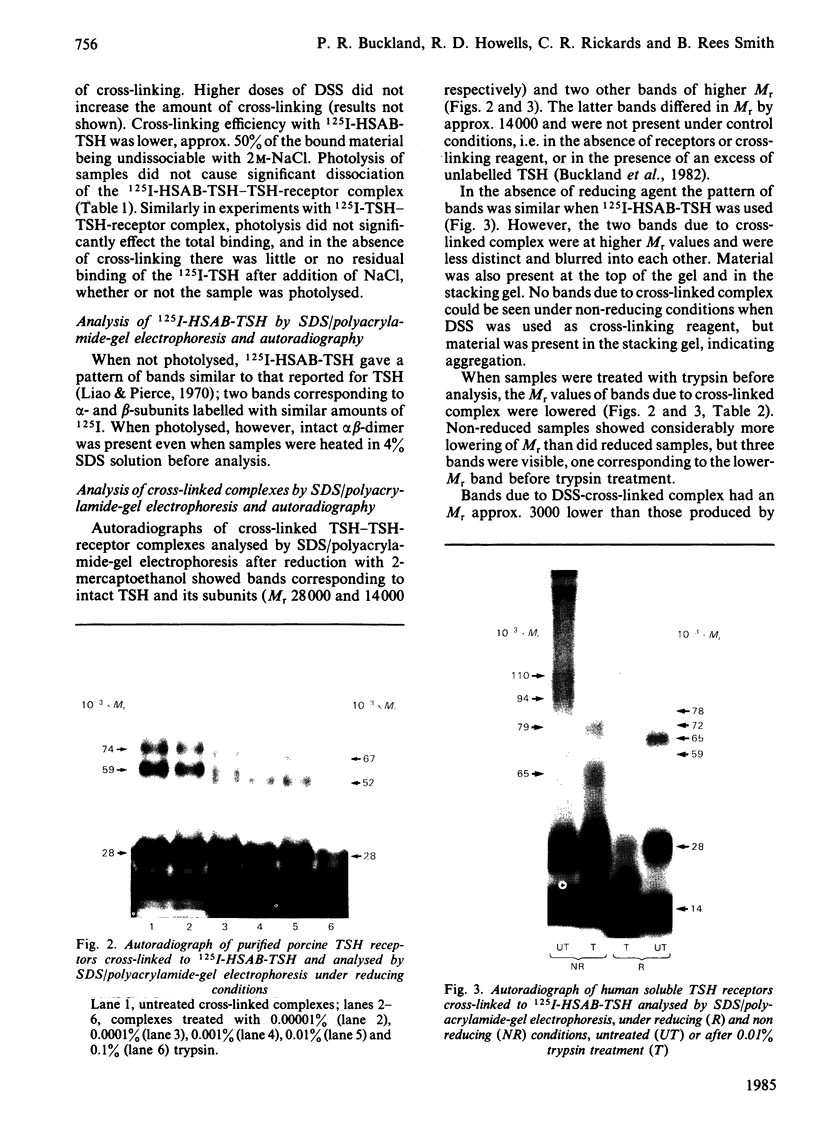
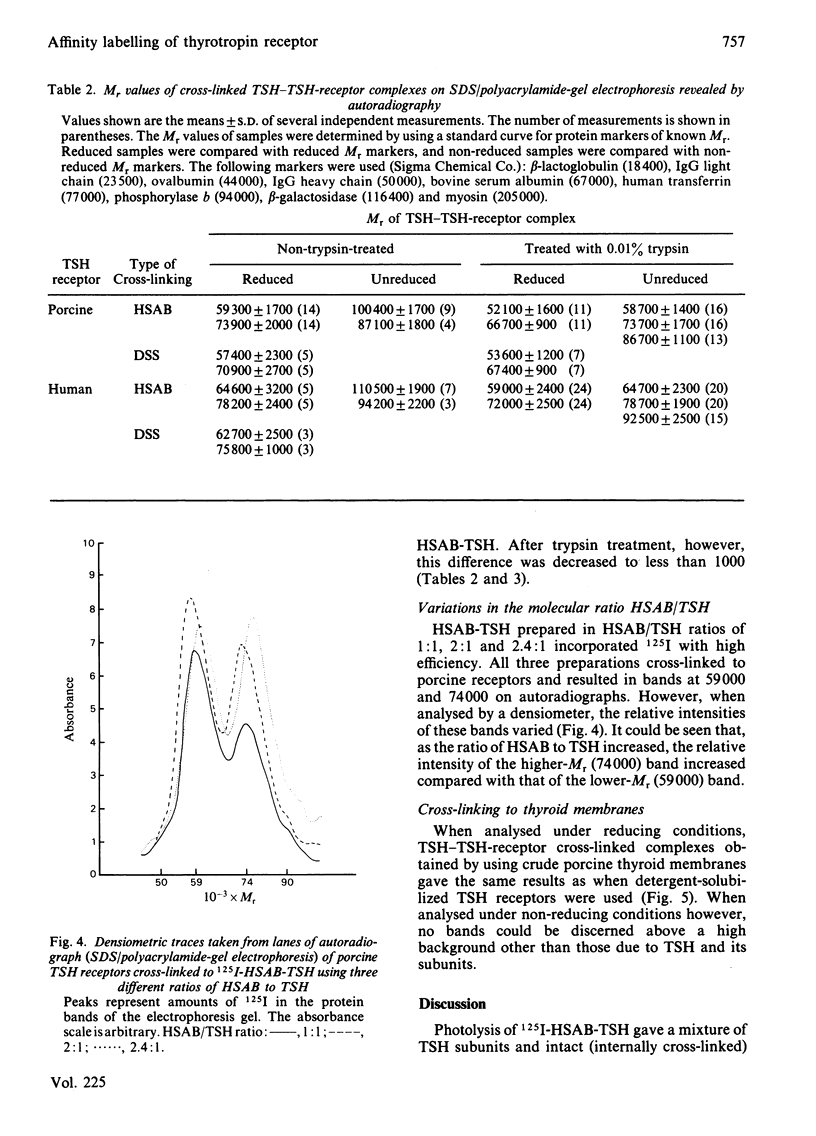
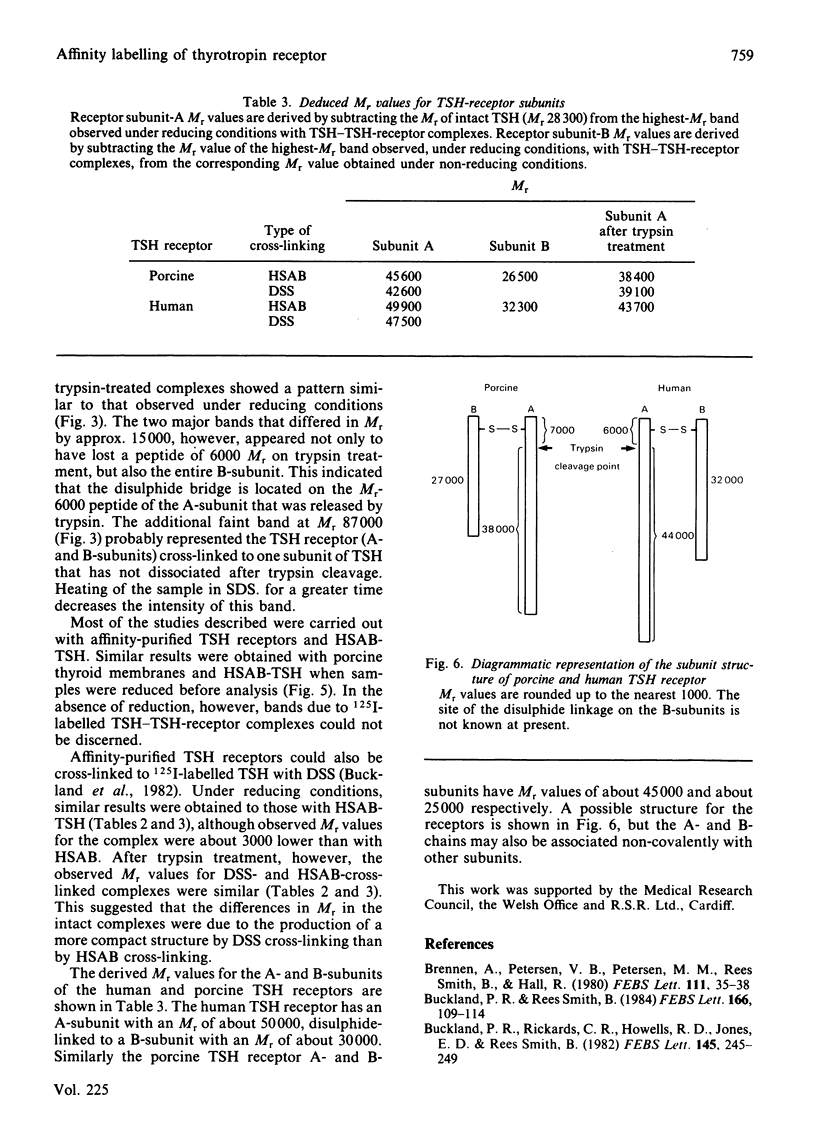
Abstract
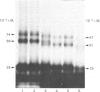
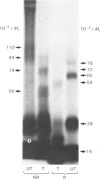
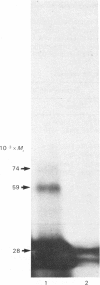
Thyrotropin (TSH) has been coupled to the photoactive heterobifunctional reagent N-hydroxysuccinimidyl 4-azidobenzoate (HSAB) and the properties of the product (HSAB-TSH) investigated. Preparations of HSAB-TSH containing two molecules of HSAB per molecule of TSH were used in most experiments and these preparations retained about 40% of the original receptor-binding activity of the TSH. HSAB-TSH could be labelled with 125I and cross-linked to porcine and human TSH receptors. Analysis of the cross-linked complexes indicated that the receptors consisted of two subunits (designated A and B) linked by a disulphide bridge. In the case of the human TSH receptor, the A- and B-subunits had approximate Mr values of 50 000 and 30 000 respectively, whereas the Mr values for porcine TSH-receptor A- and B-subunits were approx. 45 000 and 25 000 respectively. Only the A subunit was cross-linked to TSH. Comparison of the effects of trypsin and mercaptoethanol on the TSH-TSH-receptor complexes suggested that the trypsin cleavage point on the A-subunit was at a point close to the disulphide bridge.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brennan A., Petersen V. B., Petersen M. M., Smith B. R., Hall R. Time-dependent stabilisation of the TSH-TSH receptor complex. FEBS Lett. 1980 Feb 25;111(1):35–38. doi: 10.1016/0014-5793(80)80755-1. [DOI] [PubMed] [Google Scholar]
- Buckland P. R., Rees Smith B. A structural comparison of guinea pig thyroid and fat TSH receptors by photoaffinity labelling. FEBS Lett. 1984 Jan 23;166(1):109–114. doi: 10.1016/0014-5793(84)80054-x. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Griffith I. P. The effect of cross-links on the mobility of proteins in dodecyl sulphate-polyacrylamide gels. Biochem J. 1972 Feb;126(3):553–560. doi: 10.1042/bj1260553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liao T. H., Pierce J. G. The presence of a common type of subunit in bovine thyroid-stimulating and luteinizing hormones. J Biol Chem. 1970 Jul 10;245(13):3275–3281. [PubMed] [Google Scholar]
- Petersen V. B., Dawes P. J., Rees Smith B., Hall R. The interaction of thyroid-stimulating antibodies with solubilised human thyrotrophin receptors. FEBS Lett. 1977 Nov 1;83(1):63–67. doi: 10.1016/0014-5793(77)80642-x. [DOI] [PubMed] [Google Scholar]
- Pierce J. G., Liao T., Howard S. M., Shome B., Cornell J. S. Studies on the structure of thyrotropin: its relationship to luteinizing hormone. Recent Prog Horm Res. 1971;27:165–212. doi: 10.1016/b978-0-12-571127-2.50029-8. [DOI] [PubMed] [Google Scholar]
- Rickards C., Buckland P., Smith B. R., Hall R. The interaction of Graves' IgG with the thyrotrophin receptor. FEBS Lett. 1981 May 5;127(1):17–21. doi: 10.1016/0014-5793(81)80330-4. [DOI] [PubMed] [Google Scholar]
- Smith B. R., Hall R. Measurement of thyrotropin receptor antibodies. Methods Enzymol. 1981;74(Pt 100):405–420. doi: 10.1016/0076-6879(81)74029-1. [DOI] [PubMed] [Google Scholar]
- Southgate K., Creagh F., Teece M., Kingswood C., Rees Smith B. A receptor assay for the measurement of TSH receptor antibodies in unextracted serum. Clin Endocrinol (Oxf) 1984 May;20(5):539–548. doi: 10.1111/j.1365-2265.1984.tb00102.x. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]