Abstract
Isoprenaline stimulation of perfused rabbit hearts was associated with simultaneous phosphorylation of proteins in the myofilaments and phospholamban in the sarcoplasmic reticulum (SR). Hearts were perfused with Krebs-Henseleit buffer containing [32P]Pi, freeze-clamped in a control condition or at the peak of the inotropic response to isoprenaline, and myofibrils and SR were prepared from the same hearts. Stimulation of 32P incorporation in troponin I (TnI) and C-protein by isoprenaline was associated with a decrease in Ca2+-sensitivity of the myofibrillar Mg2+-dependent ATPase activity. Stimulation of 32P incorporation in SR by isoprenaline was associated with an increase in the initial rates of oxalate-facilitated Ca2+ transport, assayed with SR vesicles in either microsomal fractions or homogenates from the perfused hearts. These findings provide evidence that phosphorylation of TnI, C-protein and phospholamban in the intact cell is associated with functional alterations of the myofibrils and SR which may be responsible in part for the effects of catecholamines on the mammalian myocardium.
Full text
PDF



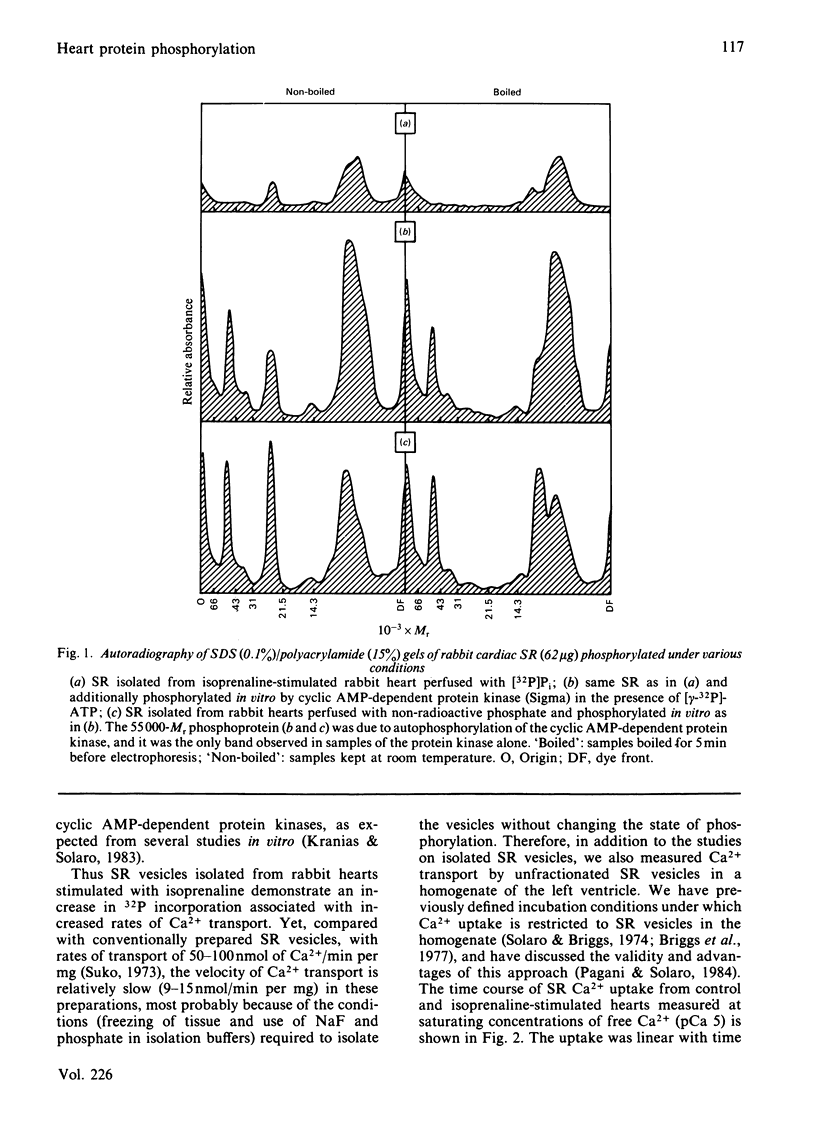
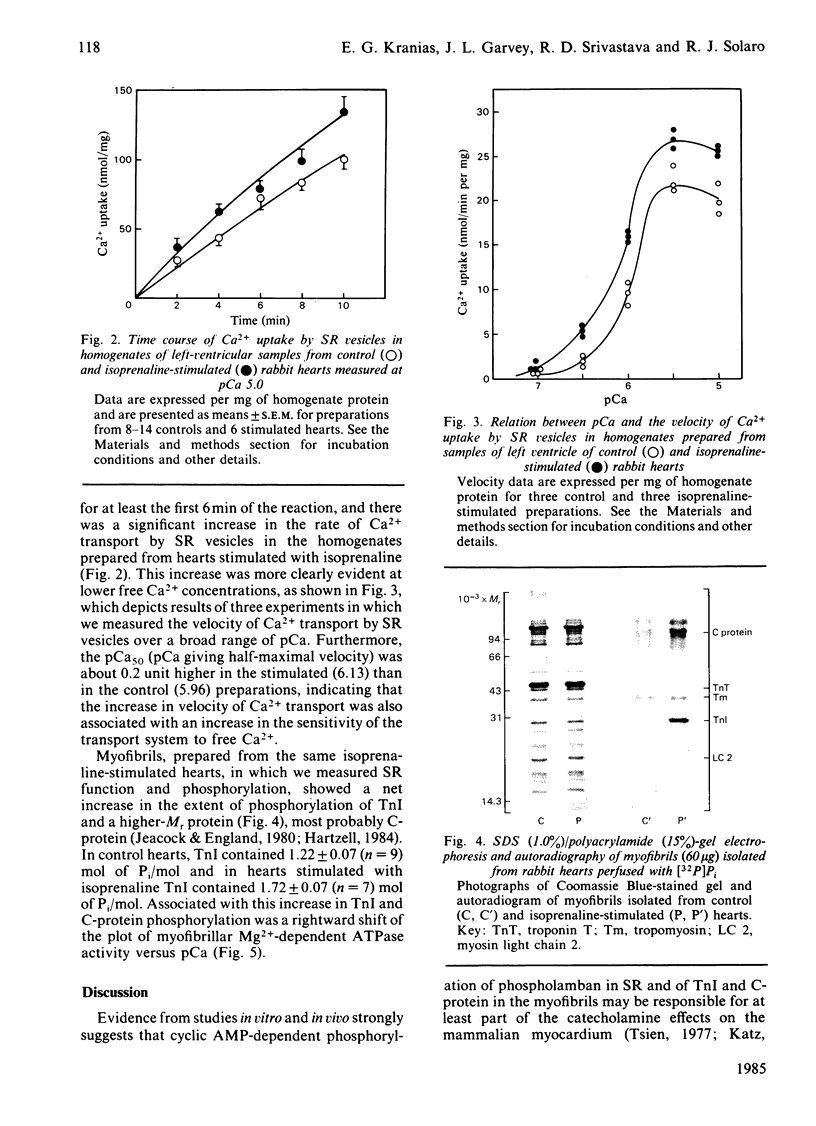
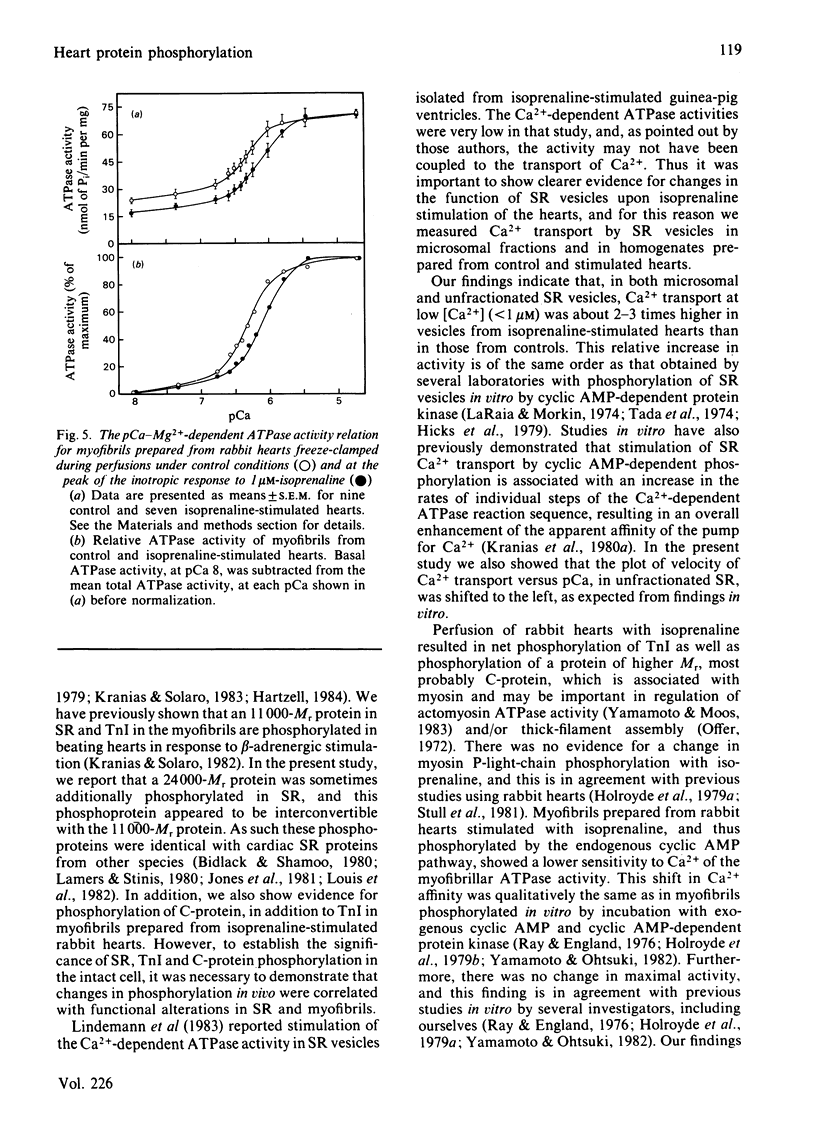


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidlack J. M., Shamoo A. E. Adenosine 3',5'-monophosphate-dependent phosphorylation of a 6000 and a 22,000 dalton protein from cardiac sarcoplasmic reticulum. Biochim Biophys Acta. 1980 Oct 1;632(2):310–325. doi: 10.1016/0304-4165(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Briggs F. N., Poland J. L., Solaro R. J. Relative capabilities of sarcoplasmic reticulum in fast and slow mammalian skeletal muscles. J Physiol. 1977 Apr;266(3):587–594. doi: 10.1113/jphysiol.1977.sp011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capony J. P., Rinaldi M. L., Guilleux F., Demaille J. G. Isolation of cardiac membrane proteolipids by high pressure liquid chromatography. A comparison of reticular and sarcolemmal proteolipids, phospholamban and calciductin. Biochim Biophys Acta. 1983 Feb 9;728(1):83–91. doi: 10.1016/0005-2736(83)90439-x. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Keely S. L. Characterization and regulation of heart adenosine 3':5'-monophosphate-dependent protein kinase isozymes. J Biol Chem. 1977 Feb 10;252(3):910–918. [PubMed] [Google Scholar]
- Corbin J. D., Sugden P. H., Lincoln T. M., Keely S. L. Compartmentalization of adenosine 3':5'-monophosphate and adenosine 3':5'-monophosphate-dependent protein kinase in heart tissue. J Biol Chem. 1977 Jun 10;252(11):3854–3861. [PubMed] [Google Scholar]
- Davis B. A., Schwartz A., Samaha F. J., Kranias E. G. Regulation of cardiac sarcoplasmic reticulum calcium transport by calcium-calmodulin-dependent phosphorylation. J Biol Chem. 1983 Nov 25;258(22):13587–13591. [PubMed] [Google Scholar]
- England P. J. Studies on the phosphorylation of the inhibitory subunit of troponin during modification of contraction in perfused rat heart. Biochem J. 1976 Nov 15;160(2):295–304. doi: 10.1042/bj1600295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galani-Kranias E., Bick R., Schwartz A. Phosphorylation of a 100 000 dalton component and its relationship to calcium transport in sarcoplasmic reticulum from rabbit skeletal muscle. Biochim Biophys Acta. 1980 Apr 3;628(4):438–450. doi: 10.1016/0304-4165(80)90393-1. [DOI] [PubMed] [Google Scholar]
- Harigaya S., Schwartz A. Rate of calcium binding and uptake in normal animal and failing human cardiac muscle. Membrane vesicles (relaxing system) and mitochondria. Circ Res. 1969 Dec;25(6):781–794. doi: 10.1161/01.res.25.6.781. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Phosphorylation of C-protein in intact amphibian cardiac muscle. Correlation between 32P incorporation and twitch relaxation. J Gen Physiol. 1984 Apr;83(4):563–588. doi: 10.1085/jgp.83.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. S., Brunton L. L., Mayer S. E. Selective activation of particulate cAMP-dependent protein kinase by isoproterenol and prostaglandin E1. J Biol Chem. 1980 Jun 10;255(11):5113–5119. [PubMed] [Google Scholar]
- Herzig J. W., Köhler G., Pfitzer G., Rüegg J. C., Wölffle G. Cyclic AMP inhibits contractility of detergent treated glycerol extracted cardiac muscle. Pflugers Arch. 1981 Sep;391(3):208–212. doi: 10.1007/BF00596172. [DOI] [PubMed] [Google Scholar]
- Hicks M. J., Shigekawa M., Katz A. M. Mechanism by which cyclic adenosine 3':5'-monophosphate-dependent protein kinase stimulates calcium transport in cardiac sarcoplasmic reticulum. Circ Res. 1979 Mar;44(3):384–391. doi: 10.1161/01.res.44.3.384. [DOI] [PubMed] [Google Scholar]
- Holroyde M. J., Small D. A., Howe E., Solaro R. J. Isolation of cardiac myofibrils and myosin light chains with in vivo levels of light chain phosphorylation. Biochim Biophys Acta. 1979 Nov 1;587(4):628–637. doi: 10.1016/0304-4165(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Jeacocke S. A., England P. J. Phosphorylation of a myofibrillar protein of Mr 150 000 in perfused rat heart, and the tentative indentification of this as C-protein. FEBS Lett. 1980 Dec 15;122(1):129–132. doi: 10.1016/0014-5793(80)80418-2. [DOI] [PubMed] [Google Scholar]
- Jones L. R., Maddock S. W., Hathaway D. R. Membrane localization of myocardial type II cyclic AMP-dependent protein kinase activity. Biochim Biophys Acta. 1981 Feb 20;641(1):242–253. doi: 10.1016/0005-2736(81)90588-5. [DOI] [PubMed] [Google Scholar]
- Katz A. M. Role of the contractile proteins and sarcoplasmic reticulum in the response of the heart to catecholamines: an historical review. Adv Cyclic Nucleotide Res. 1979;11:303–343. [PubMed] [Google Scholar]
- Kirchberger M. A., Antonetz T. Phospholamban: dissociation of the 22,000 molecular weight protein of cardiac sarcoplasmic reticulum into 11,000 and 5,500 molecular weight forms. Biochem Biophys Res Commun. 1982 Mar 15;105(1):152–156. doi: 10.1016/s0006-291x(82)80024-7. [DOI] [PubMed] [Google Scholar]
- Kirchberger M. A., Tada M., Katz A. M. Adenosine 3':5'-monophosphate-dependent protein kinase-catalyzed phosphorylation reaction and its relationship to calcium transport in cardiac sarcoplasmic reticulum. J Biol Chem. 1974 Oct 10;249(19):6166–6173. [PubMed] [Google Scholar]
- Kranias E. G., Mandel F., Wang T., Schwartz A. Mechanism of the stimulation of calcium ion dependent adenosine triphosphatase of cardiac sarcoplasmic reticulum by adenosine 3',5'-monophosphate dependent protein kinase. Biochemistry. 1980 Nov 11;19(23):5434–5439. doi: 10.1021/bi00564a044. [DOI] [PubMed] [Google Scholar]
- Kranias E. G., Schwartz A., Jungmann R. A. Characterization of cyclic 3':5'-amp-dependent protein kinase in sarcoplasmic reticulum and cytosol of canine myocardium. Biochim Biophys Acta. 1982 Dec 6;709(1):28–37. doi: 10.1016/0167-4838(82)90417-4. [DOI] [PubMed] [Google Scholar]
- Kranias E. G., Solaro R. J. Coordination of cardiac sarcoplasmic reticulum and myofibrillar function by protein phosphorylation. Fed Proc. 1983 Jan;42(1):33–38. [PubMed] [Google Scholar]
- Kranias E. G., Solaro R. J. Phosphorylation of troponin I and phospholamban during catecholamine stimulation of rabbit heart. Nature. 1982 Jul 8;298(5870):182–184. doi: 10.1038/298182a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamers J. M., Stinis J. T. Phosphorylation of low molecular weight proteins in purified preparations of rat heart sarcolemma and sarcoplasmic reticulum. Biochim Biophys Acta. 1980 Aug 21;624(2):443–459. doi: 10.1016/0005-2795(80)90086-0. [DOI] [PubMed] [Google Scholar]
- Le Peuch C. J., Guilleux J. C., Demaille J. G. Phospholamban phosphorylation in the perfused rat heart is not solely dependent on beta-adrenergic stimulation. FEBS Lett. 1980 May 19;114(1):165–168. doi: 10.1016/0014-5793(80)80885-4. [DOI] [PubMed] [Google Scholar]
- Lindemann J. P., Jones L. R., Hathaway D. R., Henry B. G., Watanabe A. M. beta-Adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J Biol Chem. 1983 Jan 10;258(1):464–471. [PubMed] [Google Scholar]
- Louis C. F., Maffitt M., Jarvis B. Factors that modify the molecular size of phospholamban, the 23,000-dalton cardiac sarcoplasmic reticulum phosphoprotein. J Biol Chem. 1982 Dec 25;257(24):15182–15186. [PubMed] [Google Scholar]
- Manalan A. S., Jones L. R. Characterization of the intrinsic cAMP-dependent protein kinase activity and endogenous substrates in highly purified cardiac sarcolemmal vesicles. J Biol Chem. 1982 Sep 10;257(17):10052–10062. [PubMed] [Google Scholar]
- Martin A. F., Pagani E. D., Solaro R. J. Thyroxine-induced redistribution of isoenzymes of rabbit ventricular myosin. Circ Res. 1982 Jan;50(1):117–124. doi: 10.1161/01.res.50.1.117. [DOI] [PubMed] [Google Scholar]
- Moir A. J., Solaro R. J., Perry S. V. The site of phosphorylation of troponin I in the perfused rabbit heart. The effect of adrenaline. Biochem J. 1980 Feb 1;185(2):505–513. doi: 10.1042/bj1850505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mope L., McClellan G. B., Winegrad S. Calcium sensitivity of the contractile system and phosphorylation of troponin in hyperpermeable cardiac cells. J Gen Physiol. 1980 Mar;75(3):271–282. doi: 10.1085/jgp.75.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura J., Wang T., Tsai L. I., Schwartz A. Properties and characterization of a highly purified sarcoplasmic reticulum Ca2+-ATPase from dog cardiac and rabbit skeletal muscle. J Biol Chem. 1983 Apr 25;258(8):5079–5083. [PubMed] [Google Scholar]
- Palmer W. K., McPherson J. M., Walsh D. A. Critical controls in the evaluation of cAMP-dependent protein kinase activity ratios as indices of hormonal action. J Biol Chem. 1980 Apr 10;255(7):2663–2666. [PubMed] [Google Scholar]
- Ray K. P., England P. J. Phosphorylation of the inhibitory subunit of troponin and its effect on the calcium dependence of cardiac myofibril adenosine triphosphatase. FEBS Lett. 1976 Nov;70(1):11–16. doi: 10.1016/0014-5793(76)80716-8. [DOI] [PubMed] [Google Scholar]
- Rinaldi M. L., Capony J. P., Demaille J. G. The cyclic AMP-dependent modulation of cardiac sarcolemmal slow calcium channels. J Mol Cell Cardiol. 1982 May;14(5):279–289. doi: 10.1016/0022-2828(82)90206-1. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Entman M. L., Kaniike K., Lane L. K., Van Winkle W. B., Bornet E. P. The rate of calcium uptake into sarcoplasmic reticulum of cardiac muscle and skeletal muscle. Effects of cyclic AMP-dependent protein kinase and phosphorylase b kinase. Biochim Biophys Acta. 1976 Feb 19;426(1):57–72. doi: 10.1016/0005-2736(76)90429-6. [DOI] [PubMed] [Google Scholar]
- Sistare F. D., Lichtenberg L., Sugg R. G., McFarland S. A., Villar-Palasi C. Effects of isoproterenol treatment of isolated perfused rat hearts on myofibrillar phosphorylation and ATPase activity. J Cyclic Nucleotide Res. 1981;7(2):85–93. [PubMed] [Google Scholar]
- Solaro R. J., Briggs F. N. Estimating the functional capabilities of sarcoplasmic reticulum in cardiac muscle. Calcium binding. Circ Res. 1974 Apr;34(4):531–540. doi: 10.1161/01.res.34.4.531. [DOI] [PubMed] [Google Scholar]
- Solaro R. J., Moir A. J., Perry S. V. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature. 1976 Aug 12;262(5569):615–617. doi: 10.1038/262615a0. [DOI] [PubMed] [Google Scholar]
- Solaro R. J., Pang D. C., Briggs F. N. The purification of cardiac myofibrils with Triton X-100. Biochim Biophys Acta. 1971 Aug 6;245(1):259–262. doi: 10.1016/0005-2728(71)90033-8. [DOI] [PubMed] [Google Scholar]
- Suko J. The calcium pump of cardiac sarcoplasmic reticulum. Functional alterations at different levels of thyroid state in rabbits. J Physiol. 1973 Feb;228(3):563–582. doi: 10.1113/jphysiol.1973.sp010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Kirchberger M. A., Katz A. M. Phosphorylation of a 22,000-dalton component of the cardiac sarcoplasmic reticulum by adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1975 Apr 10;250(7):2640–2647. [PubMed] [Google Scholar]
- Tada M., Kirchberger M. A., Repke D. I., Katz A. M. The stimulation of calcium transport in cardiac sarcoplasmic reticulum by adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1974 Oct 10;249(19):6174–6180. [PubMed] [Google Scholar]
- Tada M., Yamada M., Ohmori F., Kuzuya T., Inui M., Abe H. Transient state kinetic studies of Ca2+-dependent ATPase and calcium transport by cardiac sarcoplasmic reticulum. Effect of cyclic AMP-dependent protein kinase-catalyzed phosphorylation of phospholamban. J Biol Chem. 1980 Mar 10;255(5):1985–1992. [PubMed] [Google Scholar]
- Tsien R. W. Cyclic AMP and contractile activity in heart. Adv Cyclic Nucleotide Res. 1977;8:363–420. [PubMed] [Google Scholar]
- Wray H. L., Gray R. R. Cyclic AMP stimulation of membrane phosphorylation and Ca2+-activated, Mg2+-dependent ATPase in cardiac sarcoplasmic reticulum. Biochim Biophys Acta. 1977 Sep 14;461(3):441–459. doi: 10.1016/0005-2728(77)90232-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Moos C. The C-proteins of rabbit red, white, and cardiac muscles. J Biol Chem. 1983 Jul 10;258(13):8395–8401. [PubMed] [Google Scholar]
- Yamamoto K., Ohtsuki I. Effect of phosphorylation of porcine cardiac troponin I by 3':5'-cyclic AMP-dependent protein kinase on the actomyosin ATPase activity. J Biochem. 1982 May;91(5):1669–1677. doi: 10.1093/oxfordjournals.jbchem.a133858. [DOI] [PubMed] [Google Scholar]



