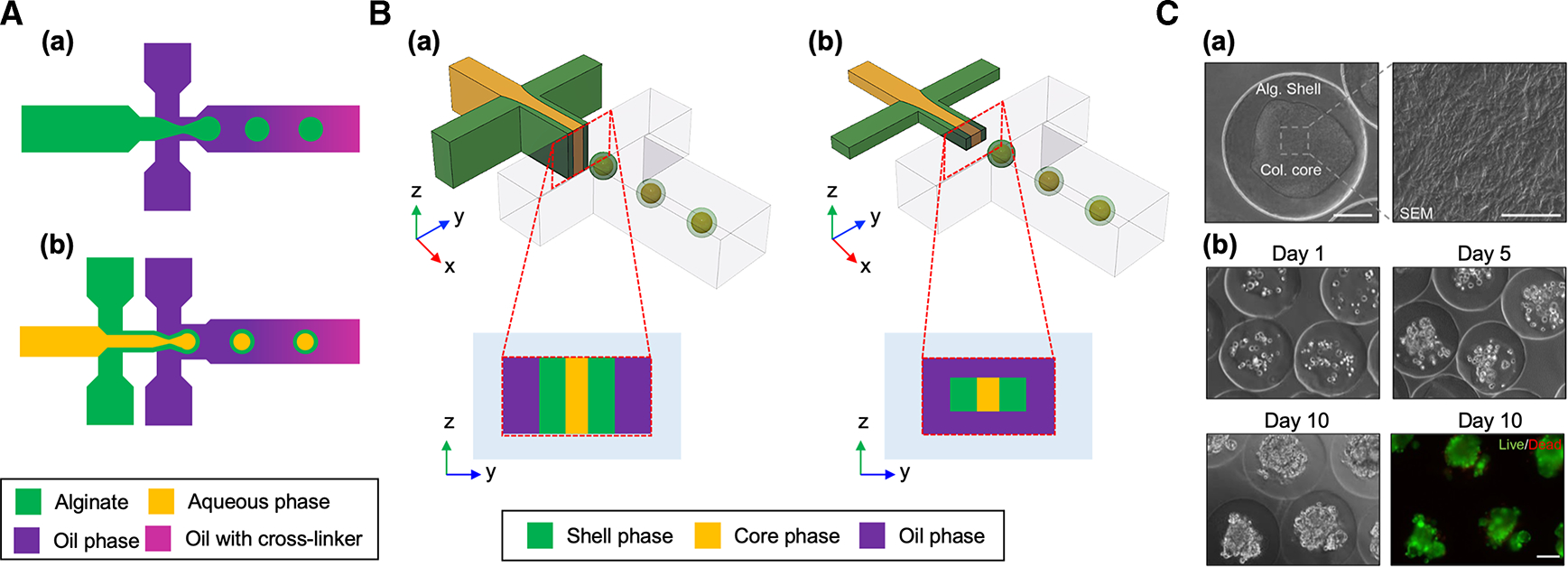
(A) Schematic of microfluidic droplet devices for generating (a) unstructured and (b) core-shell microparticles. (B) Schematic of (a) planar and (b) non-planar microfluidic droplet devices. (C) (a) Differential interference contrast (DIC) images of a collagen-alginate core-shell microparticle and scanning electron microscopy (SEM) images showing collagen fibers in the core. Scale bar: 100 μm for the DIC image and 5 μm for the SEM image. (b) Cell culture in collagen-alginate core-shell microparticles. Fluorescent image shows live (green)/dead (red) of the encapsulated MCF-7 cells. Scale bar: 100 μm. Reproduced with permission from ref. [38]. Copyright © 2017 American Chemical Society.
The gelation of alginate in the microfluidic device is a critical step for ensuring the formation of alginate-based cell-laden microparticles. Conventionally, calcium ions are added to the oil phase or insoluble calcium sources (typically CaCO3 particles) are directly added to the alginate solution to achieve the gelation of alginate in a continuous way immediately after the particle formation. Due to the rapid gelation, the direct contact of alginate solution with calcium ions often causes the issues of channel clogging in the devices. Alternatively, an acid-based method of alginate gelation (chelation of calcium by ethylenediaminetetraacetic acid (EDTA)) can be used to prevent the clogging [50], but a sudden drop in pH (from pH 6.7 to pH 4.6) potentially decreases cell viability [51]. To avoid this acid-based calcium release, the difference in affinity between ethylenediaminediacetic acid (EDDA) and EDTA to zinc ions (Zn2+) can be used to chelate calcium. However, this method requires two aqueous solutions for Zn-EDDA and Ca-EDTA solutions since zinc salt or Zn2+-chelate combination is generally not soluble in carrier fluid (typically oil phase).
These on-chip gelation methods facilitate the use of microfluidic droplet devices for the generation of cell-laden microparticles. Chen et al. [52] demonstrated in vitro 3D liver tissue modeling with alginate core-shell structure in a microfluidic device. They reconstituted the liver in a drop by assembling hepatocytes in the liquid core surrounded by fibroblasts in the alginate shell. The high permeability of the alginate enabled the encapsulated cells to be cultured for a relatively long period (two weeks) with high viability. The spatial cellular organization of hepatocytes and fibroblasts in the alginate core-shell structure showed an increased liver-specific functions compared with monotypic culture. This study showed a potential of a cell-laden core-shell microparticle for recapitulating micro-tissue environment with a specific cellular organization in 3D.
The on-chip formation of core-shell structure requires a specific control of preventing the mixing between two aqueous phases (typically alginate solution for shell and aqueous liquid for core such as culture media). A theoretical study by Huang et al. [53] provided a useful guidance to the on-chip production of alginate core-shell microparticles. Their study highlighted the displacement of two dispersed fluids (core media and shell alginate solutions) in the droplet before the gelation of alginate is highly affected by the shear induced by carrier fluid (oil phase) and the capillary effect from interfacial tension between the carrier fluid and dispersed fluids. A higher level of fluid displacement can induce the mixing of the two dispersed fluids, resulting in the failure of core-shell formation. Importantly, this fluid displacement can be minimized by increasing the viscosity of the core solution, in which the viscous effect becomes dominant than the fluid displacement momentum. Viscosity of the solutions can be controlled by adding biocompatible thickening agents (e.g. cellulose, collagen and gelatin). Another way to improve the engineering process to form alginate core-shell microparticles is by utilizing a non-planar microfluidic device (Figure 4B). Non-planar microfluidic devices can improve the core-shell microparticle generation as the cross-sectional area of the dispersed fluid orifice is smaller than the carrier fluid channel, which enables the carrier fluid to fully surround the dispersed fluid and de-wet it from the channel walls. As a result, the core-shell particles can be formed even when the dispersed fluid wets the wall in a non-planar device, whereas the particle formation in a planar device is only feasible when the wettability of the continuous fluid on the channel wall is more favorable than the dispersed fluid [54]. However, non-planar microfluidic devices require double patterning lithography, which makes the fabrication process more complex requiring microscale alignment.
By considering the above-mentioned factors, Agarwal et al. [55] demonstrated embryonic stem (ES) cell culture in alginate core-shell microparticles. ES cells were encapsulated in aqueous core thickened with cellulose to ensure the structure of microparticles with a non-planar microfluidic device. Microcapsules with liquid core and the alginate shell with porous nature supported the proliferation of cells in the core to form 3D cellular aggregates within a week with high viability (>92%). This pre-hatching embryo-mimetic miniaturized core-shell microparticles demonstrated the high levels of viability and pluripotency of the ES cells. The core-shell structure was also utilized for modeling in vitro vascularized 3D tumor tissue. Agarwal et al. [38] encapsulated the tumor cells in the collagen rich core surrounded by the alginate shell to recapitulate the 3D microenvironment of the tumor (Figure 4C). The tumor core-shell were cocultured with endothelial cells to recreate the macroscale 3D vascularized tumor tissue. This vascularized 3D tumor model more closely recapitulated in vivo tumor microenvironment and showed increased resistance to anti-cancer drug treatment than 2D cultured tumor cells. This model also serves as an effective platform for studying the impact of tumor microenvironment on progression and invasion of tumor and as an anti-cancer drug screening platform.
Non-chip-based high throughput microfluidics
Various designs of microfluidic devices were proposed to improve the limitations of conventional chip-based methods for generating cell-laden microparticles, such as relatively low yield efficiency and complexity for end-users to use on-site for testing drugs or treating patients. Morimoto et al. [58] demonstrated a centrifuge-based device for massive production of cell-laden alginate microparticles. A glass capillary nozzle, alginate-cell suspension tank, and CaCl2 solution bath for the gelation of alginate microparticle were integrated into a custom-built miniaturized system. The entire device is centrifuged, and the centrifugal force drives the flow of the alginate solution and it is ejected through the capillary nozzle to form the particles. Their system enabled oil-free high throughput generation of 45 000 uniform microparticles for 240 s. A similar concept of using centrifugal force to generate cell-laden alginate microparticles was also reported by Yoshida et al. [56]. In their study, a conventional microcentrifuge was used to generate the centrifugal force on a capillary nozzle integrated with microcentrifuge tube (Figure 5A(a)). They demonstrated the formation of compartmentalized spherical hydrogel microparticles with various types of ECM-based hemispheres and utilized the particle for 3D cell culture (Figure 5A(b–d)). During the formation of cell-laden microparticles, alginate was mixed with ECM sols as a sacrificial gelation template based on its rapid gelation property to overcome the slow gelation of ECM, which prevented diffusion and mixing of different types of ECM molecules in hemispheres. Their system eliminated the need of syringe pumps to drive the solutions, but it requires preloading of solution into the capillary before the centrifugation to generate the particles. Moreover, the total volume of the solution is limited by the volume of the capillary, which is not suitable for continuous massive production of microparticles.
Figure 5. Non-chip-based high throughput microfluidics for cell-laden microparticle generation.
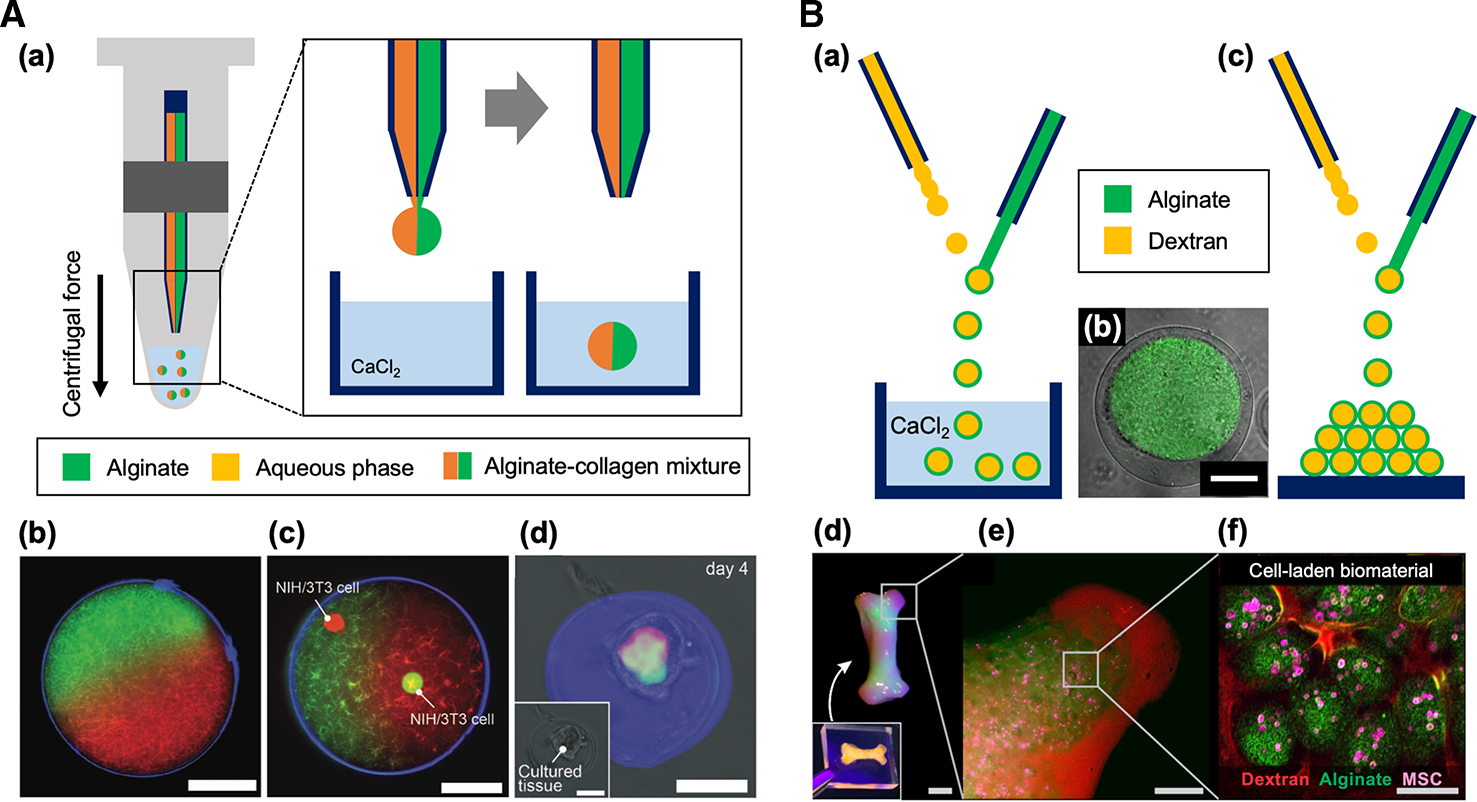
(A) (a) Schematic of a centrifuge-based microfluidic device for Janus microparticles. Anisotropic collagen microparticles (b) without and (c) with NIH/3T3 cells encapsulation. (d) Construction of microtissue in the microparticles after 4 days of culture. Scale bars: 50 μm. Reproduced with permission from ref. [56]. Copyright © 2017 John Wiley and Sons.
(B) (a) Schematic of in-air microfluidic (IAMF) device for core-shell microparticle generation. (b) Representative image of the dextran-tyramine-alginate core-shell microparticle. In this image, the core region (dextran-tyramine) can be distinguished with green color, while the shell region (alginate) is not labeled. Scale bar: 50 μm. (c, d, e & f) 3D printing application of IAMF. The 3D multiscale modular material was composed of human MSCs (mesenchymal stem cells, pink), which were incorporated in alginate microparticles (green) that are placed in dextran-tyramine hydrogel (red). Scale bars: 1 cm (d), 5 mm (e), 100 μm (f). Reproduced with permission from ref. [57]. Copyright © 2018 American Association for the Advancement of Science.
Ultra-high throughput of the microparticle fabrication was demonstrated using in-air microfluidics (IAMF) [57,59]. This IAMF was achieved by manipulating micrometer scale liquid streams in the air using a microcapillary nozzle system. Monodisperse emulsions, particles, and fibers with various compositions, shapes, and sizes were fabricated by regulating the breakup of the jet and impact of two liquid microjets that were generated from two nozzles in the air (Figure 5B(a)). Core-shell structure was formed by the in-air impact of core drop on the jet of shell solution when the core solutions have a higher surface tension than the shell solutions (Figure 5B(b)). IAMF can achieve 10 to 100 times faster production rates than typical chip-based droplet microfluidics. IAMF also allows on-the-fly production and deposition of microparticles directly into 3D multiscale modular biomaterials (3D printing) (Figure 5B(c–f)) [57]. Although the versatility of the device shows a great potential for various bio-applications, the device operation requires fine adjustment of nozzle angle for ensuring the impact between core and shell fluids and tuning of fluid property-dependent flow rates to ensure desired liquid jet.
Clinical translation of alginate microparticles
Biomedical applications of cell-encapsulated alginate microparticles include in vitro tissue modeling system to test the efficacy and safety of drugs and a cell delivery system that can be directly implanted at the defect site for treating injury and disease (Figure 6) [10]. Alginate microparticles are injectable due to their small size and can potentially be used for minimally invasive therapy to eliminate surgical operation. Alginate microparticles coimmobilized with human osteoprogenitors and endothelial cells promoted mineralization in bone, which can be used for treating bone defect [60]. Alginate microparticles can also protect cells from immune clearance and improve the residence time of infused cells. For example, mesenchymal stem cells (MSCs) encapsulated in alginate microgel exhibited longer in vivo persistence after intravenous injection compared with unencapsulated cells. Alginate gel did not compromise the diffusion of paracrine factors secreted from the embedded MSCs and significantly improved the immunomodulatory therapeutic effect of MSCs in a bone marrow transplant model [21]. In addition, pancreatic islet cells encapsulated in alginate microparticle could achieve long-term viability and glucose-responsiveness after it was laparoscopically transplanted to the bursa omentalis of non-human primate models without any immunosuppression [20]. However, despite its significant progress in research, regulation of the purity and consistency of alginate still remains a challenge to boost its clinical applications since alginate is mostly obtained from natural sources that show batch-to-batch variance. Although alginate is reported to be biocompatible, the impact of various residual impurities in alginate is still under debate [17,61]. For the successful translation of alginate microparticles, further optimization of alginate properties and encapsulation technique is needed specific to the application.
Figure 6. Schematic diagram of biomedical applications of alginate microparticles.
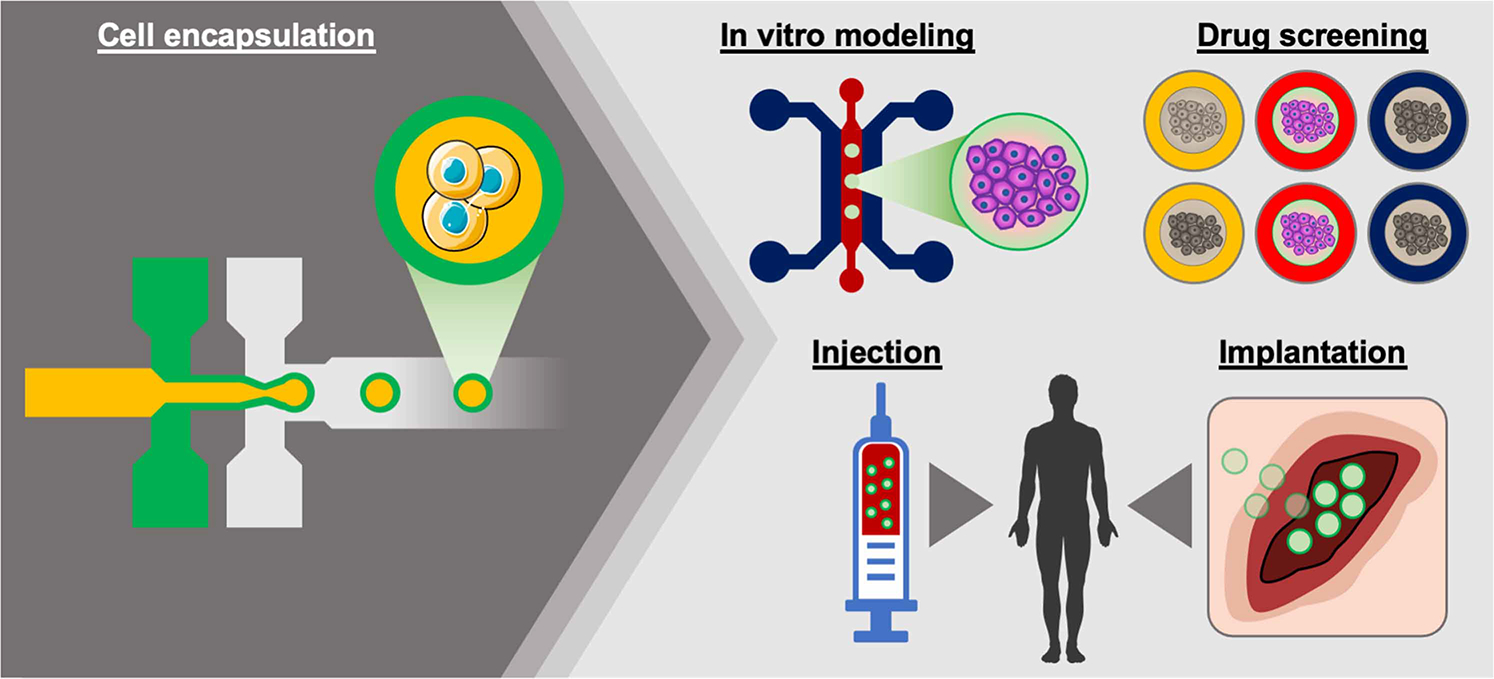
Cell-laden alginate microparticles can be used for in vitro tissue modeling and high-throughput drug screening platform. The particles can also be injectable and implantable to deliver cells in clinical applications.
Outlook
Cell-laden hydrogel microparticles can provide physiologically relevant 3D in vitro culture platforms and have high potential therapeutic applications. This technology can be utilized for large-scale drug screening, preclinical test of new therapies in relevant pathological tissue microenvironment conditions, and stem cell therapy for tissue regeneration. To translate the current platform to clinic in a reliable manner, cell-laden particles should possess good biocompatibility and scalability. There is an increased need to develop a method that minimizes cell damage during the process of particle formation as previous studies typically report a decreased cell viability of 70% after the engineering process, limiting long-term cell culture. Alginate gelation during cell encapsulation typically requires the usage of acid-based crosslinking solutions which are detrimental to the cell. The additional step for the removal of oil to collect the particles also decreases the cell viability by increasing the exposure time of cells to non-culture conditions. Although oil-free methods are available, they still require high flow rates potentially causing mechanical cell damage induced by high fluid shear stress. Most of all, high-throughput platforms are essential to scale-up the cell-laden microparticle technology for clinical translation. It is highly desirable to develop a parallelized platform integrated with automated modules for sample preparation and particle retrieval to minimize the fabrication time of cell-laden microparticles. Such an automated, highly efficient, parallelized high-throughput cell-laden microparticle generating system could further advance the clinical use of cell-laden microparticles for personalized therapeutic applications dealing with a limited amount of human patient cells.
Perspectives.
Cell-laden alginate microparticles can recapitulate 3D tissue environment, which enables microenvironmental modulation of cells and facilitates the understanding of the crosstalk between cells and their surrounding environment.
Various engineering technologies were introduced to achieve spatial organization of cells in alginate microparticles, such as microcapillary nozzles, coaxial microcapillary devices, microfluidic devices, and non-chip-based devices. These methods allow substantial control of cell encapsulation with a high level of cell viability.
Clinical translation of cell-laden hydrogel microparticles can be achieved by enabling its timely, scalable, and accurate production without damaging encapsulated cells.
Acknowledgements
This work was supported by the National Institutes of Health Awards CA236702, CA214411, AR073135, CA229772, American Lung Association Cancer Discovery Award, Department of Defense PC180355.
Abbreviations
- CLEX
Competitive ligand exchange crosslinking
- CT26
Mouse colon carcinoma cell line
- EDDA
Ethylenediaminediacetic acid
- EDTA
Ethylenediaminetetraacetic acid
- GelMa
Gelatin methacryloyl
- HE-cellulose
Hydroxy ethyl cellulose
- HepG2
Human hepatocellular carcinoma cell line
- MCF-7
Human breast cancer cell line
- MSCs
Mesenchymal stem cells
- NIH-3T3
Mouse embryonic fibroblast cell line
- pADSCs
Porcine adipose derived stem cells
- PDMS
Polydimethylsiloxane
- PEGDA
Polyethylene glycol diacrylate
- PSCs
Porcine stem cells
- SC-cellulose
Sodium carboxymethyl cellulose
Footnotes
Competing Interests
Dr. Jang is a founder and owns equity in Curer, Inc. Dr. Sengupta is a cofounder and owns equity in Vyome, Akamara, and Invictus Oncology.
References
- 1.Bissell MJ, Hall HG and Parry G (1982) How does the extracellular matrix direct gene expression? J. Theor. Biol. 99, 31–68 10.1016/0022-5193(82)90388-5 [DOI] [PubMed] [Google Scholar]
- 2.Weaver VM, Petersen OW, Wang F, Larabell C, Briand P, Damsky C et al. (1997) Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 137, 231–245 10.1083/jcb.137.1.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhadriraju K and Chen CS (2002) Engineering cellular microenvironments to improve cell-based drug testing. Drug Discov. Today 7, 612–620 10.1016/S1359-6446(02)02273-0 [DOI] [PubMed] [Google Scholar]
- 4.Bonnans C, Chou J and Werb Z (2014) Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786–801 10.1038/nrm3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soares CP, Midlej V, de Oliveira MEW, Benchimol M, Costa ML and Mermelstein C (2012) 2D and 3D-organized cardiac cells shows differences in cellular morphology, adhesion junctions, presence of myofibrils and protein expression. PLoS ONE 7, e38147 10.1371/journal.pone.0038147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schyschka L, Sánchez JM, Wang Z, Burkhardt B, Müller-Vieira U, Zeilinger K et al. (2013) Hepatic 3D cultures but not 2D cultures preserve specific transporter activity for acetaminophen-induced hepatotoxicity. Arch. Toxicol. 87, 1581–1593 10.1007/s00204-013-1080-y [DOI] [PubMed] [Google Scholar]
- 7.Kapalczynska M, Kolenda T, Przybyla W, Zajaczkowska M, Teresiak A, Filas V et al. (2018) 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch. Med. Sci. 14, 910–919 10.5114/aoms.2016.63743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edmondson R, Broglie JJ, Adcock AF and Yang L (2014) Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 12, 207–218 10.1089/adt.2014.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majumder B, Baraneedharan U, Thiyagarajan S, Radhakrishnan P, Narasimhan H, Dhandapani M et al. (2015) Predicting clinical response to anticancer drugs using an ex vivo platform that captures tumour heterogeneity. Nat. Commun. 6, 6169 10.1038/ncomms7169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daly AC, Riley L, Segura T and Burdick JA (2019) Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 5, 20–43 10.1038/s41578-019-0148-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohamed MG, Ambhorkar P, Samanipour R, Yang A, Ghafoor A and Kim K (2020) Microfluidics-based fabrication of cell-laden microgels. Biomicrofluidics 14, 021501 10.1063/1.5134060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzitelli S, Capretto L, Quinci F, Piva R and Nastruzzi C (2013) Preparation of cell-encapsulation devices in confined microenvironment. Adv. Drug Deliv. Rev. 65, 1533–1555 10.1016/j.addr.2013.07.021 [DOI] [PubMed] [Google Scholar]
- 13.Hughes CS, Postovit LM and Lajoie GA (2010) Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10, 1886–1890 10.1002/pmic.200900758 [DOI] [PubMed] [Google Scholar]
- 14.Sheu M-T, Huang J-C, Yeh G-C and Ho H-O (2001) Characterization of collagen gel solutions and collagen matrices for cell culture. Biomaterials 22, 1713–1719 10.1016/S0142-9612(00)00315-X [DOI] [PubMed] [Google Scholar]
- 15.Nemir S, Hayenga HN and West JL (2010) PEGDA hydrogels with patterned elasticity: novel tools for the study of cell response to substrate rigidity. Biotechnol. Bioeng. 105, 636–644 10.1002/bit.22574 [DOI] [PubMed] [Google Scholar]
- 16.Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N and Khademhosseini A (2015) Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 73, 254–271 10.3390/polym10111290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee KY and Mooney DJ (2012) Alginate: properties and biomedical applications. Prog. Polym. Sci. 37, 106–126 10.1016/j.progpolymsci.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neves MI, Moroni L and Barrias CC (2020) Modulating alginate hydrogels for improved biological performance as cellular 3D microenvironments. Front. Bioeng. Biotechnol. 8, 665 10.3389/fbioe.2020.00665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sodium Alginate. (2013) CFR—code of federal regulations title 21. [Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1724
- 20.Bochenek MA, Veiseh O, Vegas AJ, McGarrigle JJ, Qi M, Marchese E et al. (2018) Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques. Nat. Biomed. Eng. 2, 810–821 10.1038/s41551-018-0275-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao AS, Özkale B, Shah NJ, Vining KH, Descombes T, Zhang L et al. (2019) Programmable microencapsulation for enhanced mesenchymal stem cell persistence and immunomodulation. Proc. Natl Acad. Sci. U.S.A. 116, 15392–15397 10.1073/pnas.1819415116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rinaudo M (2008) Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 57, 397–430 10.1002/pi.2378 [DOI] [Google Scholar]
- 23.Rehm B and Valla S (1997) Bacterial alginates: biosynthesis and applications. Appl. Microbiol. Biotechnol. 48, 281–288 10.1007/s002530051051 [DOI] [PubMed] [Google Scholar]
- 24.Urtuvia V, Maturana N, Acevedo F, Peña C and Díaz-Barrera A (2017) Bacterial alginate production: an overview of its biosynthesis and potential industrial production. World J. Microbiol. Biotechnol. 33, 198 10.1007/s11274-017-2363-x [DOI] [PubMed] [Google Scholar]
- 25.Franklin MJ, Nivens DE, Weadge JT and Howell PL (2011) Biosynthesis of the pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Front. Microbiol. 2, 167 10.3389/fmicb.2011.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hay ID, Rehman ZU, Moradali MF, Wang Y and Rehm BH (2013) Microbial alginate production, modification and its applications. Microb. Biotechnol. 6, 637–650 10.1111/1751-7915.12076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Shamkhani A and Duncan R (1995) Radioiodination of alginate via covalently-bound tyrosinamide allows monitoring of its fate in vivo. J. Bioact. Compat. Polym. 10, 4–13 10.1177/088391159501000102 [DOI] [Google Scholar]
- 28.Gomez C, Rinaudo M and Villar M (2007) Oxidation of sodium alginate and characterization of the oxidized derivatives. Carbohydr. Polym. 67, 296–304 10.1016/j.carbpol.2006.05.025 [DOI] [Google Scholar]
- 29.Alessandri K, Sarangi BR, Gurchenkov VV, Sinha B, Kiessling TR, Fetler L et al. (2013) Cellular capsules as a tool for multicellular spheroid production and for investigating the mechanics of tumor progression in vitro. Proc. Natl Acad. Sci. U.S.A. 110, 14843–14848 10.1073/pnas.1309482110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao G, Liu X, Zhu K and He X (2017) Hydrogel encapsulation facilitates rapid-cooling cryopreservation of stem cell-laden core–shell microcapsules as cell–biomaterial constructs. Adv Healthc Mater. 6, 1700988 10.1002/adhm.201700988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doméjean H, Saint Pierre M.d.l.M., Funfak A, Atrux-Tallau N, Alessandri K, Nassoy P et al. (2017) Controlled production of sub-millimeter liquid core hydrogel capsules for parallelized 3D cell culture. Lab. Chip 17, 110–119 10.1039/C6LC00848H [DOI] [PubMed] [Google Scholar]
- 32.Bremond N, Santanach-Carreras E, Chu L-Y and Bibette J (2010) Formation of liquid-core capsules having a thin hydrogel membrane: liquid pearls. Soft Matter. 6, 2484–2488 10.1039/B923783F [DOI] [Google Scholar]
- 33.Utada AS, Lorenceau E, Link DR, Kaplan PD, Stone HA and Weitz D (2005) Monodisperse double emulsions generated from a microcapillary device. Science 308, 537–541 10.1126/science.1109164 [DOI] [PubMed] [Google Scholar]
- 34.Nabavi SA, Vladisavljevic GT, Gu S and Ekanem EE (2015) Double emulsion production in glass capillary micró fluidic device: parametric investigation of droplet generation behaviour. Chem. Eng. Sci. 130, 183–196 10.1016/j.ces.2015.03.004 [DOI] [Google Scholar]
- 35.Zhu K, Yu Y, Cheng Y, Tian C, Zhao G and Zhao Y (2019) All-aqueous-phase microfluidics for cell encapsulation. ACS Appl. Mater. Interfaces 11, 4826–4832 10.1021/acsami.8b19234 [DOI] [PubMed] [Google Scholar]
- 36.Raghavarao K, Rastogi N, Gowthaman M and Karanth N (1995) Aqueous two-phase extraction for downstream processing of enzymes/proteins. Adv. Appl. Microbiol. 41, 97–171 10.1016/S0065-2164(08)70309-5 [DOI] [Google Scholar]
- 37.Tang G, Xiong R, Lv D, Xu RX, Braeckmans K, Huang C et al. (2019) Gas-shearing fabrication of multicompartmental microspheres: a one-step and oil-free approach. Adv. Sci. 6, 1802342 10.1002/advs.201802342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agarwal P, Wang H, Sun M, Xu J, Zhao S, Liu Z et al. (2017) Microfluidics enabled bottom-up engineering of 3D vascularized tumor for drug discovery. ACS Nano 11, 6691–6702 10.1021/acsnano.7b00824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grosselin K, Durand A, Marsolier J, Poitou A, Marangoni E, Nemati F et al. (2019) High-throughput single-cell ChIP-seq identifies heterogeneity of chromatin states in breast cancer. Nat. Genet. 51, 1060–1066 10.1038/s41588-019-0424-9 [DOI] [PubMed] [Google Scholar]
- 40.Zilionis R, Nainys J, Veres A, Savova V, Zemmour D, Klein AM et al. (2017) Single-cell barcoding and sequencing using droplet microfluidics. Nat. Protoc. 12, 44 10.1038/nprot.2016.154 [DOI] [PubMed] [Google Scholar]
- 41.Gossett DR, Henry T, Lee SA, Ying Y, Lindgren AG, Yang OO et al. (2012) Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc. Natl Acad. Sci. U.S.A. 109, 7630–7635 10.1073/pnas.1200107109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otto O, Rosendahl P, Mietke A, Golfier S, Herold C, Klaue D et al. (2015) Real-time deformability cytometry: on-the-fly cell mechanical phenotyping. Nat. Methods 12, 199–202 10.1038/nmeth.3281 [DOI] [PubMed] [Google Scholar]
- 43.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY and Ingber DE (2010) Reconstituting organ-level lung functions on a chip. Science 328, 1662–1668 10.1126/science.1188302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sontheimer-Phelps A, Hassell BA and Ingber DE (2019) Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 19, 65–81 10.1038/s41568-018-0104-6 [DOI] [PubMed] [Google Scholar]
- 45.Selimovic Š, Oh J, Bae H, Dokmeci Mand Khademhosseini A (2012) Microscale strategies for generating cell-encapsulating hydrogels. Polymers 4, 1554–1579 10.3390/polym4031554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H, Tumarkin E, Peerani R, Nie Z, Sullan RMA, Walker GC et al. (2006) Microfluidic production of biopolymer microcapsules with controlled morphology. J. Am. Chem. Soc. 128, 12205–12210 10.1021/ja0635682 [DOI] [PubMed] [Google Scholar]
- 47.Nie Z, Seo M, Xu S, Lewis PC, Mok M, Kumacheva E et al. (2008) Emulsification in a microfluidic flow-focusing device: effect of the viscosities of the liquids. Microfluid Nanofluid 5, 585–594 10.1007/s10404-008-0271-y [DOI] [Google Scholar]
- 48.Van Steijn V, Korczyk PM, Derzsi L, Abate AR, Weitz DA and Garstecki P (2013) Block-and-break generation of microdroplets with fixed volume. Biomicrofluidics 7, 024108 10.1063/1.4801637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rahimi M, Khorrami AS and Rezai P (2019) Effect of device geometry on droplet size in co-axial flow-focusing microfluidic droplet generation devices. Colloids Surf. A Physicochem. Eng. Asp 570, 510–517 10.1016/j.colsurfa.2019.03.067 [DOI] [Google Scholar]
- 50.Utech S, Prodanovic R, Mao AS, Ostafe R, Mooney DJ and Weitz DA (2015) Microfluidic generation of monodisperse, structurally homogeneous alginate microgels for cell encapsulation and 3D cell culture. Adv Healthc Mater. 4, 1628–1633 10.1002/adhm.201500021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hati AG, Bassett DC, Ribe JM, Sikorski P, Weitz DA and Stokke BT (2016) Versatile, cell and chip friendly method to gel alginate in microfluidic devices. Lab. Chip 16, 3718–3727 10.1039/C6LC00769D [DOI] [PubMed] [Google Scholar]
- 52.Chen Q, Utech S, Chen D, Prodanovic R, Lin J-M and Weitz DA (2016) Controlled assembly of heterotypic cells in a core–shell scaffold: organ in a droplet. Lab. Chip 16, 1346–1349 10.1039/C6LC00231E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang H and He X (2015) Fluid displacement during droplet formation at microfluidic flow-focusing junctions. Lab. Chip 15, 4197–4205 10.1039/C5LC00730E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rotem A, Abate AR, Utada AS, Van Steijn V and Weitz DA (2012) Drop formation in non-planar microfluidic devices. Lab. Chip 12, 4263–4268 10.1039/C2LC40546F [DOI] [PubMed] [Google Scholar]
- 55.Agarwal P, Zhao S, Bielecki P, Rao W, Choi JK, Zhao Y et al. (2013) One-step microfluidic generation of pre-hatching embryo-like core–shell microcapsules for miniaturized 3D culture of pluripotent stem cells. Lab. Chip 13, 4525–4533 10.1039/c3lc50678a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshida S, Takinoue M and Onoe H (2017) Compartmentalized spherical collagen microparticles for anisotropic cell culture microenvironments. Adv. Healthc. Mater. 6, 1601463 10.1002/adhm.201601463 [DOI] [PubMed] [Google Scholar]
- 57.Visser CW, Kamperman T, Karbaat LP, Lohse D and Karperien M (2018) In-air microfluidics enables rapid fabrication of emulsions, suspensions, and 3D modular (bio) materials. Sci. Adv. 4, eaao1175 10.1126/sciadv.aao1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morimoto Y, Onuki M and Takeuchi S (2017) Mass production of cell-laden calcium alginate particles with centrifugal force. Adv. Healthc. Mater. 6, 1601375 10.1002/adhm.201601375 [DOI] [PubMed] [Google Scholar]
- 59.Kamperman T, Trikalitis VD, Karperien M, Visser CW and Leijten J (2018) Ultrahigh-throughput production of monodisperse and multifunctional janus microparticles using in-air microfluidics. ACS Appl. Mater. Interfaces 10, 23433–23438 10.1021/acsami.8b05227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grellier M, Granja PL, Fricain JC, Bidarra SJ, Renard M, Bareille R et al. (2009) The effect of the co-immobilization of human osteoprogenitors and endothelial cells within alginate microspheres on mineralization in a bone defect. Biomaterials 30, 3271–3278 10.1016/j.biomaterials.2009.02.033 [DOI] [PubMed] [Google Scholar]
- 61.De Vos P, De Haan B and Van Schilfgaarde R (1997) Effect of the alginate composition on the biocompatibility of alginate-polylysine microcapsules. Biomaterials 18, 273–278 10.1016/S0142-9612(96)00135-4 [DOI] [PubMed] [Google Scholar]


