Abstract
Human mutations in neuropeptide Y (NPY) have been linked to high body mass index but not altered dietary patterns1. Here we uncover the mechanism by which NPY in sympathetic neurons2,3 protects from obesity. Imaging of cleared mouse brown and white adipose tissue (BAT and WAT, respectively) established that NPY+ sympathetic axons are a smaller subset that mostly maps to the perivasculature; analysis of single-cell RNA sequencing datasets identified mural cells as the main NPY-responsive cells in adipose tissues. We show that NPY sustains the proliferation of mural cells, which are a source of thermogenic adipocytes in both BAT and WAT4–6. We found that diet-induced obesity leads to neuropathy of NPY+ axons and concomitant depletion of mural cells. This defect was replicated in mice with NPY abrogated from sympathetic neurons. The loss of NPY in sympathetic neurons whitened interscapular BAT, reducing its thermogenic ability and decreasing energy expenditure before the onset of obesity. It also caused adult-onset obesity of mice fed on a regular chow diet and rendered them more susceptible to diet-induced obesity without increasing food consumption. Our results indicate that, relative to central NPY, peripheral NPY produced by sympathetic nerves has the opposite effect on body weight by sustaining energy expenditure independently of food intake.
Subject terms: Fat metabolism, Neuronal physiology
We find that, relative to central neuropeptide Y, peripheral neuropeptide Y produced by sympathetic nerves has the opposite effect on body weight by sustaining energy expenditure independently of food intake.
Main
Sympathetic nerves within both BAT and WAT release noradrenaline locally to trigger lipolysis and thermogenesis7,8. NPY is coreleased with noradrenaline9, but its specific role in adipose tissue remains unclear. Although many reports indicate that NPY in the brain stimulates appetite10,11, knockout of NPY has no effect on daily food consumption12,13. In addition, mice without NPY receptors develop late-onset obesity despite eating less14–16. In humans, mutations in NPY have been linked to high body mass index (BMI) but not to an altered dietary pattern (Extended Data Fig. 1m) (https://hugeamp.org/gene.html?gene=NPY)1. These findings suggest contrasting roles for NPY from the brain and sympathetic nervous system in the regulation of body weight. To test this hypothesis we used animal models in which NPY was removed from sympathetic neurons while remaining intact in the brain.
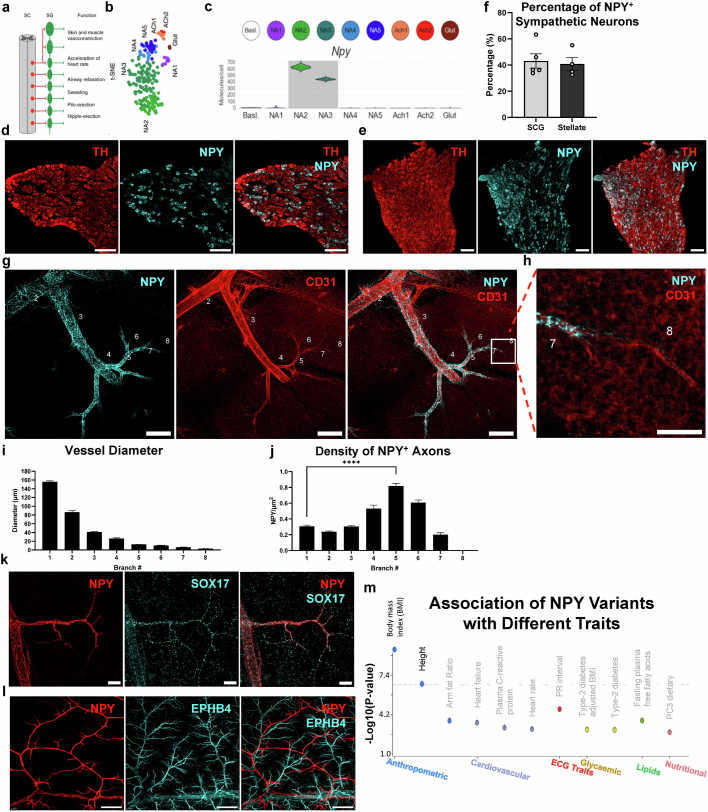
Extended Data Fig. 1. Npy is expressed by a subpopulation of sympathetic neurons, and NPY+ axons preferentially innervate the 5th order arteriole branches; NPY variants are significantly associated with human body mass index (BMI).
(a) Schematic showing the tissues innervated by each sympathetic ganglia17. (b) Single-cell RNA-Seq dataset of sympathetic ganglia chain published by Linnarsson Lab to show the clustering of neurons17. (c) Violin plot showing the expression of Npy17. (d-e) Confocal image of cryo-sectioned (d) superior-cervical ganglion (SCG) and (e) stellate ganglia (SG) of ND-treated WT male 8-week-old mice stained with anti-TH (red) and anti-NPY (cyan). Scale bar = 100 μm. (f) The percentage of NPY+ sympathetic neurons quantified based on images as in Extended Data Fig. 5d,e (n = 5 sections from 3 mice). (g) Confocal images of cleared adipose tissue stained with anti-NPY (cyan) and anti-CD31 (red). The numbers indicate the branch orders. Scale bar = 150 μm. (h) Zoom-in of Extended Data Fig. 1g, scale bar = 50 μm. (i-j) (i) The diameters of vessels and (j) the density of NPY+ axons at each branch level quantified based on images as in Extended Data Fig. 1g (n = 6 views from 2 mice); NPY+ axon density on Branch #1 vs. #5, P < 0.0001. (k) Light-sheet images of cleared adipose tissue stained with anti-NPY (red) and anti-EPHB4 (cyan). Scale bar = 500 μm. (l) Confocal images of cleared adipose tissue stained with anti-NPY (red) and anti-SOX17 (cyan). Scale bar = 100 μm. (m) Plot showing associations of human NPY gene variants with different traits1 (https://hugeamp.org/gene.html?gene=NPY). For d-h, representative images were shown from 3 experiments; for k,l, representative images were shown from 2 experiments. All values are expressed as mean ± SEM. Statistical comparisons were made using 2-tailed Student T-tests, ****P < 0.0001.
NPY+ sympathetic innervation in WAT and BAT
The public single-cell RNA-sequencing (scRNA-seq) dataset of sympathetic ganglia17 shows that only two out of five clusters of sympathetic neurons express elevated levels of NPY (Extended Data Fig. 1a–c). This proportion of NPY+ sympathetic neurons, approximately 40%, was confirmed by immunofluorescent costaining of NPY and the sympathetic neuron marker tyrosine hydroxylase (TH) in superior cervical ganglia and stellate ganglia (Extended Data Fig. 1d–f). A similar TH/NPY overlap was observed in sympathetic axon bundles dissected from inguinal white adipose tissue (iWAT; Fig. 1a). In iWAT, NPY+ axons are associated with the vasculature and form a plexus around the vessels (Fig. 1b).
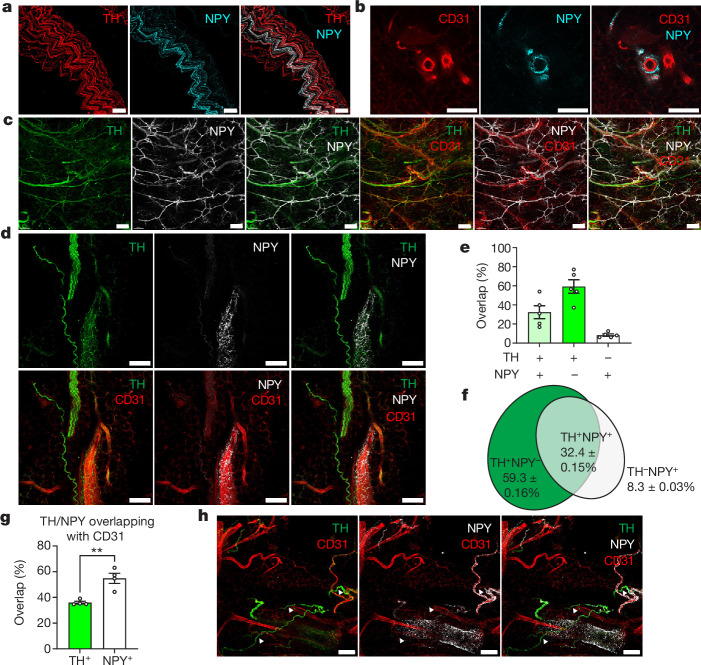
Fig. 1. One-third of sympathetic axons in iWAT and iBAT are NPY+ and preferentially innervate the vasculature.
a, Confocal images of an axonal bundle dissected from iWAT of a male lean adult mouse, stained with anti-TH (red) and anti-NPY (cyan). b, Confocal images of a capillary within cleared iWAT from a lean adult mouse, stained with anti-CD31 (red) and anti-NPY (cyan). c, Confocal images of cleared iWAT from lean adult male WT mice, stained with anti-TH (green), anti-NPY (white) and anti-CD31 (red). d, Confocal images of cleared iWAT in c from lean adult male mice, stained with anti-TH (green), anti-NPY (white) and anti-CD31 (red). e,f, Quantification of images in d for TH/NPY overlap: TH+NPY− (dark green), TH+NPY+ (light green) and TH−NPY+ (white); percentages shown on a histogram (e) and Venn diagram (f); n = 5 views from three mice. g, Quantification of images in d showing the proportions of TH+ and NPY+ axons overlapping with CD31+ endothelial cells (n = 4 views from three mice; P = 0.0035). h, Confocal images of cleared iBAT from lean adult male mice stained with anti-TH (green), anti-NPY (white) and anti-CD31 (red). Arrowheads indicate TH+NPY− axons. a–d,h, Representative images are shown from three experiments. All values shown are mean ± s.e.m. Statistical comparisons were made using two-tailed Student’s t-tests, **P < 0.01. Scale bars, 50 μm (a,c), 100 μm (b,d,h).
To determine the distribution of TH+ and NPY+ axons throughout adipose tissue, we performed tissue clearing of iWAT18 and immunolabelled for TH, NPY and CD31, the last of which marks the vascular endothelium. We confirmed that NPY+ axons are a subgroup of sympathetic axons (Fig. 1c,d). Quantifying the images, we found that TH+NPY+ axons make up 32.4% of all labelled axons whereas 59.3% are TH+NPY− (Fig. 1e,f). The images also indicate that NPY+ axons preferentially innervate CD31+ endothelium, and this was confirmed by quantification of images (Fig. 1g). Consistent with iWAT, in interscapular brown adipose tissue (iBAT), NPY+ axons mainly innervate the vasculature (Fig. 1h).
To further determine which vessel branches are innervated by NPY+ axons, we quantified light-sheet images (Extended Data Fig. 1g) and found that the density of NPY+ axons is highest in fifth-order branches (Extended Data Fig. 1i,j) whereas NPY+ axons do not innervate eighth-order branches (Extended Data Fig. 1h–j). To identify whether arteries or veins are innervated by NPY+ axons in adipose tissue, we costained iWAT for NPY and EPHB4, a vein marker19, or SOX17, an artery marker20. The images show that NPY+ axons do not innervate EPHB4+ vessels whereas they innervate SOX17+ vessels (Extended Data Fig. 1k,l). Based on these observations, we conclude that NPY+ sympathetic axons in adipose tissue preferentially innervate arterioles but not veins or venules.
In addition, our NPY stains are exclusively axonal. Previous studies suggested that adipose tissue macrophages (ATMs) can express Npy21, but this is negated by two scRNA-seq datasets of the stromal-vascular fraction (SVF) of mouse iWAT and iBAT5,22 (Supplementary Fig. 1a–d). Consistently, using quantitative PCR (qPCR), we did not detect Npy expression in sorted ATMs from Cx3cr1GFP/+ reporter mice despite the positivity of the Adgre1 (F4/80) control (Supplementary Fig. 1e,f).
NPY1R+ progenitors of thermogenic fat
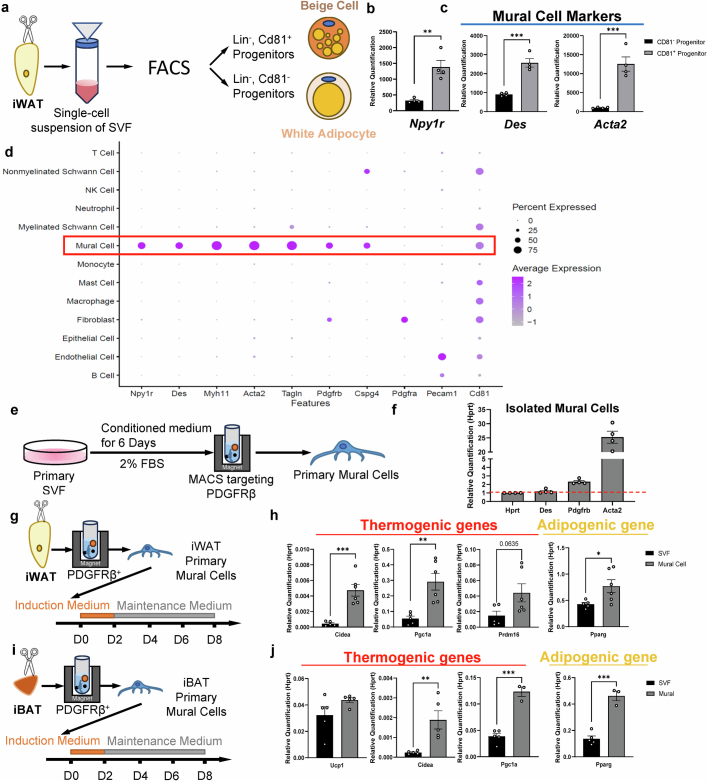
We then investigated the type of perivascular cells that are the postsynaptic targets of NPY+ axons in adipose tissues. By reanalysis of the scRNA-seq dataset of iWAT22 and iBAT5, we found that Npy1r is highly expressed in mural cells that are marked by Des, Myh11, Acta2 (αSMA), Tagln, Cspg4 (NG2) and Pdgfrb4–6, and that NPY1R is a bona fide marker of mural cells within iWAT and iBAT (Fig. 2a–d and Supplementary Fig. 1a,b). To validate the scRNA-seq datasets by qPCR we sorted mural cells (live CD31−CD45−PDGFRα−NG2+ cells) and immune cells (live CD31−CD45+ cells) from the SVF of iWAT and iBAT (Supplementary Fig. 1g) and performed qPCR. We independently confirmed that Npy1r and Des are highly expressed in mural cells but not in immune cells (Fig. 2e).
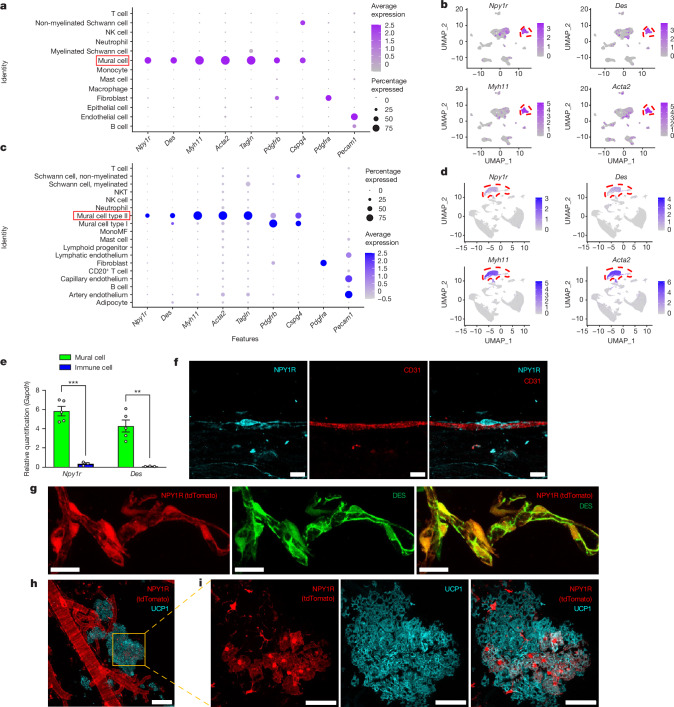
Fig. 2. Npy1r is mainly expressed in mural cells that are a source of thermogenic adipocytes.
a,c, Dot plots showing the expression of Npy1r, Des, Myh11, Acta2 (αSMA), Tagln, Cspg4 (NG2), Pdgfrb, Pdgfra and Pecam1 (CD31) in the SVF of iWAT (a)22 and iBAT (c)5. b,d, Embedding plots showing the expression of Npy1r, Des, Myh11 and Acta2 (αSMA) in the SVF of iWAT (b)22 and iBAT (d)5. Mural cell clusters are highlighted by red dashed lines. e, Expression of Npy1r (P = 0.0002) and Des (P = 0.0024) in sorted CD31−CD45−PDGFRα−NG2+ mural cells and CD45+ immune cells (n = 5 and n = 3 biologically independent samples, respectively). Gapdh was used as the reference gene. f, Confocal images of capillary dissected from iWAT of WT mice and stained with anti-NPY1R (cyan) and anti-CD31 (red). g, Confocal images of vessels dissected from Npy1rCre;Rosa26tdTomato mice stained with anti-DES (green). h, Confocal images of vessels dissected from iBAT of ND-treated, room temperature-housed, 12-week-old Npy1rCre;Rosa26tdTomato mice stained with anti-UCP1 (cyan). i, Zoomed-in images of h. f–i, Representative images from two experiments. All values mean ± s.e.m. Statistical comparisons were made using two-tailed Student’s t-tests, **P < 0.01, ***P < 0.001. NK, natural killer cells; NKT, nature killer T cells; MonoMF, monocytes and macrophages. UMAP, uniform manifold approximation and projection. Scale bars, 10 μm (f), 20 μm (g), 100 μm (h), 50 μm (i).
To confirm the existence of NPY1R in mural cells at the protein level, we dissected capillaries from adipose tissue and stained them with anti-CD31 and anti-NPY1R. We detected an NPY1R+ cell wrapping around the capillary (Fig. 2f), the morphology of which matches that of mural cells of fine capillaries, conventionally named pericytes23. To confirm the colocalization of NPY1R with multiple mural cell markers observed in scRNA-seq datasets (Extended Data Fig. 2a), we immunolabelled vessel-containing samples dissected from adipose tissues of Npy1rCre;Rosa26tdTomato mice for multiple mural cell markers including DES, αSMA and NG2. The images indicate that DES+, αSMA+ and NG2+ mural cells are all labelled with NPY1R-tdTomato (Fig. 2g and Extended Data Fig. 2b–e). Based on the observations above, we demonstrate that mural cells are the target of NPY+ axons and that they are NPY1R+.
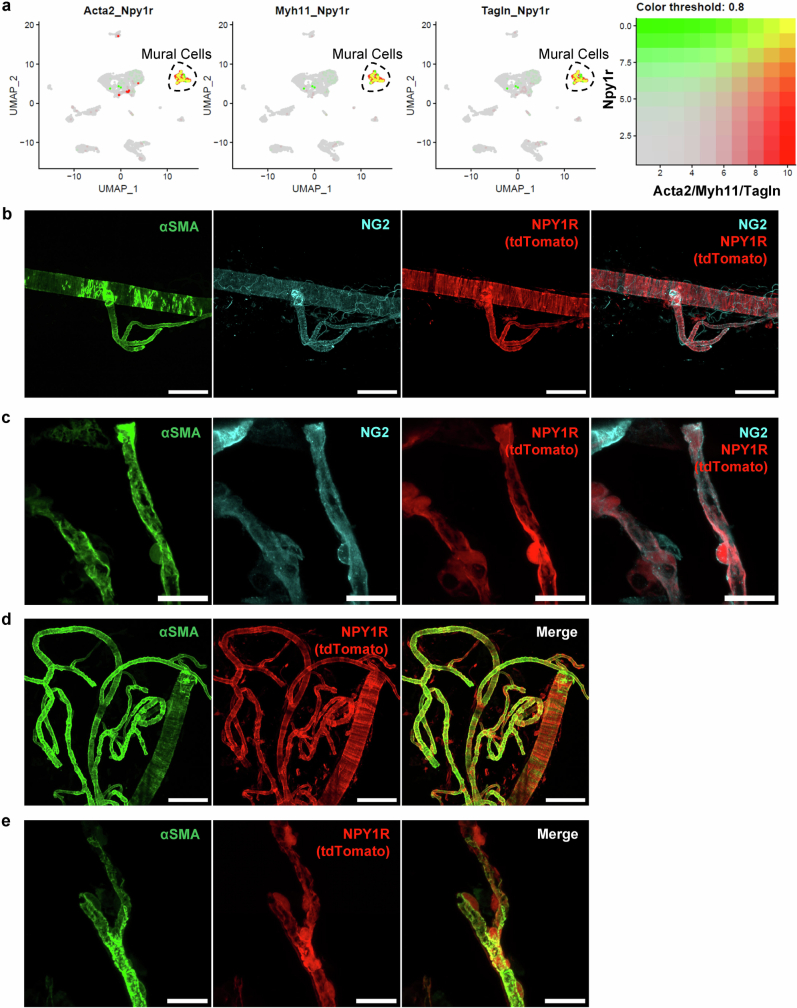
Extended Data Fig. 2. NPY1R-tdTomato is colocalised with NG2 and αSMA in mural cells.
(a) Embedding plot showing the co-expression of mural markers Acta2 (αSMA), Myh11, and Tagln (red) with Npy1r (green). Co-expression is in yellow22. (b) Confocal images of vessels dissected from adipose tissue of Npy1rCre; Rosa26tdTomato mice stained with anti-αSMA (green), scale bar = 100 μm. (c) Zoomed-in images of vessels as in Extended Data Fig. 2b, scale bar = 20 μm. (d) Confocal images of vessels dissected from Npy1rCre; Rosa26tdTomato mice stained with anti-αSMA (green) and anti-NG2 (cyan), scale bar = 100 μm. (e) Zoomed-in images of vessels as in Extended Data Fig. 2b, scale bar = 20 μm. For b-e, representative images were shown from 2 experiments.
It is worth noting that earlier reports of Npy2r and Npy5r expression by immune cells, preadipocytes and adipocytes24–27 are negated by our analysis of the scRNA-seq datasets of both iBAT and iWAT, where Npy5r is undetectable and Npy2r expression is detectable only in iWAT but negligible (Supplementary Fig. 2a,b). Also, some previous reports of Npy1r expression by adipocytes or macrophages28 are negated by a single-nucleus atlas of mouse WAT and a scRNA-seq dataset of iBAT5,29, as well as our qPCR result for sorted ATMs (Supplementary Fig. 1f). Therefore, other than mural cells, no immune cells or adipocytes are likely to be the postsynaptic targets of NPY+ axons in adipose tissue.
Mural cells can give rise to thermogenic adipocytes4–6, and previously identified progenitors of beige cells (Lin−CD81+) express Npy1r and mural cell markers6 (Extended Data Fig. 3a–c), which is further proven via our analysis of iWAT scRNA-seq data (Extended Data Fig. 3d). We confirmed that mural cells from both iWAT and iBAT are progenitors of thermogenic adipocytes by their isolation and differentiation in vitro30 (Extended Data Fig. 3e,f,j,i), and found they are more potent in differentiating into thermogenic adipocytes than SVF (Extended Data Fig. 3g–j). To demonstrate that NPY1R+ mural cells are progenitors of thermogenic adipocytes under physiological conditions, we immunolabelled iBAT of Npy1rCre;Rosa26tdTomato room temperature-housed mice for uncoupling protein 1 (UCP1). Using this lineage-tracing approach, we observed that both mural cells and a subgroup of UCP1+ adipocytes were colabelled with NPY1R-tdTomato (Fig. 2h,i). Because adipocytes do not express Npy1r (Fig. 2c), we reasoned that these NPY1R-tdTomato+UCP1+ thermogenic adipocytes are lineage traced to NPY1R+ mural cells. It is worth noting that NPY1R-tdTomato labels only multilocular UCP1+ adipocytes in iBAT and iWAT, but not UCP1− unilocular white adipocytes (Supplementary Fig. 3a–c), which indicates that NPY1R is specifically expressed in the progenitor of thermogenic adipocytes. Based on both the observations above and published literature4–6, we conclude that NPY1R+ mural cells are progenitors of thermogenic adipocytes.
Extended Data Fig. 3. Previously identified progenitors of thermogenic adipocytes (Lin−/CD81+) in iWAT express Npy1r and mural cell markers, and mural cells isolated from both iWAT and iBAT can differentiate into thermogenic adipocytes.
(a) Schematic of isolating previously identified progenitors of thermogenic adipocytes (Lin−/CD81+) in iWAT6. (b-c) The expression level of (b) Npy1r (P = 0.0027), and (c) mural cell markers Des (P = 0.0003) and Acta2 (P = 0.0009) in CD81− vs. CD81+ progenitors (n = 4 biologically independent samples). (d) Dot plot showing the expression of Npy1r, Des, Myh11, Acta2, Tagln, Pdgfrb, Cspg4, Pdgfra, Pecam1, and Cd81 in different cells in the SVF of mice iWAT. The size of the dot represents the percentage of cells expressing a certain gene, and the darkness of the dot represents the expression level. NPY1R+ mural cell clusters are highlighted with red rectangles. (e) Schematic of isolating mural cells from adipose tissue. (f) The expression level of mural cell markers Des, Pdgfrb, and Acta2 in isolated mural cells. The red dashed line indicates the expression level of reference gene Hprt (n = 4 biologically independent samples). (g,i) Schematic of isolating and differentiating mural cells from (g) iWAT and (i) iBAT. (h,j) The expression of thermogenic and adipogenic genes in adipocytes differentiated from the stromal-vascular fraction (SVF) and mural cells isolated from (h) iWAT (n = 5&6 biologically independent samples; Cidea P = 0.0006, Pgc1a P = 0.0037, Pparg P = 0.0.0364) and (j) iBAT (n = 5&5 biologically independent samples for Ucp1 and Cidea P = 0.0071; n = 5&3 biologically independent samples for Pgc1a P = 0.0001 and Pparg P = 0.0003). All values are expressed as mean ± SEM. Statistical comparisons were made using 2-tailed Student T-tests, *p < 0.05, **p < 0.01, ***p < 0.001. The icon of scissors in c,g,i is from Flaticon (https://flaticon.com). Hprt was used as the reference gene for qPCR.
NPY sustains mural cells
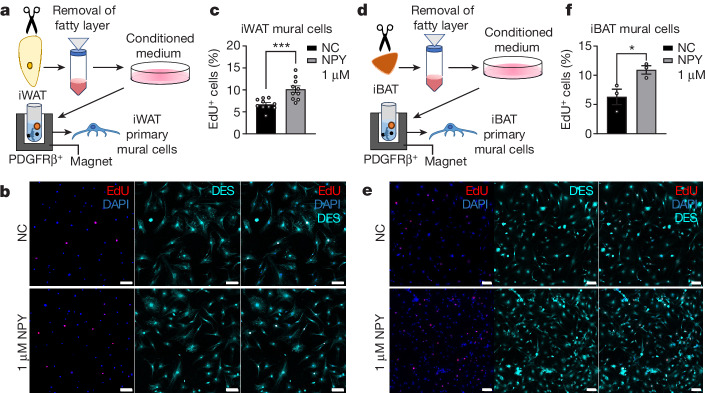
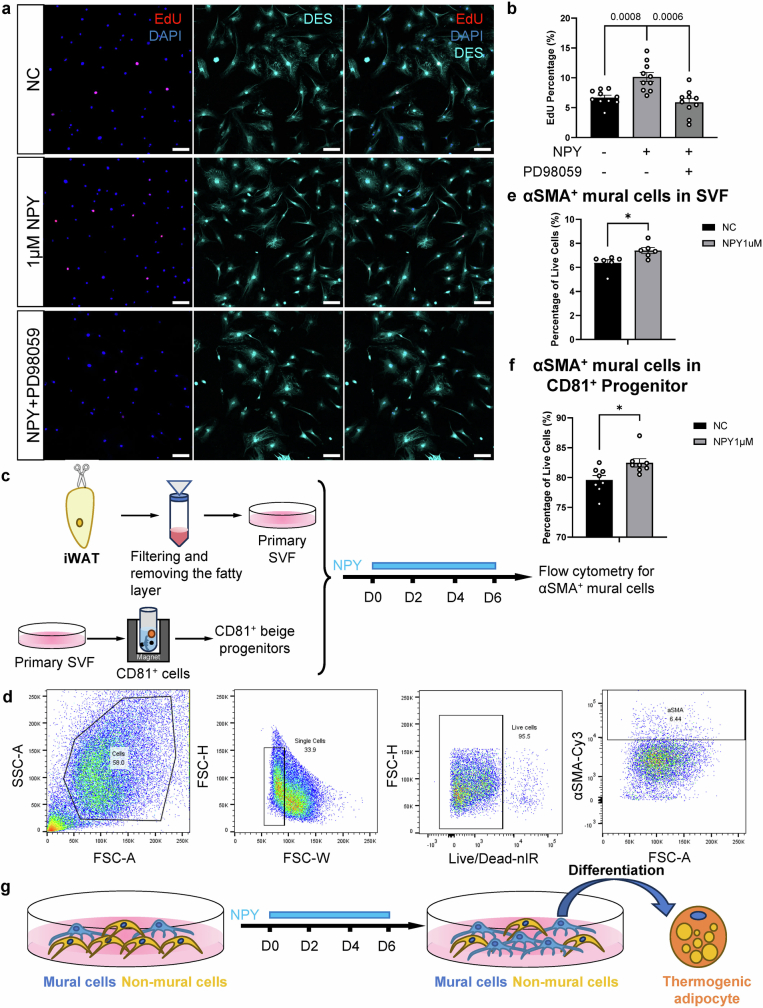
NPY1R is a type of G-protein-coupled receptor (GPCR) that can activate mitogen-activated protein kinase (MAPK) pathways following binding to NPY, with the MAPK downstream of NPY1R being extracellular signal-regulated kinase (ERK)31–33. Because the MAPK pathway is a canonical regulator of cell proliferation34, we questioned whether NPY could promote the proliferation of mural cells. By isolation of mural cells and the addition of NPY, we observed that NPY promoted the proliferation of both iWAT and iBAT mural cells (Fig. 3a–f). Furthermore, ERK inhibitor PD98059 blocked the mitogenic effect of NPY (Extended Data Fig. 4a,b), indicating that NPY promotes mural cell proliferation via ERK. Consistent with this, we found that NPY increased the proportion of αSMA+ mural cells in cultured SVF and CD81+ cells (Extended Data Fig. 4c–g).
Fig. 3. NPY promotes the proliferation of both iWAT and iBAT mural cells.
a, Schematic illustrating isolation of mural cells from iWAT. b, Confocal images showing iWAT mural cells treated with or without 1 μM NPY and stained with DES (cyan) or DAPI; 5-ethynyl-2′-deoxyuridine (EdU) (red) indicates proliferating cells. c, Percentage of EdU+ mural cells quantified based on images in b (n = 10 biologically independent samples; P = 0.0008). d, Schematic showing isolation of mural cells from iBAT. e, Confocal images showing iBAT mural cells treated with or without 1 μM NPY and stained with DES (cyan) and DAPI. EdU (red) indicates proliferating cells. f, Percentage of EdU+ mural cells quantified based on images in d (n = 3 biologically independent samples; P = 0.0370). b,e, Representative images are shown from three and two experiments, respectively. All values mean ± s.e.m. Statistical comparisons were made using two-tailed Student’s t-tests, *P < 0.05, ***P < 0.001. NC, negative control (without NPY). Scale bars, 80 μm.
Extended Data Fig. 4. NPY promotes the proliferation of mural cells via ERK1/2 and increases the proportion of mural cells ex vivo.
(a) Confocal images of isolated mural cells treated with or without 1 μM NPY and 2 μM PD98059, an ERK inhibitor, stained with anti-DES (cyan) and DAPI (blue). EdU (red) was used to indicate proliferation. Scale bar = 80 μm. (b) The percentage of EdU+ cells quantified based on images as in Extended Data Fig. 4a (n = 6 biologically independent samples). (c) Schematic of isolating iWAT SVF and CD81+ cells for NPY treatment experiments and flow cytometry analysis. (d) Gating strategy for measuring the percentage of αSMA+ mural cells in cultured SVF and CD81+ cells by flow cytometry. (e-f) The percentage of αSMA+ mural cells in (e) SVF (n = 6 biologically independent samples, P = 0.0191) and (e) CD81+ cells (n = 8 biologically independent samples, P = 0.0141) after incubating with or without 1 μM NPY. (g) Schematic showing NPY treatment increases the percentage of mural cells in cultured SVF or CD81+ cells, and mural cells are the progenitor of thermogenic adipocytes. For a, representative images were shown from 3 experiments. All values are expressed as mean ± SEM. Statistical comparisons were made using 2-tailed Student T-tests, *p < 0.05. The icon of scissors in c is from Flaticon (https://flaticon.com).
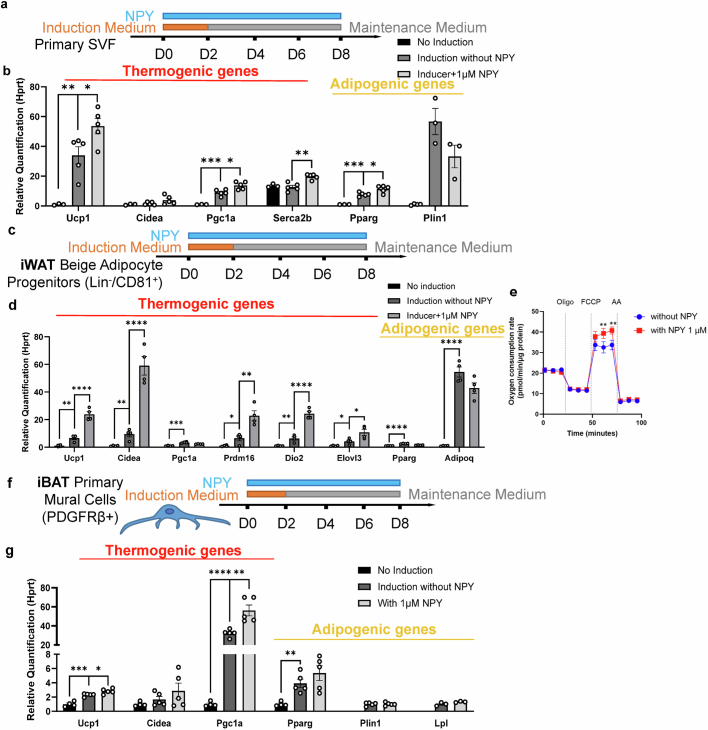
Because mural cells are proven to be the progenitors of thermogenic adipocytes (Extended Data Fig. 3g–j), we reasoned that NPY could facilitate the neogenesis of thermogenic adipocytes (Extended Data Fig. 4g). To prove this directly, we differentiated iWAT SVF and progenitors of thermogenic adipocytes isolated from iWAT and iBAT, finding that NPY facilitated their differentiation into thermogenic adipocytes by upregulation of thermogenic genes without affecting adipogenic genes (Extended Data Fig. 5a–d,f,g). In addition, NPY increased the maximal respiratory capacity of adipocytes differentiated from iWAT beige adipocyte progenitors (Extended Data Fig. 5e). We further demonstrated that NPY does not affect adipogenesis, using NPY1R+ 3T3-L1 preadipocytes (Supplementary Fig. 4a–d), confirming that NPY specifically promotes the differentiation of thermogenic adipocytes.
Extended Data Fig. 5. NPY facilitates the neogenesis of thermogenic adipocytes from iWAT SVF and mural progenitors isolated from both iWAT and iBAT.
(a) Schematic of differentiating primary stromal vascular fraction (SVF) of iWATs to thermogenic adipocytes. (b) The expression Ucp1, Cidea, Pgc1a, Serca2b, Pparg, and Plin1 (n = 4&3&3 biologically independent samples for Plin1 and n = 3&5&5 biologically independent samples for other genes) in thermogenic adipocytes differentiated from primary SVF with or without 1 μΜ NPY. No Induction vs. Induction: Ucp1, P = 0.0052; Pgc1a, P = 0.0006; Pparg, P = 0.0001. Induction vs. Induction+NPY: Ucp1, P = 0.0350; Pgc1a, P = 0.0113; Serca2b, P = 0.0012; Pparg, P = 0.0190. (c) Schematic of differentiating beige adipocyte progenitors (Lin−/CD81+) isolated from iWAT6. (d) The expression of thermogenic and adipogenic genes in adipocytes differentiated from iWAT beige adipocyte progenitors (Lin−/CD81+) with or without 1 μM NPY (n = 4 biologically independent samples). No Induction vs. Induction: Ucp1, P = 0.0027; Cidea, P = 0.0043; Pgc1a, P = 0.0001; Prdm16, P = 0.0261; Dio2, P = 0.0052; Elvol3, P = 0.0246; Pparg&Adipoq, P < 0.0001. Induction vs. Induction+NPY: Ucp1&Cidea&Dio2, P < 0.0001; Prdm16, P = 0.0071; Elvol3, P = 0.0400. (e) Oxygen consumption in adipocytes differentiated from beige adipocyte progenitors (Lin−/CD81+) with or without 1 μM NPY (n = 5 biologically independent samples per group). At 60 min, P = 0.0077; at 70 min, P = 0.0056. (f) Schematic of differentiating iBAT mural cells (PDGFRβ+) to thermogenic adipocytes. (g) The expression of thermogenic and adipogenic genes in adipocytes differentiated from iBAT mural cells or SVF (n = 4&5&5). No Induction vs. Induction: Ucp1, P = 0.0002; Pgc1a, P < 0.0001; Prdm16, P = 0.0261; Pparg, P = 0.0040. Induction vs. Induction+NPY: Ucp1, P = 0.0259; Pgc1a, P = 0.0072. All values are expressed as mean ± SEM. Statistical comparisons were made using 2-tailed Student T-tests (b,d,g) or 2-way ANOVA (e), *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Hprt was used as the reference gene for qPCR.
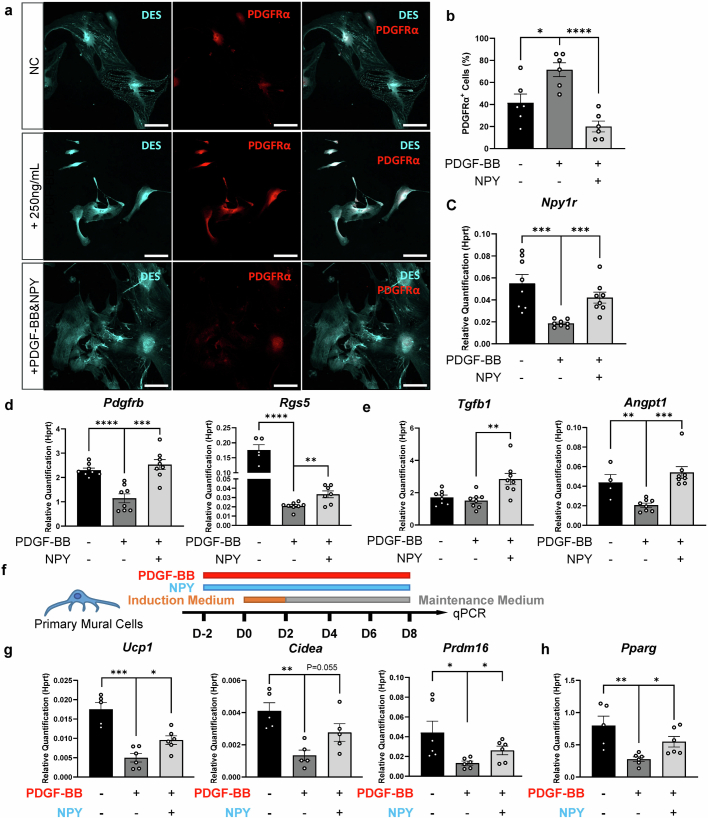
Previous reports have shown that mural cells can be regulated by PDGF-B35. PDGF-B is required for the recruitment of mural cells, but elevated levels can decrease mural cells both in vitro and in vivo by upregulation of fibroblast markers and promotion of fibroblast proliferation36,37. We questioned whether NPY could sustain mural cells when challenged with PDGF-B, which is physiologically relevant because diet-induced obesity (DIO) increases PDGF-B in adipose tissue37. By treating isolated mural cells with PDGF-BB (dimerized PDGF-B), we found that PDGF-BB decreased the proportion of mural cells and increased the number of spindle-shaped PDGFRα+ fibroblasts, an effect that is counteracted by NPY (Extended Data Fig. 6a,b). Using qPCR, we demonstrated that NPY restored the expression of Npy1r and other mural cell markers including Pdgfrb and Rgs5 that are downregulated by PDGF-BB (Extended Data Fig. 6c–e). Because mural cells are identified as the progenitor of thermogenic adipocytes, we examined whether NPY can preserve their beiging ability against PDGF-BB. We observed that PDGF-BB treatment downregulated thermogenic and adipogenic genes and that NPY restored their expression (Extended Data Fig. 6f–h). Based on the observations above, we demonstrate in vitro that NPY can sustain mural cells as the progenitors of thermogenic adipocytes.
Extended Data Fig. 6. NPY sustains mural cells and their ability to differentiate into thermogenic adipocytes against PDGF-BB.
(a) Confocal images of primary mural cells stained with anti-DES (cyan) and anti-PDGFRα (red), cultured under different conditions. Scale bar = 100 μm. (b) The percentage of PDGFRα+ cells under different stimulating conditions (n = 6 biologically independent samples). No drug vs. PDGF-BB, P = 0.0138; PDGF-BB vs. PDGF-BB + NPY, P < 0.0001. (c-e) The expression of (c) Npy1r (n = 8 biologically independent samples), (d) mural cell markers Pdgfrb (n = 8 biologically independent samples), and Rgs5 (n = 5&8&8 biologically independent samples) and (e) secretory factors in maintaining vascular integrity includingTgfb1 (n = 8) and Angpt1 (n = 4&8&8 biologically independent samples) in primary mural cells cultured with or without 1 μΜ NPY or 250 ng/mL PDGF-BB. No drug vs. PDGF-BB: Npy1r, P = 0.0005, Pdgfrb&Rgs5, P < 0.0001; Angpt1, P = 0.0032. PDGF-BB vs. PDGF-BB + NPY: Npy1r, P = 0.0004; Rgs5, P = 0.0051; Tgfb1, P = 0.0043; Angpt1, P = 0.0001. (f) Schematic of differentiating mural cells with or without 1 μΜ NPY or 250 ng/mL PDGF-BB. (g-h) The expression of (g) thermogenic and adipogenic genes in adipocytes differentiated from mural cells with or without 1μΜ NPY or 250 ng/mL PDGF-BB (n = 5&6 biologically independent samples). No drug vs. PDGF-BB: Ucp1, P = 0.0001; Cidea, P = 0.0019; Prdm16, P = 0.0260; Pparg, P = 0.0045. PDGF-BB vs. PDGF-BB + NPY: Ucp1, P = 0.0154; Prdm16, P = 0.0240; Pparg, P = 0.0134. For a, representative images were shown from 3 experiments. All values are expressed as mean ± SEM. Statistical comparisons were made using 2-tailed Student T-tests, *p < 0.05. Hprt was used as the reference gene for qPCR.
DIO depletes NPY+ axons
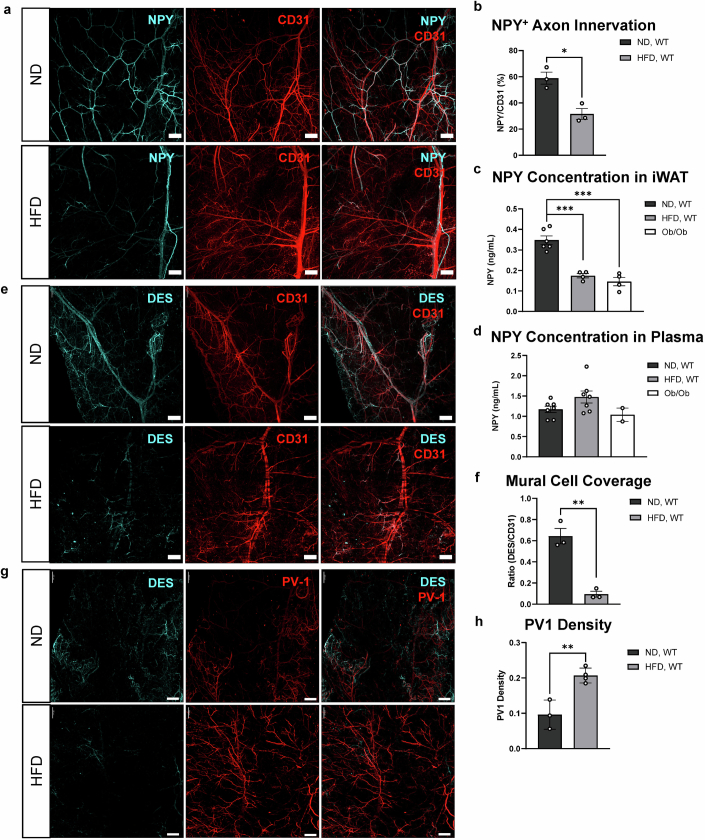
To test whether NPY+ sympathetic axons are affected by the obesity-induced sympathetic neuropathy in adipose tissue38, we immunolabelled cleared iWAT of normal-diet (ND)- and high-fat-diet (HFD)-treated mice for NPY and CD31. Our light-sheet images show a decreased density of NPY+ axons and a decreased percentage of vessels innervated by NPY+ axons in the iWAT of HFD-treated mice compared with ND-treated lean mice (Extended Data Fig. 7a,b). Consistently, NPY concentration also diminished in the iWAT of HFD-induced obese mice and leptin-deficient obese mice (Extended Data Fig. 7c). This decrease is not due to any changes in Npy expression in the ganglionic neuronal soma (Supplementary Fig. 5a,b) or systemic NPY level (Extended Data Fig. 7d). These results indicate that obesity-induced sympathetic neuropathy reduces NPY+ innervation in adipose tissue, which in turn decreases the local concentration of NPY in iWAT.
Extended Data Fig. 7. High-fat diet-induced obesity (DIO) depletes NPY+ innervation and mural cells and leads to vascular leakiness.
(a) Light-sheet images of cleared iWAT of normal diet (ND)-treated and high-fat diet (HFD)-treated 17-week-old male WT mice stained with anti-NPY (cyan) and anti-CD31 (red). Scale bar = 500 μm. (b) NPY+ innervation calculated as the ratio of NPY to CD31 quantified based on images as in Extended Data Fig. 7a (n = 3 mice, P = 0.0112). (c,d) The concentration of NPY in (c) iWAT (n = 5&4&4 mice) and (d) blood plasma of ND and HFD-treated 17-week-old male WT mice, and ND-treated Ob/Ob 17-week-old male mice (n = 6&7&2 mice). P = 0.0002 for both comparisons. (e) Light-sheet images of cleared iWAT of ND- and HFD-treated 17-week-old male WT stained with anti-DES (cyan) and anti-CD31 (red). Scale bar = 500 μm. (f) Mural cell coverage calculated as the ratio of DES+ cells to CD31+ cells quantified based on images as in Extended Data Fig. 7e (n = 3 mice, P = 0.0021). (g) Light-sheet images of iWATs of ND- and HFD-treated 17-week-old male mice stained with anti-DES (cyan) and anti-PV1 (red). Scale bar = 500 μm. (h) PV-1 density quantified based on images as in Extended Data Fig. 7g (n = 3&4 mice, P = 0.0053). For a,e,g, representative images are shown from 3 experiments. All values are expressed as mean ± SEM. Statistical comparisons were made using 2-tailed Student T-tests, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Hprt was used as the reference gene for qPCR.
We then investigated whether mural cells, the postsynaptic targets of NPY+ innervation in iWAT, are also affected by obesity. By immunolabelling cleared iWATs for DES and CD31, we found that there are fewer mural cells covering the vasculature of the iWAT of DIO mice compared with age-matched lean mice (Extended Data Fig. 7e,f). Given that mural cells are essential for vascular integrity39, we observed increased vascular leakiness in the iWAT of DIO mice, as shown by PV1 staining40 (Extended Data Fig. 7g,h). To determine whether this phenomenon is related to NPY levels, we immunolabelled sympathetic ganglia for DES to ascertain whether mural cell coverage changes when NPY levels are unaffected. We observed no significant difference in mural cell coverage of ganglionic vasculature between DIO and lean mice (Supplementary Fig. 5b–e), confirming that mural cell coverage positively correlates with local NPY level.
Sympathetic NPY regulates leakiness
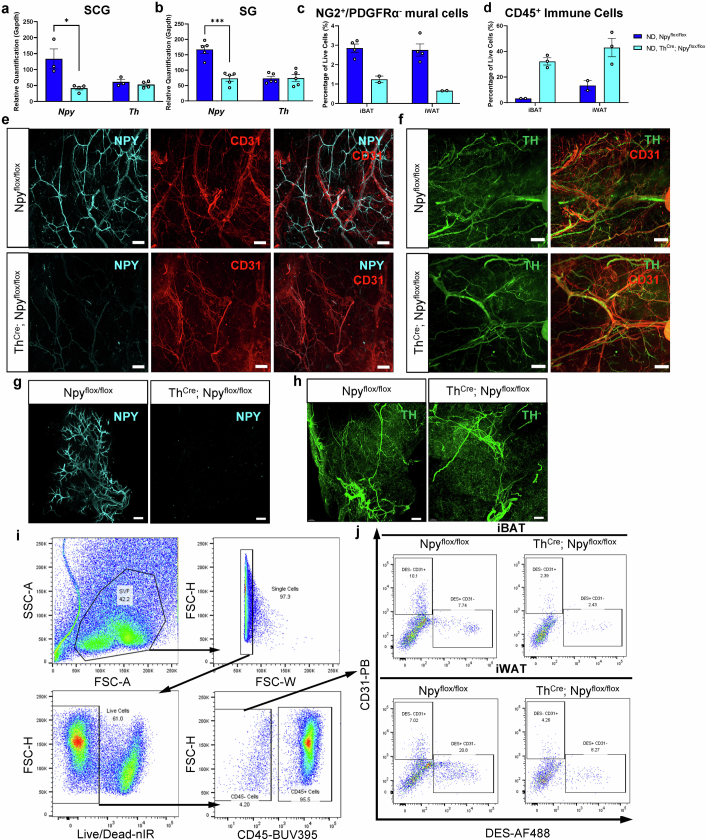
To test the hypothesis that sympathetic neuron-derived NPY is required to sustain mural cells in vivo, we generated a mouse model in which NPY was abrogated from sympathetic neurons: ThCre;Npyflox/flox mice. We first confirmed that, in ThCre;Npyflox/flox mice, NPY was abrogated from sympathetic ganglia (Extended Data Fig. 8a,b) and sympathetic axons innervating iWAT and iBAT (Extended Data Fig. 8e,g), whereas general TH+ sympathetic innervation was not affected (Extended Data Fig. 8f,h). Using this mouse model, we demonstrated that the loss of NPY in sympathetic neurons downregulated mural cell markers in iBAT of ND-treated mice (Fig. 4c) and depleted mural cells in iBAT and iWAT of both ND-treated (Fig. 4a,b and Extended Data Fig. 8c) and HFD-treated mice (Fig. 4e).
Extended Data Fig. 8. ThCre;Npyflox/flox mice have depleted NPY from the sympathetic nervous system and unchanged sympathetic innervation, central or plasma NPY level, causing mural cell depletion.
(a,b) The expression of Npy and Th in (a) SCG (n = 3&4 mice; Npy, P = 0.0190) and (b) SG (n = 5 mice; Npy, P = 0.0003) of 12-week-old ThCre;Npyflox/flox mice and Npyflox/flox male mice. (c,d) The percentage of (c) PDGFRα− /NG2+ mural cells (n = 4&2 mice) and (d) CD45+ immune cells (n = 2&3 mice) in iBATs and iWATs of 30-week-old, ND-treated mice measured by flow cytometry. (e,f) The image of cleared iWAT of 12-week-old ThCre;Npyflox/flox mice and Npyflox/flox male mice stained with (e) anti-NPY (cyan) or (f) anti-TH (green) and anti-CD31 (red). Scale bar = 500 μm. (g,h) Light-sheet image of cleared iBAT of 12-week-old ThCre;Npyflox/flox mice and Npyflox/flox male mice stained with (g) anti-NPY (cyan), scale bar = 500 μm, or (h) anti-TH (green), scale bar = 200 μm. (i) The gating strategy of flow cytometry to quantify the percentage of CD45+ immune cells DES+ mural cells, used for Fig. 4e. (j) The representative flow cytometry analysis of the percentage of mural cells (DES+) in the iBAT (top) and iWAT (bottom) of HFD-treated male ThCre;Npyflox/flox mice and Npyflox/flox mice. For e-h, representative images are shown from 2 experiments. All values are expressed as mean ± SEM. Statistical comparisons were made using 2-tailed Student T-tests, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Gapdh was used as the reference gene for qPCR.
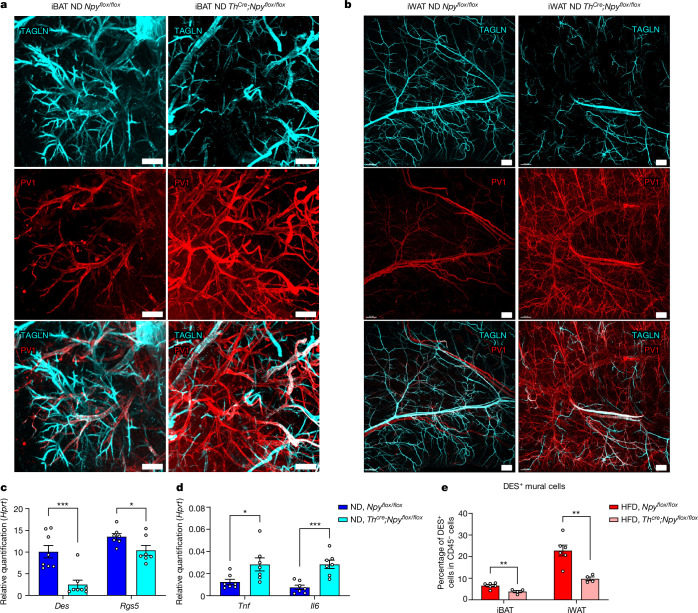
Fig. 4. Loss of NPY from sympathetic neurons depletes mural cells and increases vascular leakiness in both iBAT and iWAT.
a,b, Light-sheet images of cleared iBAT from 15-week-old ND-treated mice (a) and of iWAT from 30-week-old ND-treated mice (b) stained with anti-PV1 (red) and anti-TAGLN (cyan). c,d, Expression levels of mural cell markers Des (n = 8 mice; P = 0.0007) and Rgs5 (n = 7 mice; P = 0.0374) (c) and proinflammatory genes Tnf (n = 7 mice, P = 0.0284) and Il6 (n = 7 mice, P = 0.0004) (d) in BAT of ND-treated, 12-week-old, male ThCre;Npyflox/flox and Npyflox/flox mice. e, Percentage of DES+ mural cells in iBAT (Npyflox/flox versus ThCre;Npyflox/flox, P = 0.0084) and iWAT (Npyflox/flox versus ThCre;Npyflox/flox, P = 0.0038) of 17-week-old, HFD-treated mice, measured by flow cytometry (n = 4 mice). a,b, Representative images from three experiments. All values mean ± s.e.m. Statistical comparisons were made using two-tailed Student’s t-tests, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Scale bars, 200 μm (a), 500 μm (b). Hypoxanthine–guanine phosphoribosyltransferase (Hprt) was used as the reference gene for qPCR.
Because mural cells are required for vascular integrity39, we observed increased vascular leakiness in both iBAT and iWAT of ThCre;Npyflox/flox mice, as demonstrated by PV1 staining (Fig. 4a,b). We further evaluated vascular leakiness in epidydimal WAT using an intravital method by intravenous injection of Dextran-70kDa. Increased vascular leakiness in ThCre;Npyflox/flox mice was shown by leakage of Dextran-70kDa into the parenchyma (Extended Data Fig. 9a–d) whereas blood flow remained unchanged (Extended Data Fig. 9e). Consistent with increased vascular leakiness, we observed upregulated proinflammatory genes in iBAT (Fig. 4d) and increased immune infiltration in both iBAT and iWAT of ND-treated ThCre;Npyflox/flox mice (Extended Data Fig. 8d).
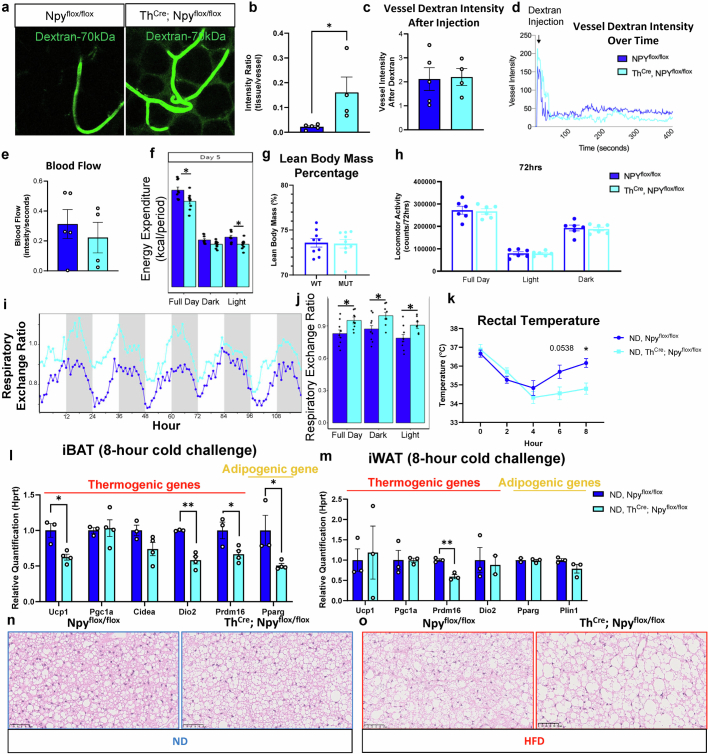
Extended Data Fig. 9. Loss of sympathetic NPY increases vascular leakiness, decreases energy expenditure (EE) and cold tolerance, inhibits thermogenesis and beiging, and increases respiratory exchange ratio (RER) and lipid droplet size.
(a) Intravital microscopy of epididymal WATs of 23-week-old male Npyflox/flox and ThCre;Npyflox/flox mice 12 min after i.v. injection of 70 kDa Dextran at the orbital plexus. (b) Fluorescence intensity ratio between tissue parenchyma and vessel calculated based on images as in Extended Data Fig. 9a (n = 5&4 mice). P = 0.0390. (c) Dextran intensity in the vessels of eWAT right after i.v. injection (n = 5&4 mice). (d) Dextran intensity in vessels over time measured with a 2-photon microscope. Arrow indicates the time when Dextran was injected. (e) Blood flow calculated using the average of the raw intensity of Dextran inside the artery over 100 s right after the stabilization of the Dextran signal following administration (n = 5&4 mice). (f) The quantification of daily energy expenditure (EE) recorded and quantified using an indirect calorimetry system (n = 9 mice). Full day, P = 0.0416; dark, P = 0.0288. (g) The percentage of lean body weight (LBM) of 8-week-old male mice measured by MiniSpec MRI (n = 9). (h) The locomotor activity measured using Multitake cage (n = 6 mice). (i) Respiratory exchange ratio (RER) of 8-week-old mice recorded using a metabolic cage. (j) The quantification of (i) (n = 9 mice). Full day, P = 0.0173; light, P = 0.0136; dark, P = 0.0243. (k) Rectal temperature of 20-week-old Npyflox/flox and ThCre;Npyflox/flox mice under cold exposure recorded using a rectal probe every hour (n = 3&4 mice). 8 h, P = 0.0158. (l,m) The expression of thermogenic and adipogenic genes in (l) iBATs (n = 3&4 mice) and (m) iWATs (n = 3 mice) of cold-challenged Npyflox/flox and ThCre;Npyflox/flox mice. iBAT: Ucp1, P = 0.0106; Dio2, P = 0.0012; Prdm16, P = 0.0385; Pparg, P = 0.0412. iWAT: Prdm16, P = 0.0024. (n,o) Histological staining of BATs of (n) ND- and (o) HFD-treated 17-week-old male ThCre;Npyflox/flox and Npyflox/flox mice. Scale bar = 50 μm. For a, representative images are shown from 4 experiments. All values are expressed as mean ± SEM. Statistic comparisons were made by 2-tailed Student T-tests (b,l,m) or 2-way ANOVA (f,j,k), *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Hprt was used as the reference gene for qPCR.
These observations confirmed that NPY is required for sustaining mural cells in adipose tissue and preventing immune infiltration.
Sympathetic NPY protects from obesity
To test the hypothesis that NPY locally sourced from sympathetic axons in adipose tissues preserves the mural cell pool and is required for energy homeostasis, we characterized the metabolism of ThCre;Npyflox/flox mice. We discovered that ND-treated, lean ThCre;Npyflox/flox mice had whitened and enlarged iBAT (Fig. 5a,g) and that their iBAT was dysfunctional in thermogenesis, as shown by lower levels of thermogenic genes (Fig. 5b). Consistent with this, ThCre;Npyflox/flox mice have lower daily energy expenditure (Fig. 5c and Extended Data Fig. 9f) and higher respiratory exchange (Extended Data Fig. 9i,j), indicating reduced usage of fat as a metabolic substrate. When food restricted, ThCre;Npyflox/flox mice showed lower iBAT temperature and lost less weight during a 14-h fast, indicative of energy conservation (Fig. 5d,e). Following 8 h cold exposure, ThCre;Npyflox/flox mice showed lower rectal temperature (Extended Data Fig. 9k) and downregulation of thermogenic genes in iBAT and Prdm16 in iWAT (Extended Data Fig. 9l,m) compared with their wild-type (WT) littermates. These alterations are not likely to be related to muscular activity because ThCre;Npyflox/flox mice have the same lean mass and locomotor activity as WT mice (Extended Data Fig. 9g,h).
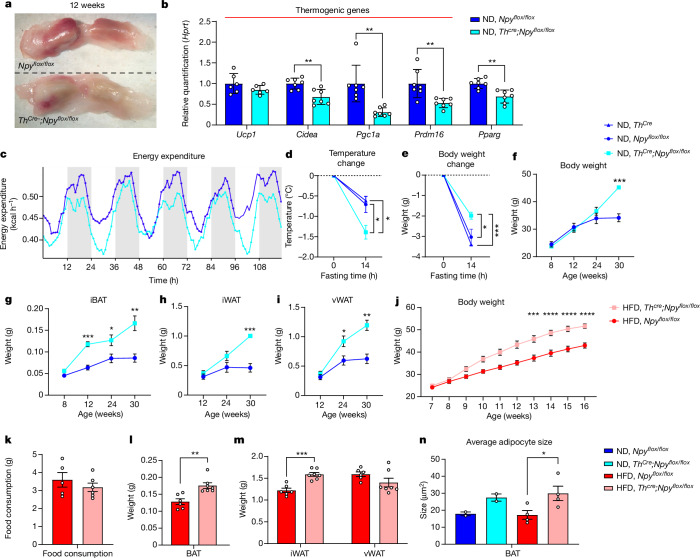
Fig. 5. Loss of NPY from sympathetic neurons whitens BAT, decreases energy expenditure and thermogenesis and renders mice more susceptible to DIO without increasing food intake.
a, iBAT dissected from ND-treated, 12-week-old, male Npyflox/flox and ThCre;Npyflox/flox mice. Dashed lines delineate iBAT lobes. b, Expression of thermogenic genes Ucp1, Cidea (P = 0.0024), Pgc1a (P = 0.0018) and Prdm16 (P = 0.0048), and of adipogenic gene Pparg (P = 0.0012), in iBAT from ND-treated, 12-week-old, male ThCre;Npyflox/flox and Npyflox/flox mice (n = 6 mice for Ucp1, n = 7 mice for other genes). c, Daily energy expenditure of 7-week-old male ThCre;Npyflox/flox and Npyflox/flox mice, measured using an indirect calorimetry system (n = 9). d,e, Changes in iBAT temperature (ThCre versus ThCre;Npyflox/flox mice, P = 0.0198; Npyflox/flox versus ThCre;Npyflox/flox mice, P = 0.0440) (d) and body weight (ThCre versus ThCre;Npyflox/flox mice, P = 0.0008; Npyflox/flox versus ThCre;Npyflox/flox mice, P = 0.0225) (e) of 12-week-old mice following a 14-h fast (n = 6 for ThCre;Npyflox/flox mice and n = 3 for ThCre and Npyflox/flox mice). f–i, Body weight (week 30, P = 0.0003) (f) and weights of iBAT (week 12, P = 0.0006; week 24, P = 0.0387; week 30, P = 0.0058) (g), iWAT (week 30, P = 0.0004) (h) and visceral WAT (vWAT) (week 24, P = 0.0338; week 30, P = 0.0030) (i) from ND-treated, male ThCre;Npyflox/flox and Npyflox/flox mice (n = 4 mice). j,k, Weekly body weight (n = 8; week 13, P = 0.0007; weeks 14–16, P < 0.0001) (j) and averaged daily food consumption (n = 4 and n = 6 cages) (k) from male, HFD-treated, ThCre;Npyflox/flox and Npyflox/flox mice. l,m, Weights of iBAT (n = 5 and n = 6 mice, P = 0.0023) (l), and iWATs (n = 6 and n = 7 mice, P = 0.0002) and vWAT (n = 6 and n = 7 mice) (m), from HFD-treated, 17-week-old, male ThCre;Npyflox/flox and Npyflox/flox mice. k–m, Colour coding as in j. n, Average adipocyte size in iBAT from ND- and HFD-treated male ThCre;Npyflox/flox and Npyflox/flox mice (n = 2 for ND-treated mice, n = 4 for HFD-treated mice, P = 0.0436). a, Representative images from three experiments. All values mean ± s.e.m. Statistical comparisons were made using two-tailed Student’s t-tests, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Hprt was used as the reference gene for qPCR.
As a cumulative result of deficient thermogenic ability and reduced energy expenditure, ND-treated ThCre;Npyflox/flox mice developed adult-onset obesity at 30 weeks, with heavier adipose depots compared with WT mice (Fig. 5f–i). When on a HFD, ThCre;Npyflox/flox mice became obese earlier and faster than WT mice without increasing food intake (Fig. 5j,k). In addition, HFD-treated ThCre;Npyflox/flox mice had significantly heavier adipose depots (Fig. 5l,m) and their iBAT contained larger lipid droplets on average (Fig. 5n and Extended Data Fig. 9n,o). Lastly, metabolic phenotypes were consistent across genders because HFD-treated female ThCre;Npyflox/flox mice also had heavier body weight, WAT and iBAT compared with Npyflox/flox control mice (Supplementary Fig. 6a–c).
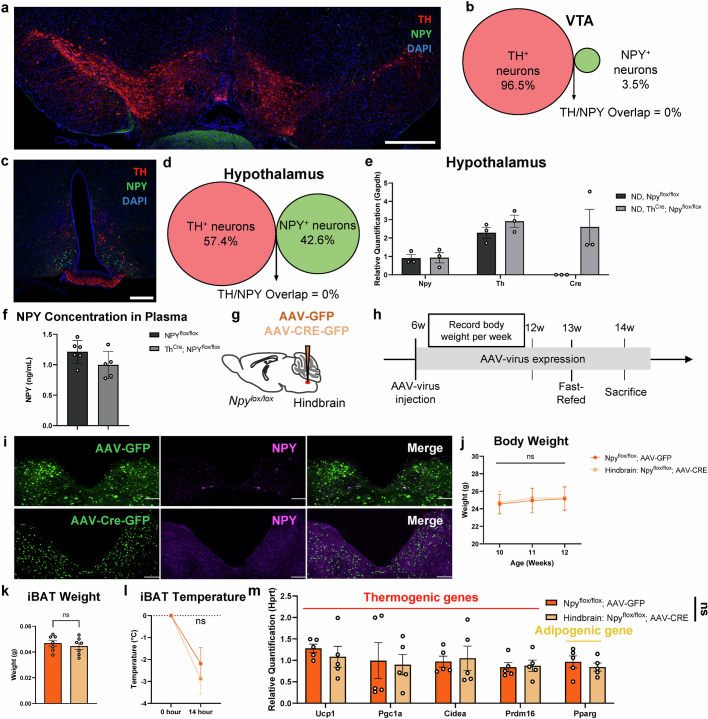
We can ascertain that the metabolic phenotypes observed in ThCre;Npyflox/flox mice were not caused by NPY+ neurons in the brain or systemic NPY, because (1) no catecholaminergic neurons coexpress NPY in the hypothalamus41 or ventral tegmental area (Extended Data Fig. 10a–d) and Npy expression was not changed in the hypothalamus of ThCre;Npyflox/flox mice (Extended Data Fig. 10e). (2) The metabolic phenotypes were not caused by systemic NPY because NPY concentration in the blood plasma of ThCre;Npyflox/flox mice was unchanged (Extended Data Fig. 10f). (3) Even though there are NPY+ catecholaminergic neurons in the hindbrain42, knocking out NPY from the hindbrain has no effect on either thermogenesis or iBAT (Extended Data Fig. 10g–m). Taken together, our findings demonstrate that sympathetic neuron-derived NPY in adipose tissue is required for sustaining energy expenditure and leanness.
Extended Data Fig. 10. Ablating NPY from sympathetic neurons using ThCre;Npyflox/flox mice does not affect NPY levels in VTA, hypothalamus or plasma, and ablating NPY from the hindbrain does not affect body weight, BAT weight or thermogenesis.
(a,c) Confocal image showing a slice of (a) ventral tegmental area (VTA, Scale bar = 500 μm) and (c) arcuate nucleus (Arc, Scale bar = 200 μm) of the hypothalamus of an 8-week-old NPY-GFP male mouse stained with anti-TH (red), anti-GFP (green), and DAPI (blue). (b,d) Quantification of TH/NPY-GFP overlap in (b) VTA and (d) hypothalamus (n = 3 mice). (e) The expression of Npy, Th, and Cre in the hypothalamus of 12-week-old ThCre;Npyflox/flox mice and Npyflox/flox male mice (n = 3). (f) NPY concentration in the blood plasma of 12-week-old ThCre;Npyflox/flox and Npyflox/flox male mice (n = 6&5). (g) Schematic of knocking out NPY in the hindbrain by AAV injection. (h) Schematic of metabolic phenotyping of mice with NPY knocked out from the hindbrain (Hindbrain: Npyflox/flox; AAV-CRE-GFP) and WT (Npyflox/flox; AAV-GFP) mice. (i) Confocal images of the sliced hindbrain of Hindbrain: Npyflox/flox; AAV-CRE-GFP and WT mice stained with anti-NPY (magenta). Scale bar = 100 μm. (j) Weekly body weight of ND-treated male Hindbrain: Npyflox/flox; AAV-CRE-GFP and WT mice (n = 7). (k) iBAT weight of 14-week-old ND-treated male Hindbrain: Npyflox/flox; AAV-CRE-GFP and WT mice (n = 7). (l) iBAT temperature change after a 14-hour fast (n = 7). (m) The expression levels of thermogenic and adipogenic genes in the iBAT of RT-housed ND-treated male Hindbrain: Npyflox/flox; AAV-CRE-GFP and WT mice (n = 5). For a,c,i, representative images are shown from 3 experiments. All values are expressed as mean ± SEM. Hprt was used as the reference gene for qPCR.
Discussion
Mural cells are contractile cells that wrap around the vasculature to regulate the diameter of vessels43 and have been described as a source of thermogenic adipocytes in both WAT and iBAT4–6, which are subject to the influence of noradrenaline released from adjacent sympathetic nerve terminals. These autonomic nerves corelease NPY2,3,9, but their function in the development and activity of thermogenic adipocytes is hitherto unknown. In humans, mutations in NPY have been linked to high BMI but not to dietary patterns1 (https://hugeamp.org/gene.html?gene=NPY; Extended Data Fig. 1m). In mice, the loss of NPY receptors leads to late-onset obesity without increase in food intake14–16. Taken together, the experimental evidence points to a role of NPY in body weight homeostasis that is independent of appetite regulation. Three studies have implicated NPY in directing lipogenic actions in white adipocytes, which favoured the growth of white adipose mass24,25,27. However, some of these studies are not replicated here24,25 and others are not supported by modern evidence provided by our imaging data and several single-cell sequencing datasets27. Specifically, we show in our supplementary data that NPY, directly, promotes neither adipogenesis24 nor the proliferation of 3T3-L1 preadipocytes25. Thus the evidence disfavours a pro-obesity role for peripheral NPY, contradicting the current perception.
This study provides anatomical maps of NPY+ innervation in both WAT and BAT that demonstrate that only one-third of sympathetic innervation is neuropeptidergic and that it preferentially targets arterioles where mural cells are present. Consistent with recent studies showing that sympathetic axons within adipose tissues retract and degenerate with the onset of obesity8,38, our data show that the axonal source of NPY, locally, within these tissues is also obliterated and this, again, refutes a direct adipogenic role of peripheral NPY. Moreover, several modern datasets omit NPY receptors in adipocytes or immune cells whereas Npy1r expression is robustly detected in mural cells, identifying these cells as the postsynaptic targets of NPY+ innervation within adipose tissues. We show that NPY sustains mural cells that are a source of thermogenic adipocytes by promotion of their proliferation in vitro. In vivo, NPY sourced from sympathetic neurons is necessary for support of mural cells and the normal development of thermogenic adipocytes. Conditional knockout of NPY (NPY-cKO) in sympathetic neurons depletes mural cells and whitens brown fat before the onset of obesity, rendering this tissue hypertrophic and with reduced thermogenic ability. These NPY-cKO mice have reduced thermogenic ability and lower energy expenditure, show cold intolerance, develop late-onset obesity on a regular chow diet and rapidly develop obesity when fed a high-fat diet, without eating more. These metabolic phenotypes match those of whole-body-NPY-receptor-knockout mice14–16, which also develop late-onset obesity on a regular chow diet even though they eat slightly less.
Notwithstanding this, disrupted mural cell proliferation and adipocyte differentiation are reasons for adipose dysfunction in NPY-cKO mice and are among many possibilities, including a reciprocal causality between vascular leakiness and adipose tissue inflammation. Taken together, we clarify that central and peripheral sources of NPY have antipodal roles in body weight homeostasis. The biological mechanism described in this study is a potential explanation for why mutations of the orexigenic NPY paradoxically associate with high BMI in humans (https://hugeamp.org/gene.html?gene=NPY)1. Lastly, it highlights that energy dissipation may be more important than appetite in some individuals via the mechanism identified herein.
Methods
Animals
ThCre mice (B6.Cg-7630403G23RikTg(Th-cre)1Tmd/J, stock no. 008601) and Cx3cr1GFP/+ mice (Cx3cr1tm1Litt/LittJ, stock no. 008451) were purchased from the Jackson Lab. Npyflox/flox mice were a donation from the I. Kalajzic Laboratory at the Department of Reconstructive Science, University of Connecticut44 under the material transfer agreement (MTA). Tissues of NPY-GFP mice (B6.FVB-Tg(Npy-hrGFP)1Lowl/J) were sourced from the Tamas Horvath Laboratory at Brandy Memorial Laboratory, Yale University. Tissues from Npy1rCre;Rosa26tdTomato mice were provided by M. Roberts at the Department of Otolaryngology–Head and Neck Surgery, University of Michigan. Sympathetic neuron-specific NPY-cKO mice were generated by crossing ThCre mice with Npyflox/flox mice. Diet-induced obesity was achieved by feeding mice an HFD (Diet Research, product no. D12492) when they were 7 weeks old, and this feeding regime lasted for 10 weeks. The body weight of each mouse and food consumption in each cage were recorded weekly. All mice were group housed in standard housing under controlled room temperature (21–23 °C) and 50% humidity under a 12/12 h light/dark cycle and given access to diet and water ad libitum. All experimental procedures were performed on living animals in accordance with the UK ANIMALS ACTS 1986 under the project licence (PPL no. P80EDA9F7) and personal licences granted by the UK Home Office. Ethical approval was provided by the Ethical Review Panel at the University of Oxford.
Energy expenditure and measurement of respiratory exchange rate
Animals aged 6 weeks were analysed for oxygen consumption, carbon dioxide production, energy expenditure and respiratory exchange rate using an indirect calorimetry system (Panlab, Harvard Apparatus, LE405 Gas Analyzer and Air Supply & Switching). Animals were maintained in individual cages following a 12/12 h light/dark cycle with water and food supplied ad libitum, and under controlled room temperature (21–23 °C) and 50% humidity. Metabolic data were collected for 5 days following a 2 day acclimatation period. Animals’ body weight (g) and food (g) were measured before entering and after exiting the cage. Results were normalized by weight, and graphs and statistical analysis were obtained using the CalR Web-based Analysis Tool for Indirect Calorimetry Experiments (v.1.3)45.
Locomotor activity and measurement of body composition
Animals aged 8–9 weeks were analysed for spontaneous locomotor activity (Panlab, Harvard Apparatus, LE001 PH Multitake Cage). Animals were maintained in individual cages following a 12/12 h light/dark cycle with water and food supplied ad libitum, and under controlled room temperature (21–23 °C) and humidity. Data were collected for 72 h following a 1 day acclimatation period. Activity was recorded using COMPULSE v.1.0 software (Panlab). Animals aged 7–9 weeks were analysed for body composition using a Minispec LF50 (Brucker).
Fasting and thermoimaging
Animals aged 12–14 weeks were used for thermoimaging. The data shown in Fig. 5e were recorded using an Optris thermocamera (Optris PI 160 with a standard 61 lens), and the data in Extended Data Fig. 10l were recorded using a FOTRIC 225 s infrared camera and analysed with FOTRIC software (v.5.0.8.214). Animals were single housed and placed under the thermocameras, with their back shaved to expose the skin above iBAT. Animals were acclimatized for 4 days in individual cages with ad libitum access to food and water at room temperature under a 12/12 h light/dark cycle before a 14-h fast. Mice had ad libitum access to water during fasting.
iBAT temperature was recorded every 1 s over a 6-day period by storing the temperature of the warmest pixel in view using the software provided by the camera manufacturer (rel. 2.0.6, Optris PIX Connect). iBAT temperature at 0 and 14 h was taken as an average of temperatures during a 1-h period.
Cold-exposure experiment
Animals aged 20 weeks were single housed in a thermoneutral environment (31 °C, 50% humidity) under a 12/12 light/dark cycle for 1 week, with ad libitum access to food and water. Mice were then cold-challenged at 4 °C and their rectal temperature measured every 1 h using a rectal probe. Animals were killed immediately following 8 h of cold exposure and their iBAT harvested for qPCR with reverse transcription analysis.
Stereotaxic injection
For viral injection, mice were anaesthetized with avertin (240 mg kg−1 intraperitoneally) and fixed on a stereotaxic holder (RWD, Life Science). Either AAV2/9-hSyn-Cre-EGFP-WPRE-pA (Taitool, catalogue no. S0230-9, 2 × 1,012 viral genomes ml−1, 200 nl per side) or AAV2/9-hSyn-EGFP-WPRE-pA (catalogue no. S0237-9, Taitool, 2 × 1,012 viral genomes ml−1, 200 nl per side) was bilaterally injected into the nucleus tractus solitarius (NTS) (NTS coordinates anteroposterior, mediolateral, dorsoventral −7.5, ±0.35 and −4.75 mm, respectively) of Npyflox/flox mice.
Immunofluorescent staining
Superior cervical ganglia and sympathetic axon bundles were dissected from adipose tissues of mice perfused with 20 ml of PBS, and the tissues were fixed in 4% paraformaldehyde (PFA, 043368.9 M, ThermoFisher) for at least 4 h. Cells collected following in vitro experiments were washed once with PBS and fixed using 4% PFA for at least 4 h. Axon bundles were first stained by incubation overnight at 4 °C with primary antibodies dissolved in the permeabilizing buffer (3% bovine serum albumin, 2% goat serum, 0.1% Triton X-100 and 0.1% sodium azide in PBS), followed by incubation with secondary antibodies and DAPI dissolved in the permeabilizing buffer for 1 h with gentle agitation. As for immunofluorescence in fixed cells, cells were first incubated with the permeabilizing buffer for 1 h at room temperature and then stained overnight at 4 °C with primary antibodies diluted in the permeabilizing buffer. Samples were then washed and stained with DAPI and secondary antibodies for 1 h at room temperature. Sympathetic fibres or fixed cells were then mounted on slides with Fluoromount-G mounting medium (ThermoFisher, catalogue no. 00-4958-02).
For cryosection, samples were first embedded in OCT (VWR, catalogue no. 361603E) and frozen at −20 °C until cryosectioning. Samples were cut into 10-µm-thick sections and mounted on charged SuperFrost Plus slides (VWR, catalogue no. 631-0108), followed by staining using the same procedure as for fixed cells.
For paraffin embedding, fixed samples were processed in 70, 80 and 90% ethanol and then in 100% ethanol, 100% xylene and 60 °C paraffin wax, three times for each condition. Samples were embedded in paraffin wax, sectioned at 7 µm, mounted on coverslips and dried in an oven at 45–50 °C overnight. Before staining, samples were deparaffinized twice in 100% xylene for 5 min, rehydrated twice in 100% ethanol and then once each in 95 and 80% ethanol and water for 10 min. Antigen retrieval was done by the addition of 0.05% trypsin to each slide and incubation at 37 °C for 20 min. After washing with running water, samples were stained using the same procedure as for fixed cells.
For staining, whole-mount, PFA-fixed ganglia were dehydrated once in each of 30, 50, 70 and 90% ethanol, twice in 100% ethanol—with shaking for 20 min at each concentration—and then rehydrated in 90, 70, 50 and 30% ethanol. Ganglia were then digested with 1.5 U ml−1 dispase-1 (Roche, catalogue no. 04942078001), 0.5 mg ml−1 collagenase (Sigma, catalogue no. C2674) and 300 μg ml−1 hyaluronidase (Sigma, catalogue no. H3884) diluted in PBS, with shaking at 37 °C in a water bath for 30 min. Ethanol-treated ganglia were blocked in blocking solution (3% bovine serum albumin, 2% goat serum, 0.1% Triton X-100 and 0.1% sodium azide in PBS) for 2 h and then incubated with primary antibodies diluted in the permeabilizing buffer for 3 days at 4 °C. Three days later, ganglia were incubated in secondary antibodies diluted in the permeabilizing buffer for a further 3 days at 4 °C. Finally, ganglia were dehydrated once each in 30, 50, 70 and 90% ethanol and twice in 100% ethanol then cleared using ethyl cinnamate (ECi). Cleared ganglia were mounted in a coverslip slide sandwich filled with ECi.
Images were acquired using a Zeiss LSM880 confocal microscope with Zen-black software (v.2.1). Samples were imaged using either (1) a ×10/0.45 numerical aperture (NA) objective with a voxel size of 1.04 μm (x), 1.04 μm (y) and 3.64 μm (z), (2) a ×20/0.8 NA objective with voxel size of 0.52 μm (x), 0.52 μm (y) and 1.00 μm (z) or (3) a ×63/1.4 NA oil-immersion objective with a voxel size of 0.13 μm (x), 0.13 μm (y) and 1.00 μm (z). Solid-state lasers of 405, 561 and 633 nm and an Argon 488 laser were used for DAPI, AF488, AF546 and AF647 fluorophores, respectively.
Clearing of adipose tissue
Whole fat pads were stained and cleared using a modified version of iDISCO as previously described18,46. In brief, WAT or BAT was dissected from mice perfused with 20 ml of PBS, and the tissues were fixed with 4% PFA overnight. Tissues were pretreated once each with 20, 40, 60 and 80% ethanol and twice in 100% methanol for 30 min, all at room temperature, and then bleached with 5% H2O2 diluted in 100% methanol for 24 h at 4 °C with shaking. Bleached samples were rehydrated using 80, 60, 40 and 20% methanol for 30 min each and then in PTwH buffer (0.2% Tween-20, 10 μg ml−1 heparin and 0.02% sodium azide in PBS) for 30 min. Afterwards, samples were incubated overnight in permeabilizing solution (20% DMSO, 0.2% Triton X-100, 0.3 M glycine in PBS) at 37 °C in a water bath and blocked using blocking buffer (10% DMSO, 2% Triton X-100, 0.02% sodium azide and 5% goat serum in PBS). For immunolabelling, samples were incubated for 5 days, with shaking, in primary antibodies diluted in antibody dilution buffer (5% DMSO, 5% goat serum, 0.2% Tween-20 and 10 μg ml−1 heparin in PBS) at 37 °C, washed with PTwH for 1 day and then incubated for a further 3 days with secondary antibodies and DAPI diluted using antibody dilution buffer at 37 °C, with shaking. Immunolabelled tissues were embedded in 1% agarose and dehydrated in 20, 40, 60, 80 and 100% methanol for 1 h each, and then in 100% methanol overnight at room temperature. Samples were incubated in dichloromethane (Sigma, catalogue no. 270997) until they sank and were then incubated in dibenzyl ether (Sigma, catalogue no. 179272) until clear. Samples were stored in dibenzyl ether at room temperature and transferred into ECi before imaging. Images of cleared tissues were acquired using a Miltenyi Biotec Ultramicroscope II light-sheet microscope; step size was 8 μm, thickness of the light sheet was 3.98 μm, horizontal dynamic focusing was set to eight steps and exposure time was 180 ms. Samples were illuminated using a bidirectional light sheet and scanned under a ×2/0.5 NA objective with voxel size 4.03 μm (x), 4.03 μm (y) and 8 μm (z). A 488-nm laser with a 525/50 filter, a 561-nm laser with a 620/60 filter and a 638-nm laser with a 680/30 filter were used for AF488, AF546 and AF647, respectively.
Dextran-70kDa injection and intravital microscopy
Animals were anaesthetized with ketamine (100 mg kg−1) and xylazine (10 mg kg−1) and a small incision was made to expose epididymal white adipose tissue for microscopy analysis. Intravital images of space resolution 1,024 × 1,024 pixels from epididymal WAT were obtained simultaneously using an optical microscope with coherent anti-Stokes Raman scattering, two-photon excitation fluorescence and a bright-field microscope, with a confocal LSM 780-NLO Zeiss inverted microscope Axio Observer Z.1 (Carl Zeiss). All procedures were performed at the National Institute of Science and Technology on Photonics Applied to Cell Biology at the State University of Campinas. Adipocyte images from coherent anti-Stokes Raman scattering were acquired using two lines of lasers in wavelengths λpump = 803 nm and λStokes = 1,040 nm, and fluorescence images were acquired by two-photon excitation fluorescence using the exogenous fluorescence dye tetramethylrhodamine isothiocyanate dextran (Sigma, catalogue no. T1162) in wavelength of excitation λStokes = 1,040 nm. Following identification of blood vessels and acquisition of an initial image the video was started and, after ten frames, 50 µl (30 mg kg−1) of Dextran-70KDa (Sigma, catalogue no. T1162) was injected into the orbital plexus of the mouse. The video continued recording for approximately 12 min to capture vessel fluorescence and leakage. Fluorescence intensity over time was analysed for the whole duration of the video. A static image was analysed following dextran administration using Fiji47. For evaluation of tissue leakage, a region of interest was selected inside the vessel and another in the tissue (outside the vessel). Fluorescence intensity was measured in both areas, and the intensity ratio between the tissue and within the vessel was calculated. Blood flow was calculated using the average of the raw intensity of dextran inside the artery over 100 s immediately following stabilization of the dextran signal after administration.
Antibodies
The following primary antibodies were used for immunofluorescent staining: rat anti-CD31 (BioLegend, catalogue no. 102501, MEC13.3, 1:100 dilution), rat anti-PLVAP (BioLegend, catalogue no. 120503, MECA32, 1:100 dilution), rabbit anti-DES (abcam, catalogue no. ab15200, 1:500 dilution), rabbit anti-NPY (Cell Signaling, catalogue no. D7Y5A, 1:500 dilution), rabbit anti-NPY (abcam, catalogue no. ab30914, 1:500 dilution), chicken anti-TH (Aves Labs, catalogue no. TYH73787982, 1:500 dilution), rabbit anti-TH (Sigma, catalogue no. Ab152, 1:500 dilution), mouse anti-NPY1R (Santa Cruz, catalogue no. sc-393192, 1:200 dilution), rat anti-PDGFRα (BioLegend, catalogue no. 135902, APA5, 1:200 dilution), rabbit anti-TAGLN (abcam, catalogue no. ab14106, 1:250 dilution), Cy3 anti-αSMA (Sigma, catalogue no. C6198, 1A4, 1:250 dilution), goat anti-SOX17 (R&D, catalogue no. AF1924, 1:250 dilution), goat anti-EPHB4 (R&D, catalogue no. AF3034, 1:250 dilution) and rabbit anti-UCP1 (abcam, catalogue no. ab10983, 1:500 dilution).
The following antibodies were used for fluorescent activated cell sorting (FACS) and flow cytometry: AF700 anti-CD45 (BioLegend, catalogue no. 103128), BUV395 anti-CD45 (BD Horizon, catalogue no. 564279), Pacific Blue anti-CD31 (BioLegend, catalogue no. 102421), APC anti-PDGFRa (BioLegend, catalogue no. 135907), AF488 anti-NG2 (Sigma, catalogue no. MAB5384A4) and AF488 anti-DES (abcam, catalogue no. ab185033, Y66). All antibodies for FACS and flow cytometry were diluted at 1:500. The LIVE/DEAD Fixable Near-IR Dead Cell Stain Kit (1:1,000, ThermoFisher, catalogue no. L10119) was used for live/dead staining.
qPCR
RNA extraction was performed using Trizol reagent (ThermoFisher, catalogue no. 15596026), complementary DNA was synthesized using SuperScript II Reverse Transcriptase (Invitrogen, catalogue no. 18064022) and qPCR was performed using Power SYBR Green PCR Master Mix (LifeTech, catalogue no. 4368706). Data were recorded using a StepOne qPCR system and analysed with StepOne Software v.2.3. Primers used in this research are listed in Supplementary Table 1.
The ΔCt method was used to quantify gene expression using the following formula: relative expression = 2^ (−(Cttarget gene − Ctreference gene)). The ΔΔCt method was used to compare the thermogenic and adipogenic gene expression shown in Fig. 5b and Extended Data Figs. 5b,d,g, 9l,m and 10m.
The qPCR data shown in Extended Data Fig. 10m were collected using the CFX96 Real-Time PCR Detection System (Bio-Rad) with TB Green Premix Ex Taq II (TaKaRa, catalogue no. RR820A).
Protein extraction and blood plasma isolation for NPY ELISA
Proteins were extracted from iWAT as previously described48. In brief, dissected iWAT was placed in homogenizing tubes filled with 300 μl 50 mg−1 RIPA lysis buffer (Sigma, catalogue no. 20188) containing 50 μM DPP-IV inhibitor (Sigma, catalogue no. DPP4-010) and 500,000 IU ml−1 aprotinin (Sigma, catalogue no. A6103-1MG) and homogenized using a Precellys 24 homogenizer. Lipid in the tissue homogenize was removed by centrifuging at 20,000× rcf at 4 °C for 15 min, and the clear portion retained. The process was repeated three times to ensure complete removal of lipid.
For terminal blood collection, mice were euthanized by intraperitoneal injection of 10 μl g−1 pentobarbital and blood was then collected from the left ventricle using a 25 G needle and a syringe coated with 100 mM EDTA. Blood was then placed in tubes with 5 μl of 100 mM EDTA and centrifuged at 1,000× rcf at 4 °C for 15 min to separate plasma from blood cells, and then the supernatant was transferred to fresh tubes with DPP-IV inhibitor (final concentration 50 μM) and aprotinin (final concentration 500,000 IU ml−1). The concentration of NPY in mouse iWAT and blood plasma was determined using an NPY ELISA kit (Merck, catalogue no. EZRMNPY-27K). ELISA data were recorded using a FLUOstar Omega microplate reader with Omega v.6.20 software.
Single-cell suspension and flow cytometry
Dissected adipose tissues were minced and digested in an enzyme mixture (for each sample, 500 μl of collagenase II (4 mg ml−1, Sigma, catalogue no. C6885), 500 μl of hyaluronidase (5.3 mg ml−1, equivalent to 40,000 U ml−1, Sigma, catalogue no. H3884) and 5 μl of Dnase I (BioLabs, catalogue no. M0303L)) in a 37 °C water bath, with shaking, for 45 min, and samples pipetted every 10 min. Digestion was stopped by the addition of FACS buffer (PBS containing 2% fetal bovine serum (FBS)), and single-cell suspensions were collected by filtering the digested sample using EASYStrainer cell sieves (70 μm mesh; Greiner, catalogue no. 542070).
For preparation of samples for sorting or flow cytometry, cells were first treated with red blood cell lysis buffer (BioLegend, catalogue no. 420301) to remove red blood cells and then treated with Fc block (ThermoFisher, catalogue no. 14-9161-73) before staining with antibodies. Before immunolabelling for intracellular markers, cells were fixed and permeabilized using the eBioscience Intracellular Fixation and Permeabilization Buffer Set (ThermoFisher, catalogue no. 88-8824-00). Cell sorting was performed using a BD FACSAria III sorter, and flow cytometry data were acquired with either a BD FACSAria III sorter or a BD LSRFortessa X20 cytometer with BD FACSDiva v.6.0 software. Cytometry data were analysed using FlowJo v.10.8.1.
Isolation and primary culture of mural cells and in vitro stimulation
Mural cells were isolated and cultured following a modified version of the protocol previously published49. In brief, mice were euthanized intraperitoneally with 10 μl g−1 pentobarbital and perfused with 15 ml of PBS to remove blood. The iBAT SVF was isolated using the method described above. Cells were then seeded in 10 cm dishes with 10 ml of DMEM with 10% FBS for 2 days for the cells to attach to the bottom. Afterwards, 5 ml of the medium was replaced with mural cell-specific medium (DMEM with 2% FBS, supplied with 1% mural cell growth supplement (Science Cell, catalogue no. 1252)). Every other day, 5 ml of medium in dishes was refreshed until the cells grew confluent. For acquisition of pure mural cells, cells were labelled with an APC anti-CD104b antibody (1:100, BioLegend, catalogue no. 136007) and mural cells were sorted using the MojoSort magnetic sorting protocol with MojoSort buffer (BioLegend, catalogue no. 480017) and anti-APC nanobeads (BioLegend, catalogue no. 480071).
To perform in vitro coculturing experiments, cells were seeded at 5 × 104 per millilitre on glass coverslips and the indicated concentrations of PDGF-BB (R&B, catalogue no. 220-BB) and NPY (Cayman Chemical, catalogue no. CAY15071) were added to cells 24 h following cell seeding. Cells were collected 5 days later and either fixed with 4% PFA for imaging or lysed with Trizol for RNA extraction and qPCR.
Cell lines
The RAW264.7 mouse macrophage cell line (Sigma, catalogue no. 91062702) was purchased without further authentication or testing for mycoplasma contamination. The complete medium for cell culture includes high-glucose DMEM with glutamate (Sigma, catalogue no. 41965039) and 10% FBS (Sigma, catalogue no. 12133 C).
The 3T3-L1 preadipocyte cell line was a gift from R. Klemm at the Department of Physiology, Anatomy and Genetics, University of Oxford, and used without further authentication or testing for mycoplasma contamination. The complete medium for cell culture includes high-glucose DMEM with glutamate (ThermoFisher, catalogue no. 41965039) and 10% calf serum (Sigma, catalogue no. 12133 C). For subculture of preadipocytes the medium was transferred, and cells were washed with 1 ml of prewarmed 0.05% trypsin (ThermoFisher, catalogue no. 25300054) and digested with 200 μl of trypsin at 37 °C for 5–10 min. Afterwards, digestion was stopped and cells resuspended using the complete medium.
Induction to white and thermogenic adipocytes
For differentiation of 3T3-L1 cells to white adipocytes, cells were seeded in 12-well plates at a density of 1 × 105 per millilitre. The medium was refreshed until cells were confluent. Two days following cell confluence, induction medium (10% FBS, 500 μM 3-isobutyl-methylxanthine and 1 μM dexamethasone in DMEM) with or without 1 μg ml−1 insulin was added to each well; 3 days later the induction medium was replaced with maintenance medium (DMEM with 10% FBS with or without 1 μg ml−1 insulin). Subsequently the medium was refreshed with maintenance medium every 2 days. Total differentiation time for each well was 8 days. NPY treatments in experimental groups started when the induction medium was added and lasted throughout the whole differentiation process.
SVF and mural cells were differentiated to thermogenic adipocytes as previously reported6,30. SVF and mural cells were isolated and seeded in 12-well plates at a density of 1 × 105 per millilitre. When cells reached 95% confluency, beige cell induction medium (DMEM with 10% FBS, 125 μM indomethacin, 5 μM dexamethasone, 500 μM 3-isobutyl-methylxanthine and 0.5 μM rosiglitazone) was added to each well; 2 days later the induction medium was replaced with maintenance medium (DMEM with 10% FBS, 5 μg ml−1 insulin and 1 nM triiodothyronine). Maintenance medium was refreshed every 2 days. Total differentiation time for each well was 8 days. NPY treatments in the experimental groups started following the addition of induction medium and lasted throughout the whole differentiation process. Oxygen consumption was measured using a Seahorse XF Analyzer.
Cell proliferation assay
The cell proliferation assay was performed with the Click-iT EdU Cell Proliferation Kit (ThermoFisher, catalogue no. C10340) for imaging. Mural cells were seeded at 0.5 × 105 per millilitre in 12-well plates on coverslips and cultured overnight before experiments. Cells were then labelled with EdU and cultured with or without 1 μM NPY or 2 μM PD98059 (Cell Signaling, catalogue no. 9900 S) for 6 h in medium for mural cells (low-glucose DMEM with 2% FBS). Cells were then fixed with 4% PFA and permeabilizing buffer for 30 min each, and EdU was labelled with AF647 using the Click-iT Plus reaction cocktail. Following immunofluorescent staining with anti-DES, cells were imaged using a Zeiss LSM880 confocal microscope with Zen-black software (v.2.1), with a ×20/0.8 NA objective and voxel size of 0.52 μm (x), 0.52 μm (y) and 1.00 μm (z). An area of 3,072 × 3,072 pixels was imaged for each biological replicate.
scRNA-seq dataset analysis
Public scRNA-seq datasets were downloaded from Gene Expression Omnibus (GEO) and analysed using the following method. Cells with fewer than 200 unique detected genes or over 5% mitochondrial counts were discarded. After filtering, the gene x cell matrix was normalized using ‘NormalizeData()’ in Seurat v.4.2.0 (ref. 50) in R (v.4.2.2). Data were then scaled using ‘ScaleData()’, and linear dimensional reduction performed by principal component analysis and calculation of UMAP coordinates for all cells using Seurat v.4.2.0. Cells were clustered using ‘FindNeighbour()’, with dimensions set to 15, and ‘FindClusters()’, with resolution set to 0.5. Each cluster was identified based on differentially expressed genes and known markers in the published literature5,22,29,51–53.
Figure quantification
Overlapping between TH, NPY and CD31 was calculated using JACoP54, a Fiji plug-in47. To make this calculation, 472.33, 472.33 and 30 μm xyz regions were randomly picked and maximally projected to z using Fiji script. Labelled areas were automatically segmented using a threshold set by default, and overlapping percentages were calculated automatically by JACoP.
Innervation of NPY+ axons was quantified using the ‘Surface’ tool in Imaris v.9.2. Labelled areas of NPY+ axons and CD31+ vessels in whole cleared iWAT were automatically segmented, and the innervation of NPY in vasculature was calculated as volumeNPY+/volumeCD31+. The coverage of DES+ mural cells in iWAT was calculated similarly, as volumeDES+/volumeCD31+. Confocal images showing NPY+ innervation were quantified using Fiji with an automatic unbiased method. AreaNPY+ and areaCD31+ were segmented using the Otsu thresholding method and measured with the ‘measure’ program.
The percentage of EdU+ cells was counted with an unbiased automatic method using ‘detect particles’ in Fiji47. Threshold was set automatically using the Otsu thresholding method.
The percentages of PDGFRα+ and DES+ cells were counted manually based on PDGFRα and DES signals. The percentages of TH+ and NPY+ neurons in the hypothalamus and ventral tegmental area were also counted manually. To calculate ganglionic mural cell coverage, 531, 531 and 30 μm xyz views were randomly picked and maximally projected to z using Fiji script. Labelled areas were segmented using a threshold set by default, and coverage was calculated as area NPY+/area CD31+.
The size of adipocytes in WAT and iBAT was quantified as previously described55 using Fiji. In brief, WAT and iBAT were processed to paraffin-embedded sections (3 μm), stained with haematoxylin and eosin and scanned into digital images using a NanoZoomer-SQ Digital slide scanner (Hamamatsu). Images were randomly picked and the plug-in Analyse Particles was used to count the number and measure the size of adipocytes and droplets in iBAT. For each iBAT, 11,000 lipid droplets were measured.
Statistics and reproducibility
GraphPad Prism v.9.5.0 was used for statistical analysis. Mean and s.e.m. were used to represent a sample. Chi-squared and paired two-tailed Student’s t-tests were used to compare two samples; analysis of variance was used to compare multiple data points. No statistical methods were used to predetermine sample sizes.
All experiments were replicated at least twice with the same conclusion, and all in vivo experiments were replicated in at least two independent cohorts. The exact numbers of replicated experiments are stated in each figure legend. Samples and animals were randomly allocated to experimental groups and proceeded in experiments, and data were collected and analysed blind and ad hoc registered to groups or genotypes.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-024-07863-6.
Supplementary information
Supplementary Figs. 1–6, Table 1 and additional references.
Acknowledgements
We thank I. Kalajzic for sharing Npyflox/flox mice; M. Silveira and M. Roberts for sharing Npy1rCre;Rosa26tdTomato mice; T. Hovarth for sharing NPY-GFP mice; R. Klemm for the 3T3-L1 cell line; V. Vyazovskiy and S. Wilcox for access to thermocamera and technical assistance; M. Dustin at the Kennedy Institute of Rheumatology for access to the light-sheet microscope, in particular C. Lagerholm for his technical assistance; the Dunn School of Pathology for confocal microscopy, in particular A. Wainman for his technical assistance; and K. Zhang for her technical assistance. We thank the National Institute of Science and Technology on Photonics Applied to Cell Biology for their assistance with the inverted microscope Axio Observer Z.1, in particular M. O. Baratti and A. T. P. Campos for their help. The icons of scissors in Extended Data Figs. 3a,g,i and 4c are from Flaticon (https://flaticon.com). We thank all members of Domingos Laboratory for ther discussions and advice on the project. Y.Z. receives a scholarship from Shineroad Industry Developments Co. Ltd. L.Y. is supported by the China Scholarship Council. J.C. is supported by the National Natural Science Foundation of China (no. 32100821). This research is funded by the Wellcome Trust–Howard Hughes Medical Institute International Research Scholar Award (no. 208576/Z/17/Z), the ERC consolidator award (no. ERC-2017 COG 771431), the Pfizer ASPIRE Obesity award and the Next Iteration of the Type 2 Diabetes Knowledge Portal (no. 2UM1DK105554).
Extended data figures and tables
Author contributions
Y.Z. designed and performed all experiments, with guidance from A.I.D., unless otherwise stated. Y.Z. wrote the first draft of the manuscript and A.D. wrote subsequent versions with Y.Z. L.Y. assisted Y.Z. and performed the experiments shown in Fig. 3 and Extended Data Figs. 1g–k, 3g–j, 4c–e, 5a,b,f,g, 6f,g and 9l,m, with guidance from Y.Z. and A.D. A.L.G.-F., B.B. and M.R.S. conducted experiments shown in Fig. 5c and Extended Data Fig. 9a–j, with guidance from L.A.V. G.S. helped Y.Z. conceive this project and provided advice on scRNA-seq data analysis. D.S.-O. made intellectual contributions to scRNA-seq dataset analysis. A.C. acquired light-sheet images at the Francis Crick Institute on samples cleared and stained by Y.Z. and shown in Extended Data Fig. 7a,e, and gave technical advice on light-sheet imaging. N.M.-S. helped with mouse colony management. C.L. imaged the first samples cleared and stained by Y.Z. (not shown in this manuscript). M.L.D. permitted access to the Kennedy Imaging Facility. T.L.H. provided the brains of NPY-GFP mice shown in Extended Data Fig. 10a,c. S.K. gave intellectual contributions pertaining to beige fat biology. I.A. conducted the experiment shown in Extended Data Figs. 3a–c and 5c–e, with guidance from S.K. J.C. conducted the experiments shown in Extended Data Fig. 10g–m, with guidance from C.Z. L.Z. helped with the histology shown in Supplementary Fig. 3a,b.
Peer review
Peer review information
Nature thanks Dritan Agalliu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The public scRNA-seq dataset of sympathetic neurons can be viewed on the Linnarson Lab website (https://linnarssonlab.org/sympathetic/), with raw data deposited at GEO under accession no. GSE78845. The scRNA-seq datasets for iWAT and iBAT were deposited at GEO under accession nos. GSE154047 and GSE160585. Genome-Wide Association Study data of the association between genomic variants and metabolic traits are publicly available at the Common Metabolic Diseases Knowledge Portal (https://hugeamp.org/). All numerical data supporting this research are available within the paper and its Supplementary Information.
Code availability
The code used for scRNA-seq analysis is deposited at GitHub (https://github.com/PLAVRVSO/scRNA-Seq-analysis-of-BAT-SVF).
Competing interests
All authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41586-024-07863-6.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-024-07863-6.
References
- 1.Dornbos, P. et al. Evaluating human genetic support for hypothesized metabolic disease genes. Cell Metab.34, 661–666 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lundberg, J. M. et al. Neuropeptide Y (NPY)‐like immunoreactivity in peripheral noradrenergic neurons and effects of NPY on sympathetic function. Acta Physiol. Scand.116, 477–480 (1982). [DOI] [PubMed] [Google Scholar]
- 3.Lundberg, J., Franco‐Cereceda, A., Hemsen, A., Lacroix, J. & Pernow, J. Pharmacology of noradrenaline and neuropeptide tyrosine (NPY)‐mediated sympathetic cotransmission. Fundam. Clin. Pharmacol.4, 373–391 (1990). [DOI] [PubMed] [Google Scholar]
- 4.Long, J. Z. et al. A smooth muscle-like origin for beige adipocytes. Cell Metab.19, 810–820 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shamsi, F. et al. Vascular smooth muscle-derived Trpv1+ progenitors are a source of cold-induced thermogenic adipocytes. Nat. Metab.3, 485–495 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oguri, Y. et al. CD81 controls beige fat progenitor cell growth and energy balance via FAK signaling. Cell182, 563–577 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caron, A., Lee, S., Elmquist, J. K. & Gautron, L. Leptin and brain–adipose crosstalks. Nat. Rev. Neurosci.19, 153–165 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng, W. et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell163, 84–94 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekblad, E. et al. Neuropeptide Y co-exists and co-operates with noradrenaline in perivascular nerve fibers. Regul. Pept.8, 225–235 (1984). [DOI] [PubMed] [Google Scholar]
- 10.Le Roux, C. & Bloom, S. Peptide YY, appetite and food intake. Proc. Nutr. Soc.64, 213–216 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Stanley, B. G. & Leibowitz, S. F. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci.35, 2635–2642 (1984). [DOI] [PubMed] [Google Scholar]
- 12.Marie, L. S., Luquet, S., Cole, T. B. & Palmiter, R. D. Modulation of neuropeptide Y expression in adult mice does not affect feeding. Proc. Natl Acad. Sci. USA102, 18632–18637 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson, J. C., Clegg, K. E. & Palmiter, R. D. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature381, 415–418 (1996). [DOI] [PubMed] [Google Scholar]
- 14.Naveilhan, P. et al. Normal feeding behavior, body weight and leptin response require the neuropeptide Y Y2 receptor. Nat. Med.5, 1188–1193 (1999). [DOI] [PubMed] [Google Scholar]
- 15.Pedrazzini, T. et al. Cardiovascular response, feeding behavior and locomotor activity in mice lacking the NPY Y1 receptor. Nat. Med.4, 722–726 (1998). [DOI] [PubMed] [Google Scholar]
- 16.Nguyen, A. D. et al. Y1 and Y5 receptors are both required for the regulation of food intake and energy homeostasis in mice. PLoS ONE7, e40191 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furlan, A. et al. Visceral motor neuron diversity delineates a cellular basis for nipple-and pilo-erection muscle control. Nat. Neurosci.19, 1331–1340 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Chi, J., Crane, A., Wu, Z. & Cohen, P. Adipo-clear: a tissue clearing method for three-dimensional imaging of adipose tissue. J. Vis. Exp. 137, 58271 (2018). [DOI] [PMC free article] [PubMed]
- 19.Wang, H. U., Chen, Z.-F. & Anderson, D. J. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell93, 741–753 (1998). [DOI] [PubMed] [Google Scholar]
- 20.Kim, D. et al. Sox17 mediates adult arterial endothelial cell adaptation to hemodynamics. Biomaterials293, 121946 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer, K. et al. Neuropeptide Y is produced by adipose tissue macrophages and regulates obesity-induced inflammation. PLoS ONE8, e57929 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henriques, F. et al. Single-cell RNA profiling reveals adipocyte to macrophage signaling sufficient to enhance thermogenesis. Cell Rep.32, 107998 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Attwell, D., Mishra, A., Hall, C. N., O’Farrell, F. M. & Dalkara, T. What is a pericyte? J. Cereb. Blood Flow Metab.36, 451–455 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosmaninho-Salgado, J. et al. Intracellular mechanisms coupled to NPY Y2 and Y5 receptor activation and lipid accumulation in murine adipocytes. Neuropeptides46, 359–366 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Yang, K., Guan, H., Arany, E., Hill, D. J. & Cao, X. Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. FASEB J.22, 2452–2464 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Chen, W.-C. et al. Neuropeptide Y is an immunomodulatory factor: direct and indirect. Front. Immunol.11, 580378 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuo, L. E. et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat. Med.13, 803–811 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Yan, C. et al. Peripheral-specific Y1 receptor antagonism increases thermogenesis and protects against diet-induced obesity. Nat. Commun.12, 2622 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emont, M. P. et al. A single-cell atlas of human and mouse white adipose tissue. Nature603, 926–933 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aune, U. L., Ruiz, L. & Kajimura, S. Isolation and differentiation of stromal vascular cells to beige/brite cells. J. Vis. Exp. 73, 50191 (2013). [DOI] [PMC free article] [PubMed]
- 31.Hansel, D., Eipper, B. & Ronnett, G. Neuropeptide Y functions as a neuroproliferative factor. Nature410, 940–944 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Medeiros, P. J., Pascetta, S. A., Kirsh, S. M., Al-Khazraji, B. K. & Uniacke, J. Expression of hypoxia inducible factor–dependent neuropeptide Y receptors Y1 and Y5 sensitizes hypoxic cells to NPY stimulation. J. Biol. Chem.298, 101645 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimada, K. et al. Neuropeptide Y activates phosphorylation of ERK and STAT3 in stromal vascular cells from brown adipose tissue, but fails to affect thermogenic function of brown adipocytes. Peptides34, 336–342 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Zhang, W. & Liu, H. T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res.12, 9–18 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Armulik, A., Genove, G. & Betsholtz, C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell21, 193–215 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Hosaka, K. et al. Pericyte-fibroblast transition promotes tumor growth and metastasis. Proc. Natl Acad. Sci. USA113, E5618–E5627 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onogi, Y. et al. PDGFRbeta regulates adipose tissue expansion and glucose metabolism via vascular remodeling in diet-induced obesity. Diabetes66, 1008–1021 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Wang, P. et al. A leptin-BDNF pathway regulating sympathetic innervation of adipose tissue. Nature583, 839–844 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Daneman, R., Zhou, L., Kebede, A. A. & Barres, B. A. Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature468, 562–566 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spadoni, I. et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science350, 830–834 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Zhang, X. & van den Pol, A. N. Hypothalamic arcuate nucleus tyrosine hydroxylase neurons play orexigenic role in energy homeostasis. Nat. Neurosci.19, 1341–1347 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen, Q. et al. Resident macrophages restrain pathological adipose tissue remodeling and protect vascular integrity in obese mice. EMBO Rep.22, e52835 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Almaca, J., Weitz, J., Rodriguez-Diaz, R., Pereira, E. & Caicedo, A. The pericyte of the pancreatic islet regulates capillary diameter and local blood flow. Cell Metab.27, 630–644 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wee, N. K. Y. et al. Skeletal phenotype of the neuropeptide Y knockout mouse. Neuropeptides73, 78–88 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mina, A. I. et al. CalR: a web-based analysis tool for indirect calorimetry experiments. Cell Metab.28, 656–666 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Renier, N. et al. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell159, 896–910 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diaz Marin, R., Crespo-Garcia, S., Wilson, A. M. & Sapieha, P. RELi protocol: optimization for protein extraction from white, brown and beige adipose tissues. MethodsX6, 918–928 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang, L. et al. Isolation and characterization of peritoneal microvascular pericytes. FEBS Open Bio12, 784–797 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell184, 3573–3587 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muhl, L. et al. Single-cell analysis uncovers fibroblast heterogeneity and criteria for fibroblast and mural cell identification and discrimination. Nat. Commun.11, 3953 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carr, M. J. et al. Mesenchymal precursor cells in adult nerves contribute to mammalian tissue repair and regeneration. Cell Stem Cell24, 240–256 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Kalucka, J. et al. Single-cell transcriptome atlas of murine endothelial cells. Cell180, 764–779 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Bolte, S. & Cordelières, F. P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc.224, 213–232 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Parlee, S. D., Lentz, S. I., Mori, H. & MacDougald, O. A. in Methods in Enzymology Vol. 537 (ed. MacDougald, O. A.) 93–122 (Elsevier, 2014). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs. 1–6, Table 1 and additional references.
Data Availability Statement
The public scRNA-seq dataset of sympathetic neurons can be viewed on the Linnarson Lab website (https://linnarssonlab.org/sympathetic/), with raw data deposited at GEO under accession no. GSE78845. The scRNA-seq datasets for iWAT and iBAT were deposited at GEO under accession nos. GSE154047 and GSE160585. Genome-Wide Association Study data of the association between genomic variants and metabolic traits are publicly available at the Common Metabolic Diseases Knowledge Portal (https://hugeamp.org/). All numerical data supporting this research are available within the paper and its Supplementary Information.
The code used for scRNA-seq analysis is deposited at GitHub (https://github.com/PLAVRVSO/scRNA-Seq-analysis-of-BAT-SVF).