Abstract
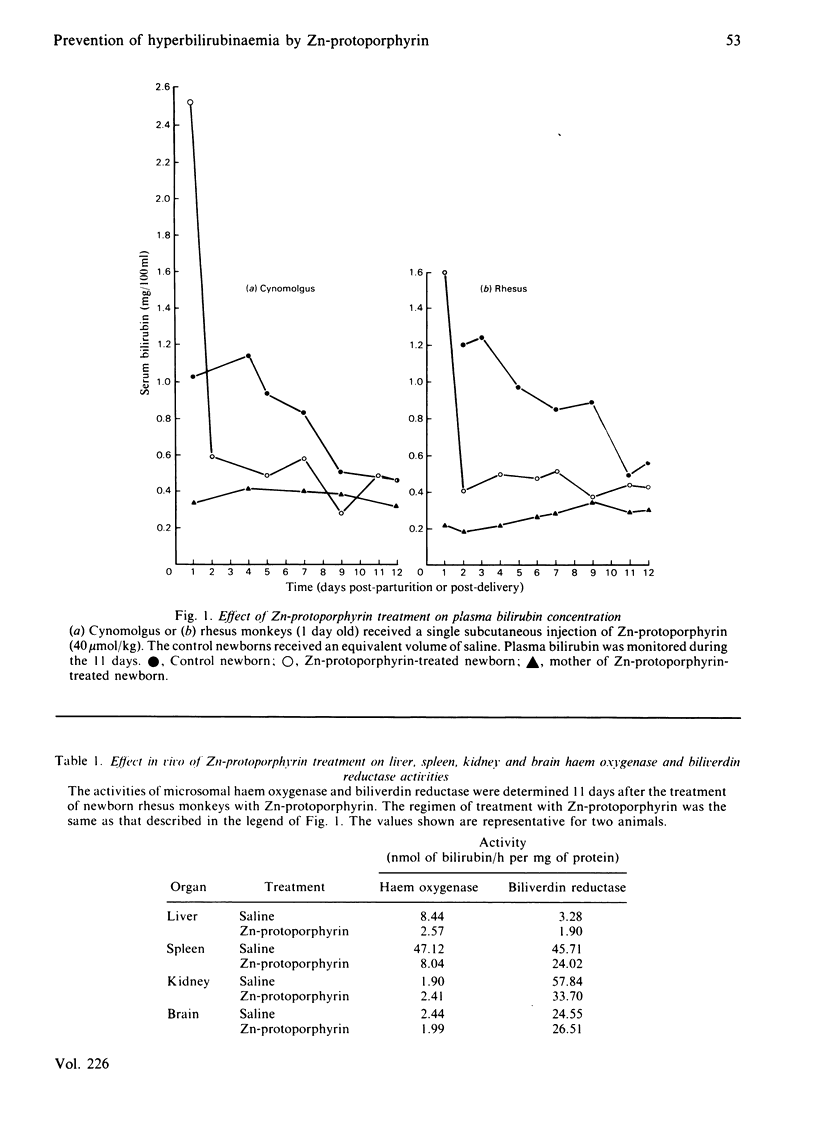
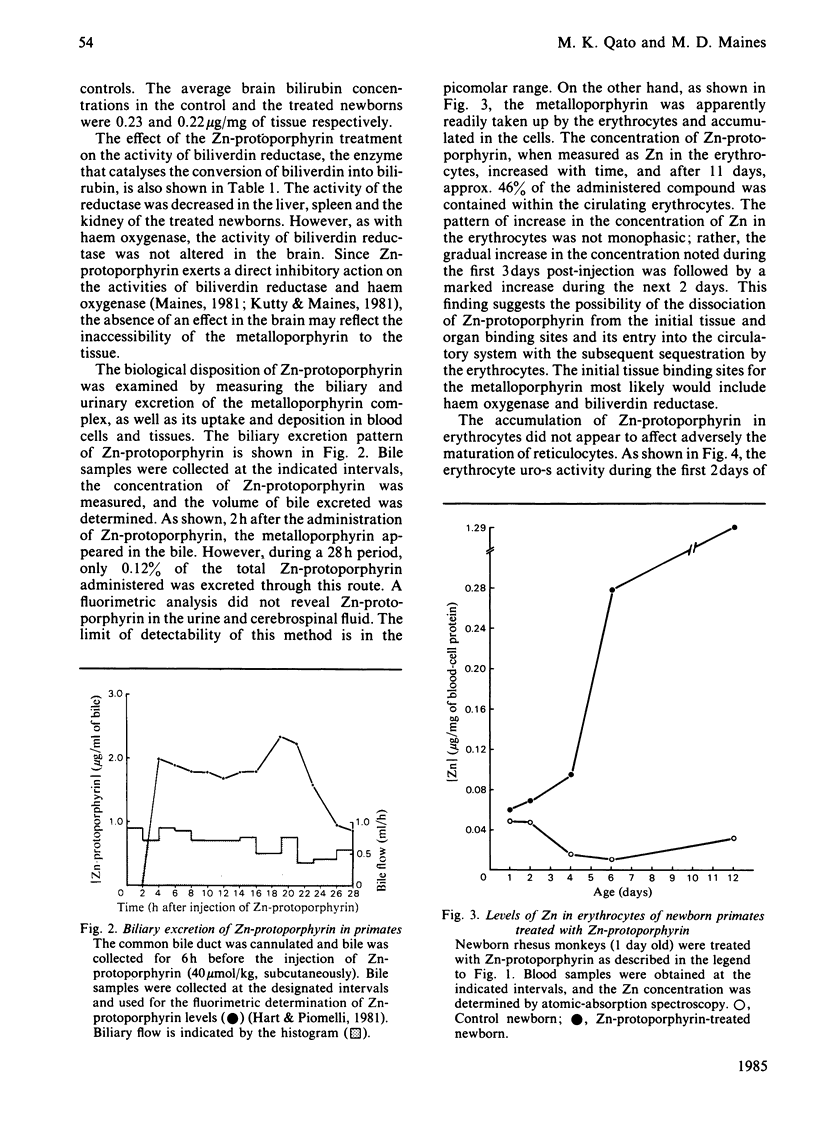
Non-human primates were used as a model of human neonatal hyperbilirubinaemia and its chemotherapeutic suppression. High levels of haem oxygenase activity were detected in the liver and the spleen of neonatal rhesus (Macaca mulatta) and cynomolgus (Macaca irus) monkeys. When 1-day-old neonatal animals were given a single injection of Zn-protoporphyrin (40 mumol/kg, subcutaneously), serum bilirubin levels declined to nearly normal adult levels within 24 h and remained suppressed throughout the postnatal period (12 days). This treatment inhibited the activities of haem oxygenase and biliverdin reductase in the liver and the spleen, without affecting that of the brain. Zn-protoporphyrin treatment did not alter the activity of brain biliverdin reductase or increase brain bilirubin levels. The biological disposition of Zn-protoporphyrin was examined by measuring the biliary and urinary excretion of the metalloporphyrin complex, as well as its uptake and deposition in blood cells and tissues. Biliary excretion of the metalloporphyrin was minimal (0.12% over a 28 h period), and no evidence was detected for the urinary excretion of Zn-protoporphyrin. However, the concentration of metalloporphyrin in erythrocytes increased over the duration of the experiment (11 days) to such an extent that 46% of the administered compound was taken up by the cells. It appeared that the molecular basis for the sustained suppression of haem oxygenase activity and bilirubin production by Zn-protoporphyrin involved the release of the metalloporphyrin in the normal process of the degradation of fetal erythrocytes. The scope of the biological activity of Zn-protoporphyrin to alter haem-dependent processes appeared limited in nature, insofar as the microsomal contents of cytochrome P-450 and b5, as well as the aniline hydroxylase, were similar to those of the control animals. Also, the concentration of glutathione in the liver was unchanged. These findings suggest the potential usefulness of Zn-protoporphyrin in experimental and perhaps clinical conditions in which hyperbilirubinaemia occurs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. E., Simionatto C. S., Drummond G. S., Kappas A. Tissue distribution and disposition of tin-protoporphyrin, a potent competitive inhibitor of heme oxygenase. J Pharmacol Exp Ther. 1984 Feb;228(2):327–333. [PubMed] [Google Scholar]
- Blumenthal S. G., Ruebner B. H., Ikeda R. M., Hanson F. W., Bergstrom D. E. Protohemin in bile during primate development. Experientia. 1977 May 15;33(5):592–593. doi: 10.1007/BF01946515. [DOI] [PubMed] [Google Scholar]
- Cohn V. H., Lyle J. A fluorometric assay for glutathione. Anal Biochem. 1966 Mar;14(3):434–440. doi: 10.1016/0003-2697(66)90286-7. [DOI] [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. Chemoprevention of neonatal jaundice: potency of tin-protoporphyrin in an animal model. Science. 1982 Sep 24;217(4566):1250–1252. doi: 10.1126/science.6896768. [DOI] [PubMed] [Google Scholar]
- Gartner L. M., Lee K. S., Vaisman S., Lane D., Zarafu I. Development of bilirubin transport and metabolism in the newborn rhesus monkey. J Pediatr. 1977 Apr;90(4):513–531. doi: 10.1016/s0022-3476(77)80360-0. [DOI] [PubMed] [Google Scholar]
- Granick S., Sassa S., Granick J. L., Levere R. D., Kappas A. Assays for porphyrins, delta-aminolevulinic-acid dehydratase, and porphyrinogen synthetase in microliter samples of whole blood: applications to metabolic defects involving the heme pathway. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2381–2385. doi: 10.1073/pnas.69.9.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARGREAVES T. THE ESTIMATION OF BILIRUBIN IN LIVER. Clin Chim Acta. 1965 Mar;11:278–280. doi: 10.1016/0009-8981(65)90075-6. [DOI] [PubMed] [Google Scholar]
- Hart D., Piomelli S. Simultaneous quantitation of zinc protoporphyrin and free protoporphyrin in erythrocytes by acetone extraction. Clin Chem. 1981 Feb;27(2):220–222. [PubMed] [Google Scholar]
- Imai Y., Ito A., Sato R. Evidence for biochemically different types of vesicles in the hepatic microsomal fraction. J Biochem. 1966 Oct;60(4):417–428. doi: 10.1093/oxfordjournals.jbchem.a128453. [DOI] [PubMed] [Google Scholar]
- Kappas A., Maines M. D. Tin: a potent inducer of heme oxygenase in kidney. Science. 1976 Apr 2;192(4234):60–62. doi: 10.1126/science.1257757. [DOI] [PubMed] [Google Scholar]
- Kutty R. K., Maines M. D. Purification and characterization of biliverdin reductase from rat liver. J Biol Chem. 1981 Apr 25;256(8):3956–3962. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labbé R. F. History and background of protoporphyrin testing. Clin Chem. 1977 Feb;23(2 Pt 1):256–259. [PubMed] [Google Scholar]
- Landaw S. A., Callahan E. W., Jr, Schmid R. Catabolism of heme in vivo: comparison of the simultaneous production of bilirubin and carbon monoxide. J Clin Invest. 1970 May;49(5):914–925. doi: 10.1172/JCI106311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt induction of hepatic heme oxygenase; with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4293–4297. doi: 10.1073/pnas.71.11.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt stimulation of heme degradation in the liver. Dissociation of microsomal oxidation of heme from cytochrome P-450. J Biol Chem. 1975 Jun 10;250(11):4171–4177. [PubMed] [Google Scholar]
- Maines M. D., Veltman J. C. Phenylhydrazine-mediated induction of haem oxygenase activity in rat liver and kidney and development of hyperbilirubinaemia. Inhibition by zinc-protoporphyrin. Biochem J. 1984 Jan 15;217(2):409–417. doi: 10.1042/bj2170409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D. Zinc . protoporphyrin is a selective inhibitor of heme oxygenase activity in the neonatal rat. Biochim Biophys Acta. 1981 Mar 18;673(3):339–350. doi: 10.1016/0304-4165(81)90465-7. [DOI] [PubMed] [Google Scholar]
- McCormack L. R., Liem H. H., Strum W. B., Grundy S. M., Muller-Eberhard U. Effects of haem infusion on biliary secretion of porphyrins, haem and bilirubin in man. Eur J Clin Invest. 1982 Jun;12(3):257–262. doi: 10.1111/j.1365-2362.1982.tb01001.x. [DOI] [PubMed] [Google Scholar]
- Mitchell J. R., Jollow D. J., Potter W. Z., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973 Oct;187(1):211–217. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- OSTROW J. D., JANDL J. H., SCHMID R. The formation of bilirubin from hemoglobin in vivo. J Clin Invest. 1962 Aug;41:1628–1637. doi: 10.1172/JCI104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Bernstein S. E. Levels of delta-aminolevulinate dehydratase, uroporphyrinogen-I synthase, and protoporphyrin IX in erythrocytes from anemic mutant mice. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1181–1184. doi: 10.1073/pnas.74.3.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]


