Abstract
INTRODUCTION:
The early detection of gastric neoplasms (GNs) leads to favorable treatment outcomes. The latest endoscopic system, EVIS X1, includes third-generation narrow-band imaging (3G-NBI), texture and color enhancement imaging (TXI), and high-definition white-light imaging (WLI). Therefore, this randomized phase II trial aimed to identify the most promising imaging modality for GN detection using 3G-NBI and TXI.
METHODS:
Patients with scheduled surveillance endoscopy after a history of esophageal cancer or GN or preoperative endoscopy for known esophageal cancer or GN were randomly assigned to the 3G-NBI, TXI, or WLI groups. Endoscopic observations were performed to detect new GN lesions, and all suspected lesions were biopsied. The primary endpoint was the GN detection rate during primary observation. Secondary endpoints were the rate of missed GNs, early gastric cancer detection rate, and positive predictive value for a GN diagnosis. The decision rule had a higher GN detection rate between 3G-NBI and TXI, outperforming WLI by >1.0%.
RESULTS:
Finally, 901 patients were enrolled and assigned to the 3G-NBI, TXI, and WLI groups (300, 300, and 301 patients, respectively). GN detection rates in the 3G-NBI, TXI, and WLI groups were 7.3, 5.0, and 5.6%, respectively. The rates of missed GNs were 1.0, 0.7, and 1.0%, the detection rates of early gastric cancer were 5.7, 4.0, and 5.6%, and the positive predictive values for the diagnosis of GN were 36.5, 21.3, and 36.8% in the 3G-NBI, TXI, and WLI groups, respectively.
DISCUSSION:
Compared with TXI and WLI, 3G-NBI is a more promising modality for GN detection.
KEYWORDS: gastric cancer, image-enhanced endoscopy, lesion detection
INTRODUCTION
Gastric cancer (GC) is the fourth most common type of cancer worldwide. Moreover, GC's early detection has been increasing owing to nationwide screening systems in Japan and Korea (1,2). In certain cases, histopathological differentiation between gastric adenoma and early GC (EGC) is challenging. Approximately 15% of gastric lesions initially diagnosed as adenomas by endoscopic biopsy are upgraded to GC after endoscopic resection (3). Furthermore, gastric adenomas gradually increase in size and become cancerous during follow-up, and some institutions treat gastric adenomas endoscopically (4). Thus, early gastric neoplasm (GN) detection, including EGC and gastric adenomas, is an ideal strategy for maximizing GC survival rates. However, endoscopic observation using white-light imaging (WLI) (5), the current standard imaging modality, is unsatisfactory because of missed lesions, with room for improvement (6).
Endoscopic observation using narrow-band imaging (NBI) is the current standard imaging method for detecting superficial head, neck, and esophageal cancers, based on the positive results of a randomized controlled trial (RCT) comparing WLI and first-generation (1G)-NBI. Therefore, in an RCT, we hypothesized that second-generation (2G)-NBI, which is brighter than 1G-NBI, would improve EGC detection compared with that obtained with WLI (6). Although 2G-NBI slightly exceeded WLI's detection rate (2.3% vs 1.9%), its superiority was not demonstrated. In addition, the rate of missed EGCs detected in the second observation was 0.8% in both groups. Therefore, further improvements are necessary for NBI to gain advantages over WLI. Thereafter, the latest endoscopic system, EVIS X1 (Olympus Medical Systems, Tokyo, Japan), was developed, which includes brighter and clearer WLI, third-generation (3G)-NBI, and texture and color enhancement imaging (TXI) as a new image-enhanced endoscopy (IEE) (7). The TXI is designed to enhance the 3 image elements of WLI (texture, brightness, and color) using Retinex-based enhancements while maintaining image naturalness (WLI appearance) clearly defines subtle tissue differences (7). This is expected to improve the detection rate of GN in terms of color difference and visibility; however, its clinical usefulness has not yet been demonstrated. Therefore, we hypothesized that 3G-NBI and TXI are promising imaging modalities that outperform WLI in EGC detection.
To identify the optimal imaging modality for identifying new GNs, excluding those already identified, we conducted a 2-arm RCT using 3G-NBI, TXI, and WLI.
METHODS
Study design and participants
This multi-institutional, randomized, open-label, 3-arm-parallel phase II trial was conducted at 6 institutions in Japan in accordance with the principles established in the Declaration of Helsinki and Clinical Trials Act. The National Cancer Center Hospital East Certified Review Board approved the study protocol in June 2021. This trial was registered in jRCT (Identifier jRCT1032210213). The article was prepared in accordance with the Consolidated Standards for Reporting Trials. All authors had access to the study data and reviewed and approved the final manuscript.
Inclusion and exclusion criteria
Patients at high risk of GNs were targeted to maximize the number of lesions detected. Moreover, the incidence of synchronous or metachronous multiple GNs is reportedly elevated in patients with GNs and esophageal cancer (8,9). Therefore, the inclusion criteria were patients aged 20–85 years with either of the following: (i) scheduled surveillance endoscopy after endoscopic resection for GN, endoscopic resection, chemotherapy, or radiotherapy for esophageal cancer, or (ii) scheduled preoperative endoscopy for known GN or esophageal cancer. Patients with known GN or esophageal cancer were evaluated for other synchronous GN that were missed at the referring hospital. The exclusion criteria were (i) previous gastrectomy or gastric tube reconstruction and (ii) assessment as inappropriate by a physician owing to a serious underlying disease, a heightened risk of bleeding stemming from the current use of antithrombotic drugs, or difficulty in communication. Written informed consent was obtained from all participants before the study.
Randomization
Patients were randomly assigned in a 1:1:1 ratio to 3G-NBI (primary 3G-NBI and secondary WLI), TXI (primary TXI and secondary WLI), and WLI (primary and secondary WLI) groups, with the WLI group set as the calibration arm by the minimization method with a random component balancing the groups regarding institution, age (<70 and ≥70 years), indication for endoscopy (surveillance and preoperative), and organ of previously treated or known lesion (stomach and esophagus). The endoscopists did not attempt to mask allocation to the study group.
Endoscopy and processor
An EVIS X1 system and high-definition gastroscope with optical zoom (GIF-XZ1200) were used in this study. The video processor settings for structural enhancement were type B, level 4 or 6 for WLI; type B, level 8 for 3G-NBI; and hard enhancement for TXI. In addition, the color-enhancement values were zero for WLI, mode 1 for 3G-NBI, and mode 1 for TXI. The use of Brightness Adjustment Imaging with Maintenance of Contrast mode was not permitted in this study.
TXI
TXI is a new IEE modality that uses Retinex-based enhancements (7). It was designed to enhance the 3 image elements of WLI (texture, brightness, and color). The TXI has 2 settings: mode 1 (texture, brightness, and color enhancement) and mode 2 (texture and brightness enhancement). Moreover, preclinical and clinical studies have shown that the TXI mode 1 has a higher color difference than the WLI and TXI mode 2 (7,10). In this study, TXI mode 1 was adopted based on a preconsultation using endoscopic imaging for EGC.
Endoscopic diagnosis criteria
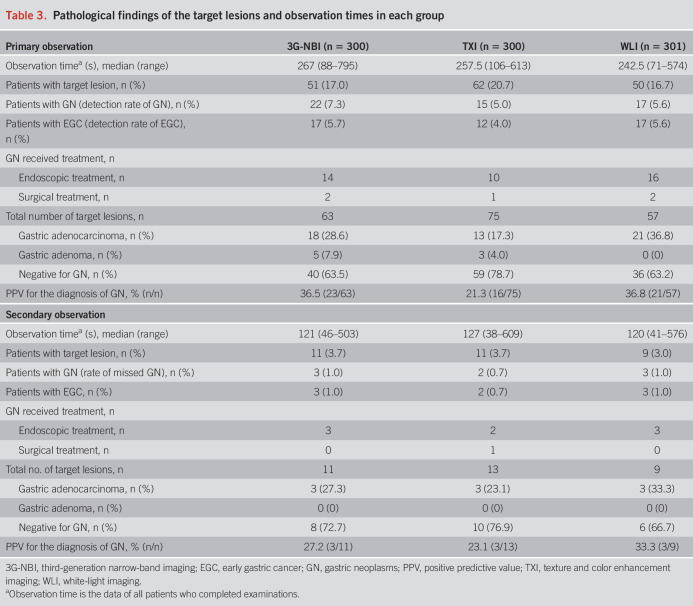
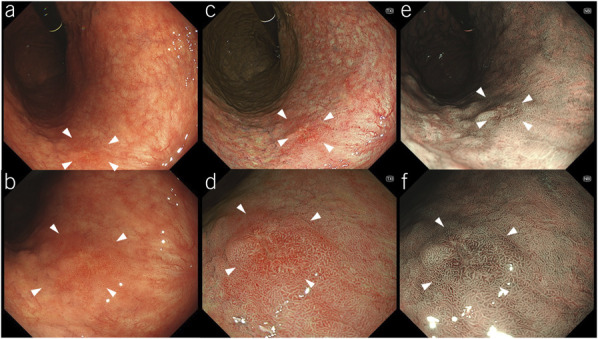
According to the Japanese Classification of Gastric Carcinoma, GN includes adenoma and EGC confined to the mucosa or submucosa, regardless of lymph node metastasis (11). Newly detected lesions suspected as GN, identified by nonmagnifying observation, were defined as “target lesions” and had at least one of the following endoscopic EGC characteristics: an area with (i) irregular margin; (ii) irregular discoloration; and/or (iii) an irregular surface or those of adenoma: (i) a villous protrusion; (ii) a smooth surface elevation with constriction; (iii) an inverted growth; (iv) nodule aggregation; and (v) a pale elevation with a clear borderline. Lesions with findings typical of advanced GC (e.g., hardness and poor extensibility) and pre-existing lesions were excluded as target lesions. The criteria for target lesions were applied to all WLI, 3G-NBI, and TXI examinations (Figures 1 and 2).
Figure 1.
Representative images of target lesions. A depressed lesion in the middle third of the stomach is shown (arrowheads). The final histopathological diagnosis was well-differentiated adenocarcinoma confined to the mucosa. (a and b) On white-light imaging (WLI), the lesion appears as a reddish area with irregular margins and surfaces. (c and d) On texture and color enhancement imaging, the lesion appears as a reddish area with irregular margin surfaces, with a greater color difference than that in WLI. (e and f) Third-generation narrow-band imaging shows the lesion as a brownish area with irregular margins and surfaces.
Figure 2.
Representative images of target lesions. A slightly elevated lesion in the middle third of the stomach is observed (arrowheads). The final histopathological diagnosis was well-differentiated adenocarcinoma confined to the mucosa. (a) On white-light imaging, the lesion appears as a same-colored area with irregular margins and surfaces. (b) On texture and color enhancement imaging, the lesion appears as a same-colored area with irregular margins and surfaces. (c and d) Third-generation narrow-band imaging: The lesion appears as a brownish area with irregular margins and surfaces.
Gastric mucosal atrophy was determined based on the Kimura–Takemoto classification as follows: non-to-mild atrophy, gastric mucosal atrophy not observed or limited to the antrum; moderate atrophy, gastric mucosal atrophy observed from the gastric angle to the lesser curvature of the upper corpus; and severe atrophy, gastric mucosal atrophy observed beyond the cardia and extending to the greater curvature (12). In addition, Helicobacter pylori infection status was divided into 4 groups based on electronic medical records and patient interviews: present, eradicated, absent, and unknown. Samples of H. pylori were collected during H. pylori examination (urease breath, serum antibody, stool, and rapid urease test and/or pathological diagnosis).
Examination protocol
The examination protocol consisted of nonmagnifying observations including primary and secondary observation, magnification with NBI, and biopsy of the target lesion. Subsequent treatments for the target lesions, such as endoscopic or surgical resection, were performed in each patient.
Primary observation of the entire stomach was performed to detect the GN lesions. Subsequently, a secondary WLI was immediately performed by the same endoscopist to detect any missed target lesions. All examinations were performed to observe the entire stomach at the esophagogastric junction according to a systematic screening protocol for the stomach (13).
If a target lesion was detected, detailed magnification with NBI was subsequently performed to differentiate between GC and noncancer, according to the magnifying endoscopy simple diagnostic algorithm for EGC, which uses irregular microvascular and/or microsurface patterns within the demarcation line (14). All detected target lesions were biopsied at the end of the examination, regardless of the diagnosis.
To maintain endoscopic quality control, all the endoscopists were board-certified fellows of the Japan Gastroenterological Endoscopy Society or had equivalent qualifications. To minimize diagnostic variability, all participating endoscopists were trained using WLI, 3G-NBI, and TXI endoscopic images of gastric lesions, and all IEEs were used for stomach observation training before the study began.
Pathological evaluation
Pathological diagnoses were made by expert pathologists at each institution based on biopsied tissues or specimens obtained through endoscopic or surgical resection. If both biopsied tissues and resected specimens had been available, the final diagnosis was determined based on the more important diagnosis.
Differentiation between GC, adenoma, and non-neoplasm was based on the Japanese Classification of GC (11) and the revised Vienna classification (15): Category 4 (mucosal high-grade neoplasia) and 5 (submucosal invasion by carcinoma) tumors were diagnosed as GCs, category 3 (mucosal low-grade neoplasia) tumors were diagnosed as gastric adenoma, and category 1 tumors were diagnosed as non-neoplasms. If diagnosed as category 2 (indefinite for neoplasia), a repeat endoscopic examination is recommended to confirm the final pathological diagnosis. In addition, even when GN was diagnosed as category 2, treatment was allowed in cases where GN was suspected based on endoscopic findings. Specifically, high-grade dysplasia in the World Health Organization classification corresponds to mucosal adenocarcinoma in the Japanese Classification of GC (4,11).
Outcomes
The primary endpoint was the detection rate of GN during the primary observation, defined as the proportion of patients with newly detected GC and adenoma, excluding previously identified lesions. Thus, new detected lesions within the esophagus were not considered. Although it is established that the risk of EGC correlates with intestinal metaplasia and atrophic gastritis (16), it is important to note that the aim of this study was not to assess the risk of GN lesion occurrence but to directly evaluate GN lesion detection. The secondary endpoints were the rate of missed GN, detection rate of EGC in the primary observation, positive predictive value (PPV) for GN diagnosis in the primary observation, observation time, and adverse events. Missed GN was defined as GN detected in the secondary examination, but not in the primary examination. The observation time was measured for each primary and secondary examination, from the passage of the endoscope through the esophagogastric junction until the end of the stomach observation period, including the time required to remove the gastric mucus. GNs were classified according to the Japanese Classification of Gastric Carcinoma (15th edition), including tumor location, and macroscopic and histological subtypes.
Statistical analysis
Based from a previous study (6), it was assumed that the primary endpoint would be 3.0% for one IEE. Considering the consensus of the experts, we expected that a better IEE would be >4.3% at the primary endpoint. Based on Simon's selection design (17), the planned sample size was set to 296 per group to ensure an 80% correct selection of the most promising IEE. Estimating that up to 4 patients would be ineligible to undergo the protocol examination after randomization, the sample size was set at 300 in each group (900 patients).
The decision rule to determine the most promising modality was set as one group with a higher detection rate of GN in the primary observation between 3G-NBI and TXI and one group that outperformed both the other and WLI by >1.0%. The target population for the primary analysis was randomized. Subgroup analyses were performed according to the indications for endoscopy (surveillance or preoperative), organs with previously treated or known lesions (stomach and esophagus), and status of H. pylori infection (present, eradicated, absent, or unknown). All statistical analyses were performed using SAS Release version 9.4 statistical software suite (SAS Institute, Cary, NC).
RESULTS
Background characteristics of patients
Between August 2021 and June 2022, 901 patients were enrolled from 6 institutions and randomized. A total of 300, 300, and 301 patients were randomly assigned to the 3G-NBI, TXI, and WLI groups. After randomization, 5 patients were excluded: 2 were ineligible, one violated the study protocol, and 2 had esophageal stenosis (Figure 3).
Figure 3.
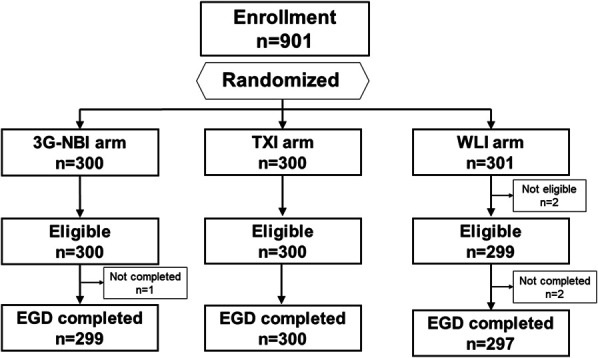
Patient flow diagram. 3G-NBI, third-generation narrow-band imaging; EGD, esophagogastroduodenoscopy; TXI, texture and color enhancement imaging; WLI, white-light imaging.
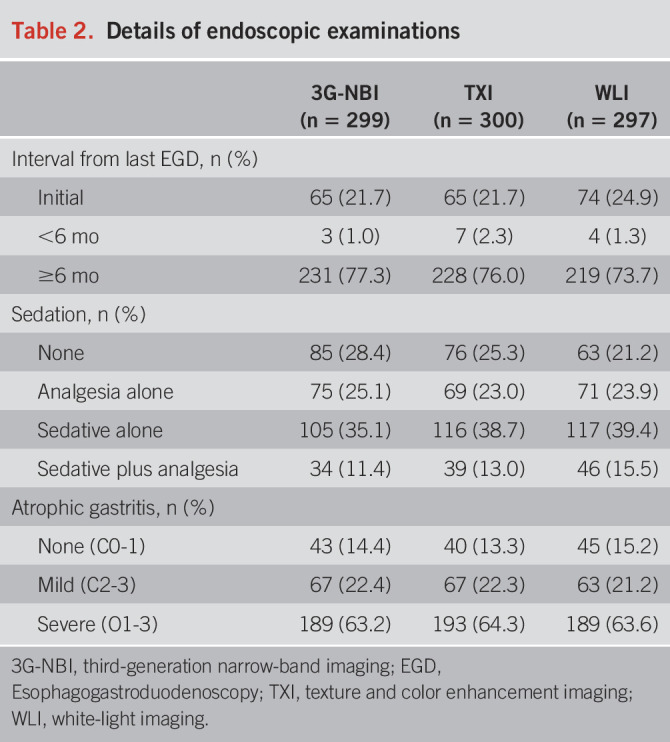
In total, 299, 300, and 297 patients were examined in the 3G-NBI, TXI, and WLI groups. As shown in Table 1, approximately 22% of the patients underwent endoscopic examination for preoperative examination of the stomach lesion. Regarding H. pylori infection status, approximately 60% of the patients were eradicated, 12% were present, and 13% were absent. Approximately 85% of patients had mild or severe atrophic gastritis (Table 2).
Table 1.
Background characteristics of patients
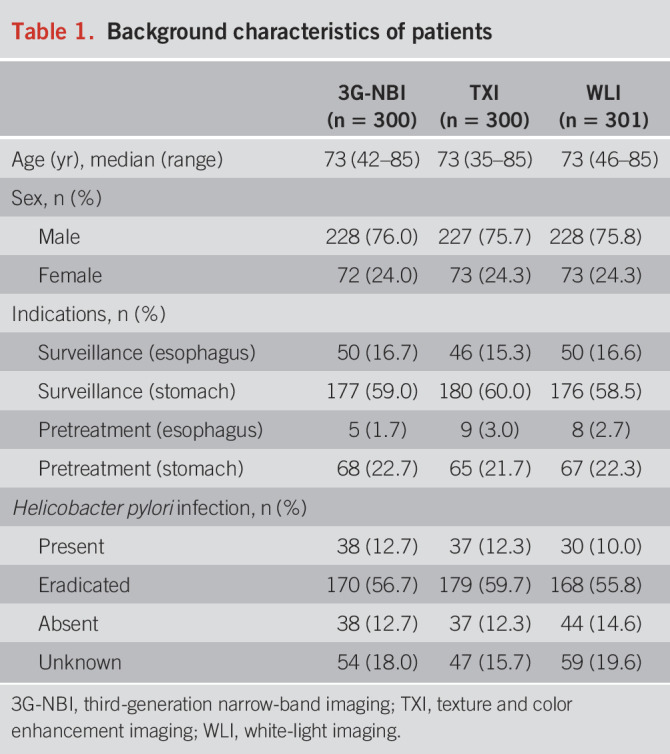
Table 2.
Details of endoscopic examinations
Outcomes
During the primary observation, target lesions were detected in 51 patients with 63 lesions in the 3G-NBI group, 62 patients with 75 lesions in the TXI group, and 50 patients with 57 lesions in the WLI group. Target lesions were pathologically diagnosed as GN in 22 patients in the 3G-NBI group, 15 in the TXI group, and 17 in the WLI group and diagnosed as EGC in 17 patients in the 3G-NBI group, 12 in the TXI group, and 17 in the WLI group (Table 3). The detection rates of GN in the 3G-NBI, TXI, and WLI groups were 7.3 (22/300), 5.0 (15/300), and 5.6% (17/301). The detection rates of EGC in the 3G-NBI, TXI, and WLI groups were 5.7 (17/300), 4.0 (12/300), and 5.6% (17/301), and the PPV for the diagnosis of GN was 36.5 (23/63), 21.3 (16/75), and 36.8% (21/57). During secondary observation, target lesions were detected in 11 patients with 11 lesions in the 3G-NBI group, 11 patients with 13 lesions in the TXI group, and 9 patients with 9 lesions in the WLI group. The missed GN rates in the 3G-NBI, TXI, and WLI groups were 1.0 (3/300), 0.7 (2/300), and 1.0% (3/301). The median observation times of the primary observation were 267 seconds (88–795 seconds), 257.5 seconds (106–613 seconds), and 242.5 seconds (71–574 seconds) in the 3G-NBI, TXI, and WLI groups.
Table 3.
Pathological findings of the target lesions and observation times in each group
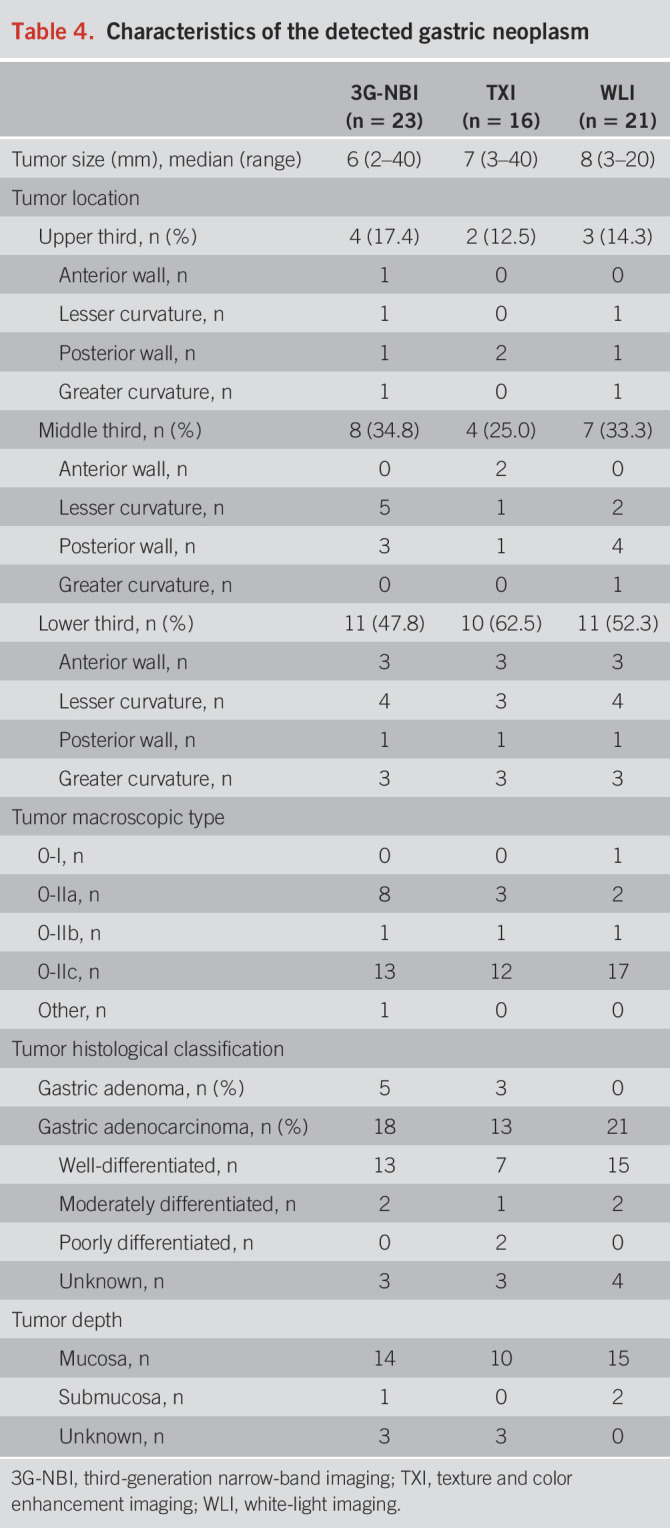
The characteristics of the detected GNs are presented in Table 4. Approximately half of the lesions were located in the lower third of each arm, and almost all were well-differentiated pT1a-M adenocarcinomas.
Table 4.
Characteristics of the detected gastric neoplasm
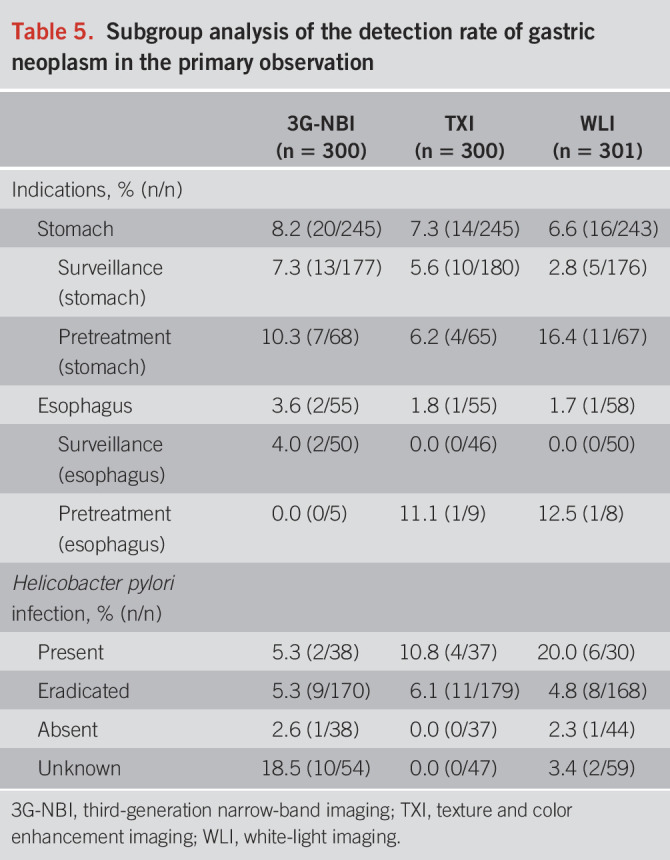
As shown in Table 5, a subgroup analysis of the detection rate of GN was conducted according to indications and H. pylori infection. In the stomach group, the detection rates of GN in the 3G-NBI, TXI, and WLI groups were 8.2 (20/245), 7.3 (14/245), and 6.6% (16/243) and 3.6 (2/55), 1.8 (1/55), and 1.7% (1/58) in the esophagus group. In addition, according to the statuses of H. pylori infection, those were 5.3 (2/38), 10.8 (4/37), and 20.0% (6/30) in the present group, 5.3 (9/170), 6.1 (11/179), and 4.8% (8/168) in the eradicated group, and 2.6 (1/38), 0.0 (0/37), and 2.3% (1/44) in the absent group.
Table 5.
Subgroup analysis of the detection rate of gastric neoplasm in the primary observation
DISCUSSION
To the best of our knowledge, this is the first randomized phase II trial comparing 3G-NBI, TXI, and WLI for GN detection. Third-generation NBI showed a higher detection rate of GNs compared with those provided by TXI and WLI. In addition, 3G-NBI maintained a PPV comparable with that of WLI, which slightly increased the observation time for the stomach. Therefore, we suggest 3G-NBI as a more promising modality than TXI for the detection of GN. Further randomized phase III trials are required to confirm the superiority of 3G-NBI to WLI.
In a study using the latest endoscopic system, which can produce brighter and higher-resolution images than those produced by previous generations of endoscopic instruments, we demonstrated higher WLI and 3G-NBI detection rates. Although the clinical background of registered patients differed from a previous study evaluating 2G-NBI (6), higher detection rates were observed in the WLI (6.6%) and 3G-NBI (8.2%) groups than in the previous study (2.2% and 2.7% in the WLI and 2G-NBI groups) (6), when focusing on gastric surveillance and gastric pretreatment cases. In other words, new endoscopic instruments may contribute to better GN detection, regardless of IEE. Major improvements in new endoscopic instruments include brighter and lower-noise image quality, achieved by changing the image sensor attached to the tip of the scope from a charge-coupled device to a complementary metal-oxide semiconductor, making it possible to reduce image noise mainly in distant views. In addition, an article study on colonic polyps demonstrated a significantly greater color difference in 3G-NBI compared with 2G-NBI. Although the target and scope are not directly applicable to our study (18), improvements in color differences may have had an important impact on our findings. Thus, the development of NBI has made it possible to produce a brightness and resolution suitable for EGC screening. However, similar to findings from previous studies, the observation time for the stomach in the 3G-NBI group tended to be longer than in the WLI group. This observation may be attributed to the unfamiliarity of the endoscopists with cleaning and inspecting the stomach during 3G-NBI observation because mucus on the gastric mucosa can be more visible with NBI compared with WLI-based imaging. Consequently, this process may have taken more time during the lavage. In addition, the actual observation of the stomach itself may have taken longer compared with WLI observation. However, it is anticipated that this issue may improve over time with increasing familiarity and experience with 3G-NBI, particularly if it becomes a standardized imaging modality.
A similar IEE, linked color imaging (FUJIFILM, Tokyo, Japan), enabled increased color differences in mucosal colors and showed the superiority of WLI for the detection of neoplastic lesions in the upper gastrointestinal tract, including the pharynx, esophagus, and stomach (4.8% vs 8.0%, P = 0.011) (19). In addition, the linked color imaging showed higher GN detection rates than those exhibited by WLI (5.5% vs 3.3%); however, the difference was not statistically significant. By contrast, TXI did not provide any clinical benefit for GN detection in this study. There are several possible explanations for these findings. First, training for TXI images of GN lesions and pretraining for stomach observation with TXI were performed before the study but may have been insufficient; thus, the study was perhaps in the middle of the learning curve. Second, the color difference, slight surface elevation, and depression in the background mucosa of the stomach could also be emphasized in the TXI images, which may have led to missing GN lesions and low PPV. Several studies have reported the usefulness of TXI in evaluating atrophic gastritis (20,21). The TXI has been reported to enhance mucosal redness with the principle of color enhancement to accentuate color tone differences in mucosal surfaces; thus, TXI accurately detects diffuse redness in patients with current H. pylori infection (20). Thus, although assessing the entire gastric background mucosa was easy, the TXI image showed a small color difference between the GN lesions and gastric mucosa with high inflammation, which may have interfered with the detection of small GN lesions, thereby explaining the lower PPV. Furthermore, although TXI has multiple modes and enhancement settings, it is possible that the optimal mode selection for detecting GN lesions was not performed in this study. Several reports have shown the usefulness of TXI mode 1 (10,22–24), whereas only one report has demonstrated the usefulness of mode 2 (25). However, most of these reports were studies on visibility, focusing only on gastric lesions; thus, there is a lack of information in detecting gastric lesions in the normal gastric mucosa. Therefore, the results of this study represent the first true TXI detection results.
This study had several limitations. First, no statistical analysis was performed because of the characteristics of a phase II study with a selection design. We originally assumed that the GN detection rate in one group was 3%, and the decision-making criterion was whether the other group could be increased by ≥1%, which was determined by consensus among researchers. The 3G-NBI group outperformed the TXI group by 2.3%, partly because the overall detection rate of GN was higher (6.0%) than expected. In addition, the ratio of the detection rate in the 3G-NBI and TXI groups was 1.46 (7.3%/5.0%), which was slightly higher than the expected 1.43 (4.3%/3.0%); therefore, we believe that our interpretation is correct. Second, gastric adenomas and EGC were included as targets for detection. Because gastric adenoma is sometimes diagnosed as GC after endoscopic resection or becomes cancerous during follow-up, some institutions perform endoscopic treatment for gastric adenoma. Therefore, we believe that detecting gastric adenomas would be beneficial to patients. However, detecting gastric adenomas may not be as important as detecting GC. Third, this study was exclusively conducted within Japanese tertiary centers and was not directly applied to community hospitals. Fourth, the study focused on patients at high risk of GC; therefore, the generalizability and reproducibility of these findings to population-based GC screening remain uncertain. Fifth, the missed lesions could not be accurately evaluated after the primary and secondary endoscopic observations because surveillance endoscopic examinations were not scheduled. Therefore, although all 3 groups had similar rates of missed GN, the quality and usefulness of the 2 rounds of observations could not be evaluated. Sixth, although it is ideal for secondary observations to be performed by another blinded expert endoscopist, operational constraints in real clinical settings required that secondary observations be performed by the same endoscopist who conducted the primary observations. Consequently, the possibility of observer bias cannot be excluded. Therefore, guided by the aforementioned limitations, we conducted a phase III RCT (jRCT1032230613) to determine the optimal imaging modality using 3G-NBI and WLI.
In conclusion, the detection rate of GN during the primary observation was higher in the 3G-NBI group than in the TXI and WLI groups. Thus, 3G-NBI is a promising candidate for subsequent phase III trials. A phase III trial comparing 3G-NBI with WLI is currently in progress.
CONFLICTS OF INTEREST
Guarantor of the article: Tomonori Yano, MD, PhD.
Specific author contributions: T.K., S.A., N.U., H.D., Y.F., M.M., N.Y., C.K., T.I., M.W., and T.Y.: designed the study. T.K., S.A., N.U., H.D., Y.F., M.M., S.N., H.T., T.M., K.N., Y.T., Y.O., A.K., N.Y., A.W., C.K., M.T., A.Y., and T.Y.: collected the data. T.I. and M.W.: analyzed the data. H.F.: managed and coordinated the study. T.K. and T.Y.: wrote the manuscript. All authors reviewed and approved the final version of the manuscript.
Financial support: This work was supported by research funding and free device loans provided by Olympus Corporation. This company was not involved in this study.
Potential competing interests: T.Y. received a research grant from Olympus as a principal investigator. S.A., N.U., H.D., Y.F., and M.M. received research grants from Olympus as facility study managers. Seiichiro Abe received personal fees from Boston Scientific, personal fees and research funding from FUJIFILM, and personal fees and research funding from Olympus. N.U. received honoraria for lectures from Olympus Co., Ltd., FUJIFILM Co., Ltd., Boston Scientific, Japan, and AI Medical Service Co., Ltd. H.D. received honoraria for lectures at Olympus. S.N. has received honoraria for lectures at Olympus, Japan. T.Y. received honoraria for lectures and research grants from Olympus and research grants from HOYA PENTAX and FUJIFILM. T.K., H.T., T.M., K.N., Y.T., Y.O., A.K., N.Y., A.W., C.K., M.T., A.Y., H.F., T.I., and M.W. have no conflict of interest.
Clinical trial registration: Japan Registry of Clinical Trials, Number: jRCT1032210213 (https://jrct.niph.go.jp/en-latest-detail/jRCT1032210213).
Study Highlights.
WHAT IS KNOWN
✓ The early detection of gastric neoplasms (GNs) leads to favorable treatment outcomes.
✓ The current standard white-light imaging method is insufficiently capable of detecting GNs.
WHAT IS NEW HERE
✓ Third-generation narrow-band imaging has the potential to detect more GNs than other imaging modalities.
ACKNOWLEDGEMENTS
The authors thank all patients and investigators for their participation in this study. The authors thank Editage (www.editage.jp) for the English language editing.
Contributor Information
Tomohiro Kadota, Email: tkadota@east.ncc.go.jp.
Seiichiro Abe, Email: seabe@ncc.go.jp.
Noriya Uedo, Email: noriya.uedo@gmail.com.
Hisashi Doyama, Email: doyama.134@gmail.com.
Yasuaki Furue, Email: yfurue@kitasato-u.ac.jp.
Manabu Muto, Email: mmuto@kuhp.kyoto-u.ac.jp.
Satoru Nonaka, Email: snonaka@ncc.go.jp.
Hiroyuki Takamaru, Email: htakamar@ncc.go.jp.
Tatsuro Murano, Email: tatmuran@east.ncc.go.jp.
Keiichiro Nakajo, Email: knakajo@east.ncc.go.jp.
Yasuhiro Tani, Email: y.tani1646@gmail.com.
Yuki Okubo, Email: ookuboyuuki01@gmail.com.
Azusa Kawasaki, Email: m03026ak@yahoo.co.jp.
Naohiro Yoshida, Email: naohilow@yahoo.co.jp.
Akinori Watanabe, Email: akinori@kitasato-u.ac.jp.
Chikatoshi Katada, Email: ckatada@kuhp.kyoto-u.ac.jp.
Masashi Tamaoki, Email: tamaoki@kuhp.kyoto-u.ac.jp.
Akira Yokoyama, Email: yokoaki@kuhp.kyoto-u.ac.jp.
Hideki Furuya, Email: hifuruya@east.ncc.go.jp.
Takashi Ikeno, Email: tikeno@east.ncc.go.jp.
Masashi Wakabayashi, Email: mawakaba@east.ncc.go.jp.
REFERENCES
- 1.Hamashima C, Ogoshi K, Okamoto M, et al. A community-based, case-control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PLoS One 2013;8(11):e79088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi KS, Jun JK, Suh M, et al. Effect of endoscopy screening on stage at gastric cancer diagnosis: Results of the national cancer screening programme in Korea. Br J Cancer 2015;112(3):608–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maekawa A, Kato M, Nakamura T, et al. Incidence of gastric adenocarcinoma among lesions diagnosed as low-grade adenoma/dysplasia on endoscopic biopsy: A multicenter, prospective, observational study. Dig Endosc 2018;30(2):228–35. [DOI] [PubMed] [Google Scholar]
- 4.Kinami S, Yamada S, Takamura H. Confusion and prospects for carcinogenesis of gastric adenoma and dysplasia: What is the correct answer currently? World J Gastroenterol 2022;28(48):6900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamashima C. Benefits and harms of endoscopic screening for gastric cancer. World J Gastroenterol 2016;22(28):6385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida N, Doyama H, Yano T, et al. Early gastric cancer detection in high-risk patients: A multicentre randomised controlled trial on the effect of second-generation narrow band imaging. Gut 2021;70(1):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato T. TXI: Texture and color enhancement imaging for endoscopic image enhancement. J Healthc Eng 2021;2021:5518948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katada C, Muto M, Tanabe S, et al. Surveillance after endoscopic mucosal resection or endoscopic submucosal dissection for esophageal squamous cell carcinoma. Dig Endosc 2013;25(Suppl 1):39–43. [DOI] [PubMed] [Google Scholar]
- 9.Hirao M, Katada C, Yokoyama T, et al. Metachronous primary gastric cancer after endoscopic resection in patients with esophageal squamous cell carcinoma. Gastric Cancer 2023;26(6):988–1001. [DOI] [PubMed] [Google Scholar]
- 10.Abe S, Yamazaki T, Hisada IT, et al. Visibility of early gastric cancer in texture and color enhancement imaging. DEN Open 2022;2(1):e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma. 15th edn Japan: Kanehara & Co., Ltd, 2017. [DOI] [PubMed] [Google Scholar]
- 12.Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1969;1(03):87–97. [Google Scholar]
- 13.Yao K. The endoscopic diagnosis of early gastric cancer. Ann Gastroenterol 2013;26(1):11–22. [PMC free article] [PubMed] [Google Scholar]
- 14.Muto M, Yao K, Kaise M, et al. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc 2016;28(4):379–93. [DOI] [PubMed] [Google Scholar]
- 15.Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut 2002;51(1):130–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Correa P. Human gastric carcinogenesis: A multistep and multifactorial process: First American Cancer Society award lecture on cancer epidemiology and prevention. Cancer Res 1992;52(24):6735–40. [PubMed] [Google Scholar]
- 17.Simon R, Wittes RE, Ellenberg SS. Randomized phase II clinical trials. Cancer Treat Rep 1985;69(12):1375–81. [PubMed] [Google Scholar]
- 18.Imai KKH, Hotta K, Ito S, et al. Narrow-band imaging with a new imaging processor can detect color difference of colorectal polyps. Tech Innov Gastrointest Endosc 2022;24(3):309–11. [Google Scholar]
- 19.Ono S, Kawada K, Dohi O, et al. Linked color imaging focused on neoplasm detection in the upper gastrointestinal tract: A randomized trial. Ann Intern Med 2021;174(1):18–24. [DOI] [PubMed] [Google Scholar]
- 20.Kitagawa Y, Koga K, Ishigaki A, et al. Endoscopic diagnosis of Helicobacter pylori gastritis using white light imaging and texture and color enhancement imaging. Endosc Int Open 2023;11(2):E136–E141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugimoto M, Kawai Y, Morino Y, et al. Efficacy of high-vision transnasal endoscopy using texture and colour enhancement imaging and narrow-band imaging to evaluate gastritis: A randomized controlled trial. Ann Med 2022;54(1):1004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abe S, Makiguchi ME, Nonaka S, et al. Emerging texture and color enhancement imaging in early gastric cancer. Dig Endosc 2022;34(4):714–20. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa T, Matsumura T, Okimoto K, et al. Efficacy of texture and color Enhancement imaging in visualizing gastric mucosal atrophy and gastric neoplasms. Sci Rep 2021;11(1):6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawasaki A, Yoshida N, Nakanishi H, et al. Usefulness of third-generation narrow band imaging and texture and color enhancement imaging in improving visibility of superficial early gastric cancer: A study using color difference. DEN Open 2023;3(1):e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koyama Y, Sugimoto M, Kawai T, et al. Visibility of early gastric cancers by texture and color enhancement imaging using a high-definition ultrathin transnasal endoscope. Sci Rep 2023;13(1):1994. [DOI] [PMC free article] [PubMed] [Google Scholar]