Abstract
Objective
This study aimed to explore the efficacy of the clonidine adhesive patch for participants with Tourette syndrome (TS).
Methods
This randomized, double-blind, placebo-controlled, multicenter phase IV clinical trial included participants with TS at 20 centers between May 2012 and March 2015. Treatment efficacy at week 8 was the primary outcome. The Clinical Global Impression–Severity scale and Improvement scale were the secondary endpoints.
Results
This trial included 488 participants, with 121 participants in the 2.0-mg/wk group, 119 participants in the 1.5-mg/wk group, 126 participants in the 1.0-mg/wk group, and 122 participants in the placebo group. For Yale Global Tic Severity Scale score reduction rate, compared with the placebo group (39.60 ± 25.56), those of the 2.0-mg/wk group (63.21 ± 32.60) and the 1.5-mg/wk group (68.16 ± 25.88) were statistically significantly different (all P < 0.001). For total Yale Global Tic Severity Scale score, compared with the placebo group (17.0 ± 8.03), the score for the 2.0-mg/wk group was 9.9 ± 8.36 (P < 0.001); 1.5-mg/wk group, 9.6 ± 8.03 (P < 0.001); and 1.0-mg/wk group, 10.5 ± 9.28 (P < 0.001). The Clinical Global Impression–Severity scale and Improvement scale scores were statistically significantly different in the 3 clonidine (or experimental) groups compared with the placebo group (all P < 0.001).
Conclusions
Larger doses of the clonidine adhesive patch such as 1.5 and 2.0 mg/wk are effective in improving the symptoms and overall function of participants with TS.
Key Words: clonidine adhesive patch, Tourette syndrome, double-blind method, tic disorder
Tourette syndrome (TS) is a neuropsychiatric disorder defined by both motor and vocal tics with the onset in childhood or adolescence, lasting a cumulative total of at least 1 year, mainly manifesting as involuntary, repetitive, rapid, and multiple motor and vocal tics. If left untreated, TS can impair the daily activities of participants and affect their quality of lives.1–3 Additionally, participants with TS tend to have neuropsychiatric comorbidities, such as attention-deficit/hyperactivity disorder, obsessive-compulsive disorder, depression, and anxiety disorders.4 The current pharmacological treatment for TS includes typical and atypical antipsychotics.5 Although these drugs can be effective,6 they often bring adverse effects such as extrapyramidal side effects and inability to sit still, resulting in poor tolerability7–9; therefore, further exploration of effective drugs for TS is needed.
Clonidine is an α2-adrenoceptor agonist that is recommended as a first-line drug for the treatment of TS in the United States and Canada due to its association with mild side effects and efficacy.10,11 Clonidine can improve the symptoms of twitching by activating presynaptic autoreceptors in the locus ceruleus and then reducing norepinephrine release and turnover. It is available as tablet (at dosages of 0.1 and 0.2 mg) and transdermal patch (at dosages of 0.1, 0.2, and 0.3 mg). The recommended daily dose of the tablet is 0.1 to 0.4 mg/d.12 Clonidine adhesive patch is a transdermal treatment system that releases drugs at a constant speed successively for 1 week. Compared with the ordinary preparation, a clonidine adhesive patch has no peak or valley plasma concentration, which is absorbed into the blood through the skin,13 reducing fluctuations in blood levels.14 Thus, it can achieve full efficacy and reduce adverse effects. At the same time, because it is a weekly patch, the compliance of the patient is also relatively high.
Previous studies have shown that clonidine, by both oral and transdermal routes of absorption, is effective and well tolerated in children and adolescents with TS. However, these studies have had shortcomings as they were not placebo-controlled or multicenter, double-blind studies, or their duration was short, with only 4 weeks of observation and treatment.15–17 A study by Wang et al suggested that clonidine adhesive patch seems to be a promising therapy for the treatment of tic disorders in children. Nevertheless, further well-conducted randomized controlled trials are necessary to extend the evidence base.18 A review by Pringsheim et al suggested moderate confidence in use of clonidine for reducing tics. However, tics have a tendency to wax and wane over time, which could be misinterpreted as an effect of treatment. Therefore, further research with modest sample size is required to understand long-term efficacy and safety of this treatment method.19
This was a multicenter, randomized, double-blind, placebo-controlled trial to investigate the effectiveness of the clonidine adhesive patch at different dose ranges (1.0, 1.5, and 2.0 mg/wk) in participants with TS, aimed to explore the efficacy of the clonidine adhesive patch for participants with TS.
MATERIALS AND METHODS
Study Program
This multicenter, randomized, double-blind, placebo-controlled clinical trial study enrolled participants diagnosed with TS at 20 centers between May 2012 and March 2015 (Supplemental Digital Content, Table S1, http://links.lww.com/CNP/A45). Participants were recruited from sites across provinces or city in China. This trial was registered at a drug clinical trial registration and information disclosure platform (http://www.chinadrugtrials.org.cn/, registration no. CTR20132112).
Participants
Inclusion criteria were as follows: (1) 6–18 years old, (2) 20 kg < weight ≤ 40 kg, (3) meeting the clinical diagnostic criteria in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) for TS (combined vocal and motor tic disorder)20; and (4) written informed consent was obtained from the participant (if 7 years or older) and the participant's parent (or guardian).
Exclusion criteria were as follows: (1) participants with other concurrent mental disorders (except those with hyperactivity disorder); (2) those with diagnosed or suspected intellectual deficits, IQ ≤69; (3) those with obvious physical illness (especially lower than normal blood pressure and heart disease); (4) those with clinically significant abnormal laboratory and electrocardiographic (ECG) findings; (5) those with other comorbidities or using drugs that may affect the safe application of the tested drug; (6) those who are allergic to clonidine adhesive patch, who have had severe allergic reactions, or who are allergic to more than 2 drugs; (7) those with regulated use of antipsychotics, antidepressants, antimanic drugs, and anticonvulsants 1 week prior to enrollment or use of long-acting agents in 1 cycle; (8) those who are systematically using medications directed at the treatment of tic disorders 2 weeks prior to enrollment; (9) those who have been systematically treated with colistin transdermal patches 4 weeks prior to enrollment; (10) those who have participated in a clinical trial of another drug within the last 3 months; (11) those who are unwilling or unable to complete clinical studies; (12) those who are unsupervised or unable to administer the drug as prescribed by the physician; (13) those who are pregnant or breastfeeding; and (14) those who are not suitable to participate in this trial in the opinion of the investigator.
Criteria for termination of a participant from the trial were as follows: (1) found to be in clear violation of the trial protocol after enrollment; (2) withdrew the informed consent; (3) lost to follow-up; (4) discontinued treatment for nonpharmacological reasons such as serious physical illness after enrollment; (5) not used the patch for more than 3 consecutive days (including 3 days); (6) have any blood pressure measurement suggestive of being at risk based on the judgment of the investigator; or (7) experienced any serious adverse event.
This study was ethically approved by the ethics committee of Shanghai Mental Health Center (RFL201001). All participants included in the study signed informed consent.
Randomization and Blinding
Randomization and blinding were entrusted to independent statisticians at Tigermed Pharmaceuticals, Inc. The SAS version 9.2 (SAS Institute, Cary, NC) plan process and zone group randomization were used to generate drug blind bottom based on the predefined seed number, zone group length, and zone group number, and drug numbers were randomly assigned to the 1.0-, 1.5-, and 2.0-mg/wk group and the placebo group (0 mg/wk) in 4 groups. Participants, investigators, and sponsors were blinded to the grouping of drugs.
Intervention
Participants were randomly assigned in a 1:1:1:1 ratio to 4 clonidine (China National Pharmaceutical Group Shanxi Rfl Pharmaceutical Co, Ltd) dose groups of 1.0, 1.5, and 2.0 mg/wk and a placebo group (0 mg/wk). They underwent 1 scheduled screening visit and 5 scheduled treatment visits, with ECG, routine blood and urine analyses, and biochemical tests performed at each visit. The assessment scale scores (Yale Global Tic Severity Scale [YGTSS]21 and Clinical Global Impression–Severity scale [CGI-S])22 were obtained during screening and weeks 0, 2, 4, 6, and 8 of treatment. Adverse events, concomitant medications, and participant compliance were recorded at each follow-up visit. Trial medication (1 patch per week), instruction to participants and guardian on proper use, and follow-up with participant and guardian after 2 weeks were conducted as planned. Participants who did not show effective results after 4 weeks of treatment (YGTSS reduction rate <30% and Clinical Global Impression–Improvement scale [CGI-I] score ≥4) were withdrawn from the trial.
Endpoints and Measurement
The primary outcome for this trial was the treatment efficacy at week 8. The YGTSS score was used to evaluate the treatment efficacy. The total YGTSS score for each visit was counted for each group of participants using the descriptive method of measures, and the missing YGTSS scores of participants at postbaseline visits were carried forward as last observation carried forward (LOCF). The study protocol required withdrawal from the trial if efficacy was not evident at 4 weeks, and the endpoint analysis was done using data from 8 weeks, so the LOCF method was used. The total YGTSS score was the sum of motor twitches and vocal twitches, ranging from 0 to 50, with lower scores being better.23–25
Each indicator was defined as follows: (1) total YGTSS score = total motor twitching + total vocal twitching; (2) value of change in YGTSS score = total pretreatment score − total posttreatment score; and (3) YGTSS reduction rate = change value of YGTSS score ÷ total score before treatment × 100%.
The treatment efficacy was defined as follows: (1) treatment effectiveness rate at each visit = (number of participants with clinical recovery + significant improvement within the current visit) ÷ number of participants with YGTSS scores at the current visit × 100% and (2) posttreatment efficacy = (number of participants that had clinical recovery + significant improvement after treatment) ÷ number of participants in the analysis set × 100%.
The correspondence between point reduction rates and treatment outcomes is as follows: reduction rate ≥80% means clinically cured; reduction rate ≥50% but <80% means significant improvement; reduction rate ≥30% but <50% means improvement; reduction rate <30% means ineffective.
The secondary outcome is the CGI-S and CGI-I, CGI-S scores at each visit, and CGI-I scores at week 2. The CGI-S is used to assess the severity of disease, and the CGI-I is used to assess clinical efficacy and has good reliability and validity.26 The CGI scale is rated on an 8-point scale ranging from 0 to 7; the higher the score, the more severe the condition. The change value was calculated as change value = baseline CGI-S score − posttreatment CGI-S score.
Sample Size
According to the results of the previous study,15 the treatment effective rate was 68%, placebo effect was 40%, α was 0.05 (bilateral), and test efficacy was 80% with the sample size of 95 participants in each group; 17% intergroup difference was observed in the detectable effective rate. Therefore, considering factors such as shedding, 120 participants were planned to be enrolled in each group, with a total of 480 participants in the current trial.
Statistical Analysis
Statistical analysis was performed using SAS version 9.2 (SAS Institute, Cary, NC). It included descriptive statistics and hypothesis testing. Statistical analysis of the population was conducted using the full analysis set (FAS). The FAS refers to participants who have taken the trial drug at least once and have data on at least 1 primary index measurement at baseline and posttreatment. The FAS is the primary analysis set for efficacy analysis.
The Kolmogorov-Smirnov test was used for the normality test. Continuous variables that conformed to a normal distribution were expressed as mean ± SD, continuous variables that did not conform to a normal distribution were expressed as median (range), and categorical variables were expressed as frequency and percentage (%).
The intergroup comparison of total YGTSS scores for each posttreatment visit was performed using analysis of covariance. The differences in the total YGTSS score reduction rates between the groups were evaluated using a mixed model for repeated measures, the treatment group, and center as fixed effects.
The efficacy rate and its 95% confidence interval (CI) were calculated for each group, and the differences between each test group and placebo group using the CMH method and their 95% CI were calculated. We used Cohen (d) statistics to analyze the effect size of each of the 3 test groups compared with the placebo group.
Enumeration data were analyzed by frequency analysis (constituent ratio). Nonparametric tests were used. The CGI-S scores of participants were tested using the Wilcoxon rank sum test. The between-group differences in the change values of CGI-S scores and CGI-I scores at each visit were tested using the Kruskal-Wallis H test.
The LOCF strategy was used for missing YGTSS scores after the baseline visit. In this method, for each dimension of motor or vocal tics or functional impairment scores, missing values were imputed using the last observation values, accordingly, and then, the total score was calculated. Additionally, the LOCF imputation strategy was performed for missing secondary efficacy indicators.
For the side effect event statistics, the number of cases and percentage of corresponding cases are given for the count indicators, A χ2 test and Fisher exact probability test were used to analyze the different rates of adverse events between the 4 groups.
RESULTS
In this study, 498 participants who underwent treatment for TS at 20 centers were enrolled. Of these, 5 cases who did not receive the study medication and 5 cases who did not have a posttreatment YGTSS score were excluded from the study. Therefore, a total of 488 participants were finally included in this trial, with 121 participants in the 2.0-mg/wk group, 119 participants in the 1.5-mg/wk group, 126 participants in the 1.0-mg/wk group, and 122 participants in the placebo group. None of the participants required early emergency unblinding or broken blinding (Fig. 1). The baseline characteristics are shown in Table 1.
FIGURE 1.
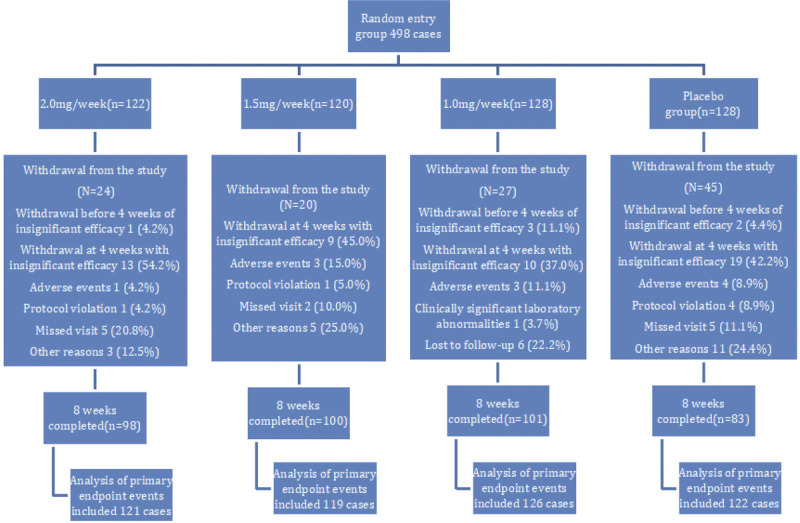
Grouping of study participants.
TABLE 1.
Demographic Characteristics of Participant Groups
| Indicators | 2.0 mg/wk (n = 121) | 1.5 mg/wk (n = 119) | 1.0 mg/wk (n = 126) | Placebo (n = 122) | Total (n = 488) |
|---|---|---|---|---|---|
| Age, mean ± SD, y | 9.04 ± 1.95 | 8.92 ± 2.08 | 9.16 ± 2.17 | 8.88 ± 1.93 | 9.00 ± 2.03 |
| Gender | |||||
| Male | 111 (91.7%) | 99 (83.2%) | 104 (82.5%) | 105 (86.1%) | 419 (85.9%) |
| Female | 10 (8.3%) | 20 (16.8%) | 22 (17.5%) | 17 (13.9%) | 69 (14.1%) |
| Ethnicity | |||||
| Han Chinese | 120 (99.2%) | 117 (98.3%) | 123 (97.6%) | 116 (95.1%) | 476 (97.5%) |
| Hui | 0 | 0 | 1 (0.8%) | 0 | 1 (0.2%) |
| Manchu | 1 (0.8%) | 0 | 1 (0.8%) | 4 (3.3%) | 6 (1.2%) |
| Tibetan | 0 | 0 | 0 | 1 (0.8%) | 1 (0.2%) |
| Other | 0 | 2 (1.7%) | 1 (0.8%) | 1 (0.8%) | 4 (0.8%) |
| Education level | |||||
| Kindergarten | 10 (8.3%) | 19 (16.0%) | 16 (12.7%) | 14 (11.5%) | 59 (12.1%) |
| Primary school | 103 (85.1%) | 92 (77.3%) | 103 (81.7%) | 103 (84.4%) | 401 (82.2%) |
| Junior high school (including preparatory classes) | 7 (5.8%) | 7 (5.9%) | 4 (3.2%) | 5 (4.1%) | 23 (4.7%) |
| High school | 1 (0.8%) | 1 (0.8%) | 3 (2.4%) | 0 | 5 (1.0%) |
| Accompanied by ADHD | |||||
| No | 86 (71.1%) | 92 (77.3%) | 90 (71.4%) | 93 (76.2%) | 361 (74.0%) |
| Yes | 35 (28.9%) | 27 (22.7%) | 36 (28.6%) | 29 (23.8%) | 127 (26.0%) |
At week 8, the YGTSS score reduction rate in the 2.0-mg/wk group (63.21 ± 32.60, d = −0.806; 95% CI, −1.068 to −0.545), 1.5-mg/wk group (68.16 ± 25.88, d = −1.11; 95% CI, −1.382 to −0.839), and 1.0-mg/wk group (63.26 ± 33.20, d = −0.797; 95% CI, −1.056 to −0.538) were statistically significantly higher than the placebo group (39.60 ± 25.56) (all P < 0.001; Table 2). But the results of the mixed model analysis of the total YGTSS reduction score showed significantly better score reduction in both the 1.5-mg/wk group (F = 28.10,P < 0.001) and the 2.0-mg/wk group (F = 11.10, P = 0.001) than in the placebo group, except for a nonsignificant difference between the 1.0-mg/wk group (F = 2.98, P = 0.086) and placebo group (Supplemental Digital Content, Table S2, http://links.lww.com/CNP/A46).
TABLE 2.
YGTSS Total Score at Week 2 and YGTSS Score Reduction Rate at Week 8 (%)
| Visits | 2.0 mg/wk (n = 121) | 1.5 mg/wk (n = 119) | 1.0 mg/wk (n = 126) | Placebo (n = 122) | |
|---|---|---|---|---|---|
| Week 2 | Mean ± SD | 28.6 ± 8.47 | 30.2 ± 8.82 | 29.4 ± 8.28 | 28.4 ± 8.46 |
| P* | 0.33 | ||||
| Week 8 | Mean ± SD | 63.21 ± 32.60 | 68.16 ± 25.88 | 63.26 ± 33.20 | 39.60 ± 25.56 |
| P† | <0.001 | <0.001 | <0.001 | ||
| Cohen (d)‡ | −0.806 | −1.11 | −0.797 | NA | |
| 95% CI | −1.068 to −0.545 | −1.382 to −0.839 | −1.056 to −0.538 | NA |
Two-factor ANOVA was used for between-group tests, with group and center as fixed effects.
*Differences between groups at baseline were tested using the ANOVA test.
†ANOVA test was conducted for the difference between the groups and the placebo group.
‡We used Cohen (d) statistics to analyze the effect size of each of the 3 test groups compared with the placebo group.
ANOVA indicates analysis of variance.
The results of LOCF for FAS cases showed that the efficacy of each group after 8 weeks of treatment was as follows: 98 cases in the 2.0-mg/wk group were effective, with an efficacy of 81.0% (95% CI, 72.9%–87.6%); 100 cases in the 1.5-mg/wk group were effective, with an efficacy of 84.0% (95% CI, 76.2%–90.1%); 101 cases in the 1.0-mg/wk group were effective, with an efficacy of 80.2% (95% CI, 72.1%–86.7%); and 34 cases in the placebo group were effective, with an efficacy of 27.9% (95% CI, 20.1%–36.7%). P value for the 4 groups was <0.0001, indicating a statistical difference in efficacy among the 4 groups. The P value of CMH test for each treatment group relative to the placebo group was also <0.0001, and the effective rate was higher than that of the placebo group, indicating that the effective rate of each test group was better than that of the placebo group. In addition, the effective rate tended to increase as the duration of treatment increased (Table 3).
TABLE 3.
Posttreatment Efficacy Summary
| Visit | Group | P* | ||||
|---|---|---|---|---|---|---|
| 2.0 mg/wk | 1.5 mg/wk | 1.0 mg/wk | Placebo | |||
| (n = 121) | (n = 119) | (n = 126) | (n = 122) | |||
| Week 2 | Effective | 15 (12.4%) | 14 (11.8%) | 10 (7.9%) | 6 (4.9%) | 0.0648 |
| Invalid | 106 (87.6%) | 105 (88.2%) | 116 (92.1%) | 116 (95.1%) | ||
| 95% CI | 7.1%–19.6% | 6.6%–19.0% | 3.9%–14.1% | 1.8%–10.4% | ||
| P† | 0.0258 | 0.0528 | 0.2049 | |||
| Week 4 | Effective | 87 (71.9%) | 79 (66.4%) | 85 (67.5%) | 20 (16.4%) | <0.0001 |
| Invalid | 34 (28.1%) | 40 (33.6%) | 41 (32.5%) | 102 (83.6%) | ||
| 95% CI | 63.0%–79.7% | 57.2%–74.8% | 58.5%–75.5% | 10.3%–24.2% | ||
| P† | <0.0001 | <0.0001 | <0.0001 | |||
| Week 6 | Effective | 91 (75.2%) | 90 (75.6%) | 91 (72.2%) | 33 (27.0%) | <0.0001 |
| Invalid | 30 (24.8%) | 29 (24.4%) | 35 (27.8%) | 89 (73.0%) | ||
| 95% CI | 66.5%–82.6% | 66.9% ~ 83.0% | 63.5%–79.8% | 19.4%–35.8% | ||
| P† | <0.0001 | <0.0001 | <0.0001 | |||
| Week 8 | Effective | 98 (81.0%) | 100 (84.0%) | 101 (80.2%) | 34 (27.9%) | <0.0001 |
| Invalid | 23 (19.0%) | 19 (16.0%) | 25 (19.8%) | 88 (72.1%) | ||
| 95% CI | 72.9%–87.6% | 76.2%–90.1% | 72.1%–86.7% | 20.1%–36.7% | ||
| P† | <0.0001 | <0.0001 | <0.0001 | |||
The differences between each test group and placebo groups using the CMH method and their 95% CI were calculated.
*The CMH method was used to compare the difference in treatment efficacy and its 95% CI between the 4 groups at each visit; with the model accounting for center effects, the difference between each test group and placebo groups using CMH method and their 95% CI were calculated.
There was a significant improvement in investigator-rated severity from week 2 onward, and the CGI-S score was statistically significantly lower in the 2.0-mg/wk group, the 1.5-mg/wk group, and the 1.0-mg/wk group than the placebo group (all P < 0.001). Despite all improvements, differences between the test groups and placebo groups began to emerge from week 6 onward. These differences became more significant at week 8, when the CGI-S scores of the 3 test groups (2.0, 1.5, and 1.0 mg/wk) were 2.5 ± 1.18, 2.4 ± 1.08, and 2.6 ± 1.21, respectively, compared with 3.0 ± 0.098 in the placebo group. However, the Kruskal-Wallis test showed that the change value at 8 weeks was statistically significantly different between the groups, and the change values were significantly greater in the 3 test groups than in the placebo group (P < 0.001) (Table 4).
TABLE 4.
Analysis of CGI-S Score Results
| Visits | 2.0 mg/wk (n = 121) | 1.5 mg/wk (n = 119) | 1.0 mg/wk (n = 126) | Placebo (n = 122) | |
|---|---|---|---|---|---|
| Baseline | Mean ± SD | 4.2 ± 0.99 | 4.3 ± 0.98 | 4.2 ± 1.04 | 4.2 ± 1.05 |
| Week 2 | Mean ± SD | 3.7 ± 0.86 | 3.7 ± 0.90 | 3.7 ± 1.05 | 3.7 ± 0.99 |
| P* | <0.001 | <0.001 | <0.001 | <0.001 | |
| Week 4 | Mean ± SD | 3.2 ± 0.87 | 3.1 ± 0.94 | 3.3 ± 1.04 | 3.3 ± 0.93 |
| P* | <0.001 | <0.001 | <0.001 | <0.001 | |
| Week 6 | Mean ± SD | 2.9 ± 1.01 | 2.8 ± 0.93 | 2.9 ± 1.13 | 3.1 ± 1.02 |
| P* | <0.001 | <0.001 | <0.001 | <0.001 | |
| Week 8 | Mean ± SD | 2.5 ± 1.18 | 2.4 ± 1.08 | 2.6 ± 1.21 | 3.0 ± 0.098 |
| P* | <0.001 | <0.001 | <0.001 | <0.001 |
The denominator of the percentages is the number of participants with nondeficient CGI-S at each visit in each group. Within-group tests were performed using the signed rank sum test, starting at week 2, and each subsequent visit was compared with baseline (visit 1).
*Compared with baseline.
The results of CGI-I scores showed differences between the 4 groups from week 2, with the 1.5-mg/wk group outperforming the placebo group in week 2. At week 4, all test groups had significantly better scores than the placebo group, which continued until week 8, and the difference became increasingly large and statistically significant (P < 0.01; Table 5).
TABLE 5.
Analysis of CGI-I Score Results
| Visits | 2.0 mg/wk (n = 121) | 1.5 mg/wk (n = 119) | 1.0 mg/wk (n = 126) | Placebo (n = 122) | P* | |
|---|---|---|---|---|---|---|
| Week 2 | Mean ± SD | 3.0 ± 0.77 | 2.8 ± 0.74 | 3.0 ± 0.88 | 3.1 ± 0.67 | 0.017 |
| P† | 0.1182 | 0.0011 | 0.0569 | |||
| Week 4 | Mean ± SD | 2.4 ± 0.97 | 2.2 ± 0.82 | 2.4 ± 1.09 | 2.8 ± 0.88 | <0.001 |
| P† | <0.001 | <0.001 | <0.001 | |||
| Week 6 | Mean ± SD | 2.2 ± 1.06 | 2.1 ± 0.94 | 2.2 ± 1.18 | 2.8 ± 1.101 | <0.001 |
| P† | <0.001 | <0.001 | <0.001 | |||
| Week 8 | Mean ± SD | 1.9 ± 1.16 | 1.8 ± 0.95 | 2.0 ± 1.24 | 2.8 ± 1.08 | <0.001 |
| P† | <0.001 | <0.001 | <0.001 |
*The test conducted between the 4 groups was the Kruskal-Wallis test.
†The comparisons for the 3 treatment and placebo groups were performed using the Wilcoxon rank sum test.
For the side effect event statistics, we used 493 cases that were included in the safety set. The safety set is all cases that were randomized and have used the study drug at least once. Systemic skin conditions (dermatitis, rash, urticaria) were abnormal in 32 cases (6.5%) in all the participants, 4 (3.3%), 9 (7.6%), and 6 (4.8%) and 13 (10.6%) in the 2.0-, 1.5-, and 1.0-mg/wk group and placebo, respectively, but no subject withdrew from the study due to adverse events. Skin conditions at the patch (rash at the administration site, painful sensitization at the administration site, and pruritus at the administration site) were abnormal in 39 (7.9%) of the all participants, 6 (5.0%), 11 (9.2%), and 7 (5.6%) and 15 (12.2%) in the 2.0-, 1.5-, and 1.0-mg/wk group and placebo, respectively. In both cases, the placebo group was slightly higher.
In the ECG examination, there were 37 cases with normal pretreatment and abnormal posttreatment, including 8 cases in the 2.0-mg/wk group, 5 cases in the 1.5-mg/wk group, 13 cases in the 1.0-mg/wk group, and 11 cases in the placebo group. The one case in which the abnormality was clinically significant was a case in the 1.0-mg/wk group with left ventricular hypervoltage and T-wave hyperacusis at week 8. All other cases showed abnormalities that were not clinically significant.
DISCUSSION
This study found that the YGTSS score reduction rate in the 2.0- and 1.5-mg/wk groups was significantly higher than that in the placebo group. The CGI-S scores showed significant improvement in investigator-rated severity from week 2 onward, and the change value was statistically significant at week 8. The CGI-I scores differed between the 4 groups from week 2, and all test groups scored significantly better than did the placebo group at weeks 4 and 8.
In the longitudinal analysis, a continuous decrease in the mean total YGTSS score from week 2 to week 8 was observed in all 3 groups, indicating that clonidine adhesive patch can continuously improve the total YGTSS score in participants with TS, maintaining the effects of treatment and further continuously improving these effects. This improvement in treatment effect is clinically significant, especially for chronic conditions such as TS that require long-term treatment. These results are consistent with that of studies involving other oral medications for TS, such as haloperidol, aripiprazole, and thiopride,27,28 as well as oral clonidine for TS. The results of a previous 4-week double-blind clinical study by our team,15 which included 437 participants with tic disorders, in which the study drug was also clonidine adhesive patch, 55% of whom had TS, showed a 4-week efficacy rate of 68.85%, which is not consistent with the findings of the current study. The efficacy rate of the 3 clonidine adhesive patch treatment groups in the study were more than 80%. This inconsistency in findings could probably be due to the fact that the previous study was conducted for only 4 weeks, and the duration of treatment was shorter, suggesting that the longer the duration of clonidine adhesive patch use, the better the improvement in TS. There may also be a relationship with the weight of this subject population being in the range of 20 to 40 kg. Another study with an open-ended self-control 12-week treatment period found that the efficacy began to improve significantly at week 8 and reached a maximum effect at week 12. However, no significant difference was observed in the efficacy between weeks 8 and 12,29 which may indicate that the effect of the clonidine adhesive patch starts after 4 weeks, but the maximum effect occurs at weeks 8 to 12, suggesting the use of the clonidine adhesive patch, clinically, as opposed to other treatment methods. This result suggests that it takes longer to achieve clinical effects with clonidine adhesive patch compared with other therapeutic agents such as haloperidol.27 Furthermore, the YGTSS score reduction rate of the clonidine adhesive patch when compared with placebo shows a difference from week 2, and by week 8, this difference is even more significant. However, when we used a mixed model to analyze the effect of the 3 doses, the results showed that the score reduction rate of the 1.0-mg/wk treatment group does not show clinical significance compared with the placebo group, which indicates that in general the dose of 1.0 mg/wk may be less effective in TS participants (considering the participant's weight and age), as a larger dose of clonidine adhesive patch needs to be used as much as possible in clinical practice. In this study, the CGI-I and CGI-S scores were used to assess the improvement of overall function in participants with TS. Although there was improvement at week 2, a significant change was observed in scores of CGI-S from week 6 to a maximum difference at week 8. Moreover, a significant change was seen in scores of CGI-I in test groups from week 4 compared with that of the placebo group. This finding indicates that the effect of the clonidine adhesive patch on the improvement of TS symptoms was seen at week 2; however, as the improvement in overall function occurs at a later stage after the improvement in symptoms, the improvement in overall function of TS participants became more pronounced as the treatment with clonidine adhesive patch progressed. Therefore, long-term maintenance medication is clinically needed to benefit the participant's recovery.
No group comparisons of the incidence of various adverse events showed statistical differences, although the placebo group was slightly higher. The incidence of common adverse reactions such as skin diseases (6.5%) and that of skin conditions at the patch site (7.9%) was slightly higher than in the previous study,15 probably because the population in this study was restricted to 20 to 40 kg, whereas the population in the previous study was not restricted to a low weight range, which may have contributed to the higher incidence of skin diseases. No serious adverse events occurred during the present trial. For the effects of ECG, there were no adverse reactions such as low blood pressure as previously thought, and only 1 case showed significant abnormalities of ECG. Hence, the clonidine adhesive patch appears safe and well-tolerated in children and adolescents with TS. At the same time, because clonidine adhesive patch is more convenient to use compared with the oral medications, leading to better treatment adherence.
This study has some limitations. TS is often associated with other disorders, which may affect the effectiveness of medications in treating the disorder. Therefore, it is difficult to assess accurately the effect of treatment specifically on TS. Moreover, this study did not distinguish between participants treated in the hospital for the first time and those who had poor results after repeated use of other clinical medications, which could affect the results. Additionally, there is also a need for future research on the effects of clonidine adhesive patch on patients' academic performance and self-esteem.
Clonidine adhesive patch can effectively improve the symptoms and overall functions of participants with TS, which is a combined vocal and motor tic disorder. The treatment was effective in all 3 dose groups of 1.0, 1.5, and 2.0 mg/wk. Further, this study concludes that the longer the treatment time, the better the effect.
ACKNOWLEDGMENT
The authors thank Liu Yi, Zhou Lin-Lin, Jiang Wen-Qing, Xu Yun, Yu Shun-Ying, and Sun Jin-Hua from Shanghai Mental Health Center; Zhu Yan, Geng Yao-Guo, Su QiaoRong, Huang Guang-Fei, and Li Fei from the Mental Health Research Institute of Central South University; Chen Yi-Xin, Yu Lin, Zou Bing, Zhang Jiu-Ping, Luo Shuo-Jun, Jiao Gong-Kai, and Hang Yue-Yue from Nanjing Brain Hospital; Wang Lei and Liu Li from Harbin Maternity and Child Care Hospital; Zhong You-Quan, Tao Xuan-Hua, Wu Ju, and Zhou Wen-Zhi from Chengdu Children's Hospital; and Zhang Feng-Ling for their hard work and dedication.
Footnotes
Z.Z. and Y.Q. contributed equally to this work.
Z.Z. contributed to design and acquisition, analysis, and interpretation of data and drafted the manuscript. Y.Q. contributed to design, acquisition and analysis of data. Y.D. contributed to conception; design; and acquisition, analysis, and interpretation of data and critically revised the manuscript. J.H. contributed to design and acquisition of data. H.C., Y.C., X.W., J.M., S.S., H.W., and F.J. contributed to acquisition of data. All authors read and approved the final manuscript.
That study was approved by the ethics committee of each center (ethical approval no. RFL201001) and registered in the National Medical Products Administration (CTR20132112).
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest and Source of Funding: J.H. was employed by China National Pharmaceutical Group Shanxi Rfl Pharmaceutical Co Ltd, China. The other authors have no conflicts of interest to declare. This research was supported at the Shanghai Jiaotong University and contracts between China National Pharmaceutical Group Shanxi Rfl Pharmaceutical Co Ltd and Shanghai Mental Health Center. The study drug was provided by the China National Pharmaceutical Group Shanxi Rfl Pharmaceutical Co. Ltd. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.clinicalneuropharm.com).
Contributor Information
Zhimin Zhao, Email: zzmsky@163.com.
Yun Qian, Email: dawuwanwan@163.com.
Hong Chen, Email: hong6644@163.com.
Jie He, Email: 1276931068@qq.com.
Yanhui Chen, Email: yanhui_0655@126.com.
Xiuxia Wang, Email: Wangxiuxia868@163.com.
Jianning Mai, Email: gzchmjn@126.com.
Suzhen Sun, Email: sunsuzhen2004@126.com.
Huimei Wang, Email: fyek2008@163.com.
Fuyong Jiao, Email: jiaofy@yeah.net.
REFERENCES
- 1.Jankovic J, Jimenez-Shahed J, Brown LW. A randomised, double-blind, placebo-controlled study of topiramate in the treatment of Tourette syndrome. J Neurol Neurosurg Psychiatry 2010;81:70–73. [DOI] [PubMed] [Google Scholar]
- 2.Pringsheim T. Tic severity and treatment in children: the effect of comorbid attention deficit hyperactivity disorder and obsessive compulsive behaviors. Child Psychiatry Hum Dev 2017;48:960–966. [DOI] [PubMed] [Google Scholar]
- 3.Eapen V, Cavanna AE, Robertson MM. Comorbidities, social impact, and quality of life in Tourette syndrome. Front Psychiatry 2016;7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganos C, Martino D, Pringsheim T. Tics in the pediatric population: pragmatic management. Mov Disord Clin Pract 2017;4:160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizzo R Pellico A Silvestri PR, et al. A randomized controlled trial comparing behavioral, educational, and pharmacological treatments in youths with chronic tic disorder or Tourette syndrome. Front Psych 2018;9:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egolf A, Coffey BJ. Current pharmacotherapeutic approaches for the treatment of Tourette syndrome. Drugs Today (Barc) 2014;50:159–179. [DOI] [PubMed] [Google Scholar]
- 7.Rizzo R Eddy CM Calí P, et al. Metabolic effects of aripiprazole and pimozide in children with Tourette syndrome. Pediatr Neurol 2012;47:419–422. [DOI] [PubMed] [Google Scholar]
- 8.Wang S Wei YZ Yang JH, et al. The efficacy and safety of aripiprazole for tic disorders in children and adolescents: a systematic review and meta-analysis. Psychiatry Res 2017;254:24–32. [DOI] [PubMed] [Google Scholar]
- 9.Stiede JT, Woods DW. Pediatric prevention: tic disorders. Pediatr Clin North Am 2020;67:547–557. [DOI] [PubMed] [Google Scholar]
- 10.Ganos C, Martino D. Tics and Tourette syndrome. Neurol Clin 2015;33:115–136. [DOI] [PubMed] [Google Scholar]
- 11.Hedderick EF, Morris CM, Singer HS. Double-blind, crossover study of clonidine and levetiracetam in Tourette syndrome. Pediatr Neurol 2009;40:420–425. [DOI] [PubMed] [Google Scholar]
- 12.Mechler K Banaschewski T Hohmann S, et al. Evidence-based pharmacological treatment options for ADHD in children and adolescents. Pharmacol Ther 2022;230:107940. [DOI] [PubMed] [Google Scholar]
- 13.Ehrlich J Beck B Thiedmann R, et al. Bioequivalence and adhesion evaluation of transdermal clonidine following a change in excipient supplier. Int J Clin Pharmacol Ther 2016;54:816–824. [DOI] [PubMed] [Google Scholar]
- 14.Li AY Cong S Lu H, et al. Clinical observation on treatment of Tourette syndrome by integrative medicine. Chin J Integr Med 2009;15:261–265. [DOI] [PubMed] [Google Scholar]
- 15.Du YS Li HF Vance A, et al. Randomized double-blind multicentre placebo-controlled clinical trial of the clonidine adhesive patch for the treatment of tic disorders. Aust N Z J Psychiatry 2008;42:807–813. [DOI] [PubMed] [Google Scholar]
- 16.Jiao F Zhang X Zhang X, et al. Clinical observation on treatment of Tourette syndrome in Chinese children by clonidine adhesive patch. Eur J Paediatr Neurol 2016;20:80–84. [DOI] [PubMed] [Google Scholar]
- 17.Yang C Kang B Yu D, et al. Effectiveness and safety of a clonidine adhesive patch for children with tic disorders: study in a real-world practice. Front Neurol 2020;11:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S Wei YZ Yang J, et al. Clonidine adhesive patch for the treatment of tic disorders: a systematic review and meta-analysis. Eur J Paediatr Neurol 2017;21:614–620. [DOI] [PubMed] [Google Scholar]
- 19.Pringsheim T Holler-Managan Y Okun MS, et al. Comprehensive systematic review summary: treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology 2019;92:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). Arlington County, VA: American Psychiatric Association; 2000. [Google Scholar]
- 21.Zhong Youquan TX, Wu JU. Yale Global Tic Severity Scale for children with tic disorders: a report on its preliminary application. Sichuan Med J 2000;21:110–111. [Google Scholar]
- 22.Wenyuan W. Clinical Global Impression. Shanghai Arch Psychiatry 1984;2:76–77. [Google Scholar]
- 23.Leckman JF Riddle MA Hardin MT, et al. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry 1989;28:566–573. [DOI] [PubMed] [Google Scholar]
- 24.Storch EA Murphy TK Geffken GR, et al. Reliability and validity of the Yale Global Tic Severity Scale. Psychol Assess 2005;17:486–491. [DOI] [PubMed] [Google Scholar]
- 25.Storch EA Murphy TK Fernandez M, et al. Factor-analytic study of the Yale Global Tic Severity Scale. Psychiatry Res 2007;149:231–237. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M. Rating Scales Manual for Mental Health. Hunan, China: Hunan Science and Technology Press; 1998. [Google Scholar]
- 27.Gaffney GR Perry PJ Lund BC, et al. Risperidone versus clonidine in the treatment of children and adolescents with Tourette's syndrome. J Am Acad Child Adolesc Psychiatry 2002;41:330–336. [DOI] [PubMed] [Google Scholar]
- 28.Leckman JF Hardin MT Riddle MA, et al. Clonidine treatment of Gilles de la Tourette's syndrome. Arch Gen Psychiatry 1991;48:324–328. [DOI] [PubMed] [Google Scholar]
- 29.Song P-P Jiang L Li X-J, et al. The efficacy and tolerability of the clonidine transdermal patch in the treatment for children with tic disorders: a prospective, open, single-group, group, self-controlled study. Front Neurol 2017;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]


