Abstract
Tyrosine phosphorylation has been shown to play a role in the replication of several herpesviruses. In this report, we demonstrate that bovine herpesvirus 1 infection triggered tyrosine phosphorylation of proteins with molecular masses similar to those of phosphorylated viral structural proteins. One of the tyrosine-phosphorylated viral structural proteins was the tegument protein VP22. A tyrosine 38-to-phenylalanine mutation totally abolished the phosphorylation of VP22 in transfected cells. However, construction of a VP22 tyrosine 38-to-phenylalanine mutant virus demonstrated that VP22 was still phosphorylated but that the phosphorylation site may change to the C terminus rather than be in the N terminus as in wild-type VP22. In addition, the loss of VP22 tyrosine phosphorylation correlated with reduced incorporation of VP22 compared to that of envelope glycoprotein D in the mutant viruses but not with the amount of VP22 produced during virus infection. Our data suggest that tyrosine phosphorylation of VP22 plays a role in virion assembly.
The tegument is a unique feature of herpesviruses and remains the least well-characterized virion compartment (41). There are approximately 15 virally encoded proteins that participate in the assembly of the amorphous tegument structure, and these tegument proteins occupy the majority of the mass in the virion (18, 25, 40). Recent studies have shown that at least a portion of the tegument structure has an ordered organization and interacts with the capsid (37, 42, 43, 47, 48); however, little is known regarding the acquisition of the viral tegument process (14, 15, 36, 38, 41). The incorporation of herpes simplex virus type 1 (HSV-1) tegument protein VP22 is increased more than twofold when the VP22 protein expression level is increased fivefold (21). This observation is consistent with the hypothesis that the incorporation of tegument protein is partly determined by local protein concentration. In contrast, the amount of HSV-1 tegument protein UL37 in virions is strictly controlled despite a 20-fold increase of UL37 in infected cells (25). Thus, multiple mechanisms to control the incorporation of different tegument proteins may exist. In addition, evidence suggests that acquisition of the tegument is independent of capsid or envelope (26, 36). The tegument retains its structural integrity in the absence of the capsid and envelope, indicating strong intermolecular interactions that must exist between these tegument proteins to support the seemingly amorphous structure (7, 27, 40).
Most of the herpesvirus tegument proteins are phosphoproteins (3, 11, 12, 20, 41). Phosphorylation of tegument proteins is believed to play a role in tegument protein dissociation (28). Both cellular and virally encoded kinases are involved in the phosphorylation of tegument proteins (5, 11, 12), and serines of tegument protein HSV-1 VP22 are phosphorylated in infected cells (11, 12). Phosphorylation of VP22 coincides with the translocation of VP22 into the nuclei of HSV-1-infected cells (10, 19, 28, 32, 33). Interestingly, only nonphosphorylated VP22 is present in HSV-1 virions (11, 12, 28). Evidence also suggests that tyrosine phosphorylation is involved in HSV-1 replication because (i) HSV-1 penetration triggers tyrosine phosphorylation of cellular proteins (1, 35), (ii) many viral proteins are tyrosine phosphorylated during infection (3, 30), and (iii) HSV-1 replication is inhibited in the presence of tyrosine kinase inhibitors (13, 44–46). Also, bovine herpesvirus 1 (BHV-1) glycoprotein E (gE) is tyrosine phosphorylated during viral replication and the titer of virus is proportional to the level of phosphorylation of this envelope protein (39). However, the exact role that tyrosine phosphorylation plays during herpesvirus infection is still unknown.
Among the tegument proteins, VP22, a heavily modified phosphoprotein (2), is of particular interest to us (16). VP22 is capable of intercellular trafficking (4, 6, 8, 31), induces microtubule acetylation, and stabilizes the microtubule bundles (9, 16). VP22 relocates to a novel subcellular site with another tegument protein, VP16, in coexpressing cells (7). In addition, a BHV-1 VP22 deletion mutant is asymptomatic and avirulent (22), suggesting that VP22 plays a functional role in virus replication in vivo.
In this report, we find that (i) several BHV-1 structural proteins are tyrosine phosphorylated, one of which is the tegument protein VP22; (ii) VP22 is tyrosine phosphorylated in transfected cells, suggesting that a cellular kinase is able to phosphorylate VP22, and tyrosine 38 is the major site for phosphorylation; (iii) a VP22 tyrosine-to-phenylalanine mutant virus possesses patterns of VP22 tyrosine phosphorylation different from those of VP22 expressed in transfected cells, suggesting that viral factors may be involved; (iv) BHV-1 infection induces the tyrosine phosphorylation of several proteins with molecular masses similar to those of tyrosine-phosphorylated virus structural proteins; and (v) the loss of VP22 tyrosine phosphorylation correlates with reduced VP22 incorporation into virions but not a reduction in VP22 expression in virus-infected cells. These findings suggest that VP22 tyrosine phosphorylation plays a major role in virion assembly.
MATERIALS AND METHODS
Cells, virus, and antibodies.
Madin-Darby bovine kidney (MDBK) cells (ATCC CCL-22) and F17 primary cultured bovine fibroblasts (16) were passaged in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum. BHV-1 (Cooper strain ATCC VR-864) and BHV-1 VP22 deletion mutant virus dvUL49 (gift from Lorne Babiuk, University of Saskatchewan, Saskatoon, Saskatchewan, Canada) stocks were prepared by infecting the MDBK cells at a multiplicity of infection (MOI) of 0.01 for 3 days at 37°C in 5% CO2. Virus titers were determined on MDBK cells, and aliquots were stored at −80°C. Purified virions were prepared as described by others (26). The cell-released virus was harvested and separated on 5 to 15% Ficoll gradients. Banded virions were pelleted, resuspended in phosphate-buffered saline, and stored at −80°C. Antiphosphotyrosine polyclonal antibody (P11230) and monoclonal antibody (PY54) were purchased (Transduction Laboratories, Lexington, Ky.). Anti-VP22 polyclonal antibody was raised in mice immunized with purified bacterially expressed VP22 proteins. Anti-VP22 antibody 114 specific for carboxyl-terminal peptides 235 to 258 (114235–258) was a gift from Lori Babiuk.
Plasmids and PCR-mediated mutagenesis.
BHV-1 VP22 sequence was amplified by PCR from BHV-1 genomic DNA using the 5′ primer GGGGAATTCCCATGGCCCGGTTCCACAGG and the 3′ primer GGGGTCGACCTAGTGGTGGTGGTGGTGGTGCGGCCGGGCCCGCTCGCC. The PCR products were digested and ligated into the EcoRI and SalI sites of the mammalian expression vector pCI-neo (Promega, Madison, Wis.) to generate plasmid pCIVP22. The primer design provided a His tag at the carboxyl end of the VP22 protein for purification. Site-specific mutations were introduced into the BHV-1 VP22 gene by overlap extension PCR (17). In brief, the first-round PCR products overlapped at the mutation site. Then, the outer primer pair was used to amplify and produce the desired amino acid substitution mutation using a mixture of the first two reaction products as the template. Multiple-site mutagenesis was done by repeating the above-described PCR procedure within the VP22 gene. The PCR products were digested and ligated into the pCI-neo vector and sequenced to verify that only the correct mutation was introduced into the VP22 gene. To generate the transfer vector for homologous recombination, a 2.8-kbp DNA fragment containing the full-length VP22 gene was isolated from pSD57 (24) and ligated into the pSP73 vector (Promega) to generate plasmid pSPVP22tr. Then, the sequence containing the amino acid mutation was isolated from pCIVP22 constructs and substituted for the corresponding sequence in plasmid pSPVP22tr.
Transfections, affinity purification of His-tagged proteins, and immunoblotting.
Transient transfections were performed using LipofectAMINE reagent (Life Technologies, Gaithersburg, Md.) as described by the manufacturer. Bovine F17 fibroblasts were transfected with pCIVP22 or constructs with different amino acid substitutions for 2 days, lysed in lysis buffer (1% Triton X-100, 5 mM imidazole, 500 mM NaCl, 20 mM Tris-HCl [pH 7.9], 0.2 mM sodium ortho-vanadate, 0.2 mM phenylmethylsulfonyl fluoride, 0.5% NP-40), and purified using Ni2+ resin according to the instructions of the manufacturer (Novagen, Madison, Wis.). Protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane. The membrane was incubated with appropriate antibodies and visualized by enhanced-chemiluminescence reaction (Pierce Chemical Company, Rockford, Ill.).
Generation of VP22 tyrosine-to-phenylalanine mutant virus and screening.
The transfer vector pSPVP22tr containing a tyrosine mutation(s) was electroporated simultaneously with VP22 deletion mutant virus vdUL49 genomic DNA into MDBK cells using an Eletroporator II (Invitrogen, Carlsbad, Calif.). After 4 to 5 days, individual virus plaques were screened using a pair of primers outside of the VP22 gene to detect successful homologous recombination and production of mutant virus. The correctly sized PCR product was verified by DNA sequencing for desired mutagenesis. The mutant virus was plaque purified three times before virus stock was prepared and stored at −80°C.
Peptide sequencing and mass spectral analysis.
Briefly, BHV-1 structural proteins were separated by SDS-PAGE. The viral protein band of interest was excised, followed by an in-gel trypsin digestion. The digested peptide mixture was eluted, purified by high-pressure liquid chromatography, and sequenced. The peptide (1 μg/μl) was mixed with a 104-fold molar excess of the matrix 2,5-dihydroxybenzoic acid in an aqueous 30% acetonitrile solution containing 0.1% trifluoroacetic acid. Peptide sequencing using matrix-assisted laser desorption ionization-mass spectrometry was performed by the Protein and Nucleic Acid Shared Facility, Medical College of Wisconsin at Milwaukee. Analysis of tyrosine phosphorylation was accomplished using VP22s isolated from viruses by SDS-PAGE as described above and performed by liquid chromatography (LC)-mass spectroscopy at the University of Wisconsin—Madison Biotechnology Facility.
RESULTS
One of the tyrosine-phosphorylated BHV-1 structural proteins is tegument protein VP22.
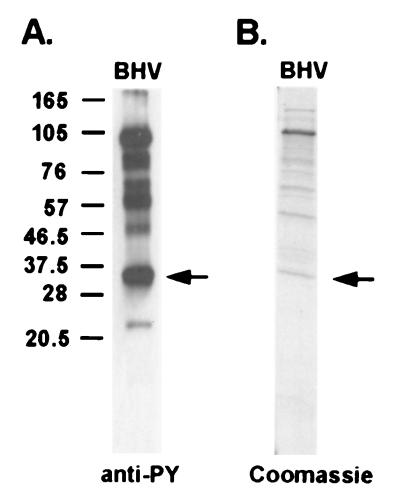
To identify the tyrosine-phosphorylated viral proteins, BHV-1 virions were purified using a 5 to 20% Ficoll gradient and analyzed by 12% SDS-PAGE. The separated viral proteins were transferred to a nitrocellulose membrane, and the tyrosine-phosphorylated virion structural proteins were detected by a monoclonal antiphosphotyrosine antibody. Several viral structural proteins with molecular masses of 96, 60 to 70, and 32 kDa were identified with antiphosphotyrosine antibody (Fig. 1A), suggesting tyrosine phosphorylation. The 32-kDa protein was selected for further analysis. This 32-kDa virion structural protein band was excised from the SDS-PAGE gel (Fig. 1B), in-gel trypsin digested, and analyzed by matrix-assisted laser desorption ionization-mass spectrometry to determine the peptide sequence. The peptide sequence APPGANAVASGRPLAFS is 100% identical to BHV-1 structural protein VP22. VP22 is a viral tegument protein encoded by the UL49 gene. The VP22 gene is dispensable for virus growth in vitro, but the attenuation of the VP22 deletion mutant virus in infected cattle suggests that it may play a functional role in vivo (22).
FIG. 1.
Tyrosine-phosphorylated BHV-1 structural proteins. (A) BHV-1 proteins were subjected to SDS-PAGE, immunoblotted, and probed with an antiphosphotyrosine antibody (PY54). Molecular mass markers are noted at the left in kilodaltons. (B) Coomassie blue staining of the purified BHV-1 structural proteins using the same sample preparation as that used for panel A. The protein band isolated and identified as VP22 is marked by arrows.
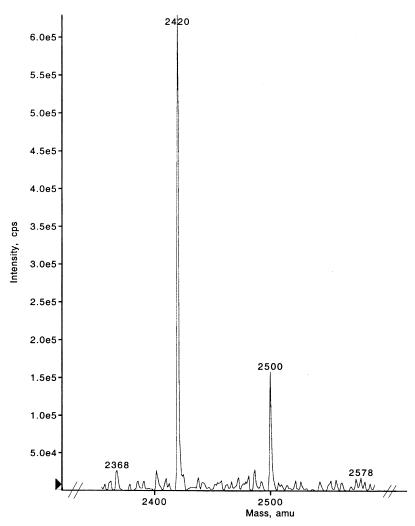
To identify the region of tyrosine phosphorylation, LC-mass spectroscopy was performed on trypsin-digested VP22 isolated from virus. Figure 2 illustrates several of the VP22 peptide fragments following trypsin digestion. Importantly, residues 23 to 42 of the peptide sequence ENSLYDYESGSDDHVYEELR, comprising tyrosines 27, 29, and 38, demonstrated a shift in position from the expected mass of 2,420 to 2,500 m/z. A shift in mass of 80 m/z correlates with the expectation of phosphorylation of one of the three tyrosines in the native protein; however, the phosphorylation of a serine in this peptide fragment should also be considered. A spectral shift associated with phosphorylation was not evident in other trypsin-digested peptides, and additional trypsin-digested peptides matched the expected peptide masses of VP22.
FIG. 2.
LC-mass spectroscopy of trypsin-digested VP22 illustrating residues 23 to 42 of the peptide sequence ENSLYDYESGSDDHVYEELR, which contains tyrosines 27, 29, and 38. A shift in mass from 2,420 to 2,500 m/z correlates with the phosphorylation of one of the three tyrosines in the peptide. amu, atomic mass units.
Because tyrosine kinase inhibitors block HSV-1 replication in vitro (13, 44–46), the tyrosine kinase inhibitors genistein and herbymycin were evaluated for inhibition of BHV-1 replication. MDBK cells were infected by BHV-1 at an MOI of 1 for 24 h with or without a tyrosine kinase inhibitor. Cells were frozen and thawed to release cell-associated viruses, and virus titer was determined by plaque assays. A dose-dependent decline in BHV-1 titer was observed (data not shown), suggesting the importance of tyrosine phosphorylation to virus production.
Mapping the tyrosine phosphorylation site(s) by site-directed mutagenesis.
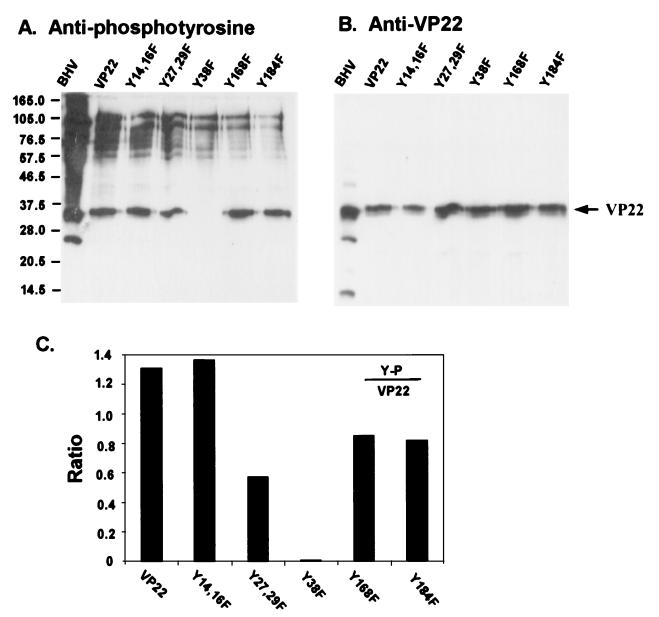
There are a total of seven tyrosine residues in the VP22 sequence for possible phosphorylation. To further study phosphorylation of tyrosines at positions 27, 29, and 38, substitutions of these three tyrosine residues by phenylalanine, as well as additional tyrosine residues of VP22, were produced using PCR-based site-directed mutagenesis. The VP22 gene was cloned into the mammalian expression vector pCI-neo, and the VP22 protein was fused with a C terminus His tag to facilitate purification. Primary cultured bovine fibroblasts were transfected with pCIVP22, and tyrosine-to-phenylalanine mutants were constructed. Two days following transfection, the cells were lysed and purified with Ni2+ columns. The purified proteins were analyzed by immunoblotting and probed with antiphosphotyrosine antibody. As shown in Fig. 3A, among the purified VP22 mutant constructs, the tyrosine 38-to-phenylalanine mutation abolished tyrosine phosphorylation while the remaining constructs retained phosphorylation. The same membrane was stripped and reprobed with anti-VP22 antibodies (Fig. 3B), indicating the presence of VP22 protein in each construct. Two additional bands were noted in the BHV lane at 22 and 14 kDa that likely represent catabolic products of the N and C termini of VP22, respectively, as supported by later antibody evidence. To semiquantify the phosphorylation level in each construct, the amount of tyrosine phosphorylation compared to the corresponding amount of VP22 protein was plotted. Figure 3C showed that the tyrosine 38-to-phenylalanine mutation totally abolished the tyrosine phosphorylation of VP22. In contrast, the tyrosine 14- and 16-to-phenylalanine double mutation apparently did not affect tyrosine phosphorylation, while the tyrosine 27- and 29-to-phenylalanine double mutation, tyrosine 168-to-phenylalanine mutation, and tyrosine 184-to-phenylalanine mutation all caused varied losses of phosphorylation. These findings suggest that, in transfected cells, tyrosine 38 is a major tyrosine phosphorylation site. These data also support the mass spectroscopy data indicating that the peptide containing Y38 was the major phosphorylation site of native VP22.
FIG. 3.
Mapping of the VP22 phosphorylation sites. Bovine fibroblast cells were transfected with VP22 or its tyrosine-to-phenylalanine mutant constructs for 2 days. The His-tagged VP22 proteins were purified using Ni2+ columns. The partially purified proteins were analyzed by immunoblotting and probed with polyclonal antiphosphotyrosine (A) or anti-VP22 (B) antibody. Note that the tyrosine 38-to-phenylalanine mutation abolished the phosphorylation of VP22. (C) The blotted signals were quantified using NIH Image software, and the ratios of phosphorylation were normalized to the amount of VP22. Molecular masses are noted at the left in kilodaltons.
Generation and characterization of the VP22 tyrosine 38-to-phenylalanine substitution mutant virus.
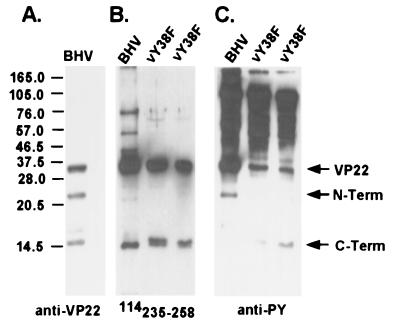
To further evaluate the role of VP22 tyrosine phosphorylation, a VP22 tyrosine-to-phenylalanine substitution mutant virus was constructed. Based on our in vitro data that VP22 tyrosine 38 is a major site of tyrosine phosphorylation in transfected cells, we first constructed a VP22 tyrosine 38-to-phenylalanine substitution mutant virus by homologous recombination. Viral genomic DNA from the VP22 deletion mutant virus and the transfer vector pSPVP22tr containing the tyrosine 38-to-phenylalanine mutation were simultaneously electroporated into MDBK cells. Individual viral plaques were picked and screened for successful homologous recombination. The PCR product was sequenced to verify that only the correct mutation was introduced. The mutant virus, designated vY38F, was further plaque purified three times and reconfirmed by sequencing before the mutant virus stock was prepared. Immunoblot analysis using an anti-VP22 antibody revealed that, in BHV-1 virions, the full-length VP22 peptide was cleaved into two smaller peptides. The larger 22-kDa peptide contained the N terminus of VP22, as it was recognized by VP22 polyclonal antibody (Fig. 4A) but not the VP22 carboxyl-terminal-peptide-specific antibody 114235–258 (Fig. 4B). The smaller 14-kDa peptide comprised the carboxyl terminus of VP22, as it reacted to antibody 114235–258 (Fig. 4B) as well as the polyclonal antibody (Fig. 4A). The tyrosine phosphorylation of VP22 in vY38F mutant virus was compared with that of the wild type using an immunoblot probed with polyclonal antiphosphotyrosine antibody. Tyrosine-phosphorylated VP22 was present in vY38F at a reduced level but was still strongly detectable compared to that in wild-type virus (Fig. 4C). Interestingly, the 22-kDa N-terminal peptide of VP22 was tyrosine phosphorylated in wild-type virus while the 14-kDa C-terminal peptide was not (Fig. 4C, BHV lane). In contrast, the C-terminal peptide of vY38F VP22 appeared to be tyrosine phosphorylated while the N terminus was not (Fig. 4C, vY38F lane). Levels of VP22 tyrosine phosphorylation in the transfected cells were different from that in the mutant virus, which may have resulted from the two different cell types used in these assays. Transfection was done on bovine fibroblasts, while infection was done with MDBK cells; therefore, cell-type-specific kinase activities may exist. To address this concern, mutant vY38F virus was passaged in F17 bovine fibroblasts. A similar level of tyrosine phosphorylation of the VP22 C terminus was detected (data not shown), suggesting that the difference in the levels of VP22 tyrosine phosphorylation in transfected cells and virions was not cell type related. Another possibility is that BHV-1 virus infection affects tyrosine kinase activities by augmenting kinase activity or activating different kinases.
FIG. 4.
VP22 is still tyrosine phosphorylated in vY38F mutant virus. VP22 and two degradation peptides are indicated by arrows. The N-terminal peptide (N-term) has a molecular mass of 22 kDa, and the C-terminal peptide has a molecular mass of 14 kDa. The purified BHV-1 and vY38F mutant viruses were analyzed by immunoblotting and probed with anti-VP22 antibody (A), anti-C-terminal-peptide antibody 114235–258 (B), or polyclonal antiphosphotyrosine antibody (C). The two lanes of vY38F represent two isolates. Note that the N terminus was phosphorylated in wild-type virus but that the C terminus was phosphorylated in vY38F. The ∼60- and 50-kDa bands observed in panel B may represent multimers of VP22 and the C-terminal peptide or nonspecific bands. Molecular masses are noted at the left in kilodaltons.
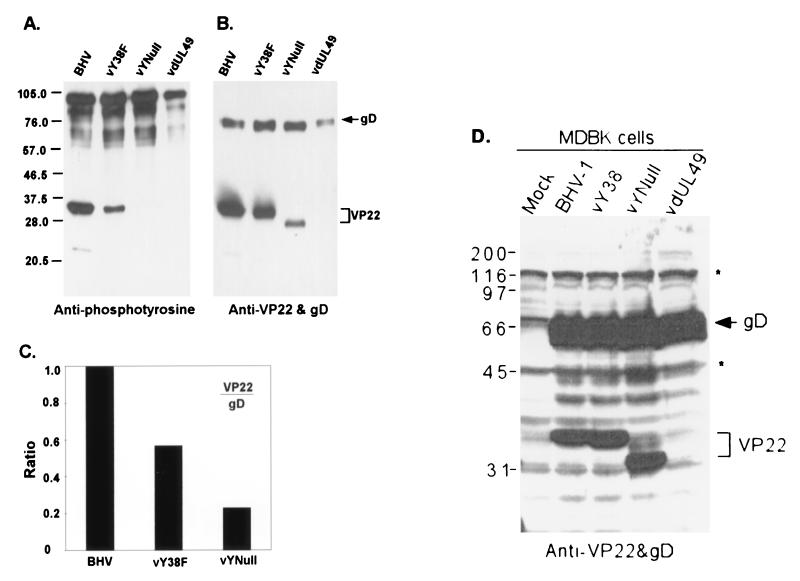
Tyrosine phosphorylation of VP22 correlates with VP22 incorporation into the virion but not in VP22 production from infected cells.
In vY38F mutant virus, VP22 was still tyrosine phosphorylated, but phosphorylation of different sites occurred. To determine the role of VP22 tyrosine phosphorylation during virus replication, replacement of all tyrosines with phenylalanines in VP22 was performed as described above to produce a mutant virus designated vYNull. The mutated sequence was verified by DNA sequencing. When the purified virions of wild-type BHV-1, vY38F, vYNull, and VP22 deletion mutant vdUL49 were analyzed by immunoblotting and probed with antiphosphotyrosine antibody, the tyrosine phosphorylation of VP22 protein in vYNull was indeed abolished (Fig. 5A) as predicted. With the gradual decrease of VP22 tyrosine phosphorylation, the amount of VP22 present in the virions also decreased in vY38F and vYNull mutant viruses (Fig. 5B), suggesting that the incorporation of VP22 correlated with VP22 tyrosine phosphorylation. Alternatively, vYNull virus may not accumulate as much VP22 as BHV or vY38F, irrespective of tyrosine phosphorylation. To examine the role of tyrosine phosphorylation, viral envelope protein glycoprotein D (gD) was chosen as an internal comparison. We reasoned that the amount of a glycoprotein present in the virion was relatively stable and not likely affected by the modification of tegument protein VP22. The ratio of tegument protein VP22 to the envelope protein gD in the virions reflected the relative incorporation of tegument protein VP22 into the virions. Decreased VP22 tyrosine phosphorylation correlated with reduced incorporation of VP22 proteins into virions (Fig. 5C). vY38F incorporated approximately half the amount of VP22 into the virions that was incorporated into the wild type, while vYNull incorporated only 23% of VP22 into the virions.
FIG. 5.
The loss of tyrosine phosphorylation correlates with the decreased incorporation of VP22 into virions but not in VP22 production from infected cells. Purified viruses were analyzed by immunoblotting and probed with polyclonal antiphosphotyrosine (A) or anti-VP22 and anti-gD (B) antibodies. Note the gradually decreased phosphorylation and incorporation of VP22 in vY38F and vYNull mutant viruses. The shift in VP22 in the vYNull lane likely represents the non-tyrosine-phosphorylated form of VP22. (C) The ratio of incorporation of VP22 normalized to the amount of gD was plotted using NIH Image software. (D) MDBK cells infected with BHV-1, vY38, vYNull, or vdUL49 virus or mock infected for 24 h. Cell lysates were collected, and SDS-PAGE was performed. Proteins were analyzed by Western blotting using anti-gD and anti-VP22 antibodies simultaneously. Note in panel D that, regardless of tyrosine mutations (vY38 or vYNull), the amounts of VP22 in the infected cell lysates were similar; in contrast, the amounts of VP22 in the virions (B and C) were reduced depending on the loss of tyrosines or phosphorylated tyrosines, suggesting the inability of the virus to incorporate tyrosine mutant VP22. Molecular masses are noted at the left of the gels in kilodaltons.
In contrast to VP22 incorporation into mutant viruses based on tyrosine phosphorylation, VP22 production during cell infection was not altered based on selected VP22 tyrosine mutations (Fig. 5D). Similar amounts of VP22 protein were produced in cells infected with wild-type, vY38, or vYNull virus. Likewise similar amounts of gD protein were produced in these infected cells.
The tyrosine-to-phenylalanine mutant virus caused only a slight drop in titer in vitro.
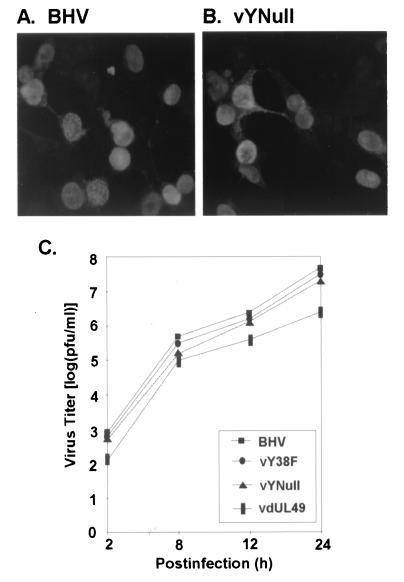
To determine whether the mutation of tyrosines in VP22 would affect the subcellular localization of this protein, MDBK cells were infected with BHV-1 or vYNull mutant virus at an MOI of 0.01 for 18 h, fixed with 4% paraformaldehyde, labeled with anti-VP22 antibodies, and visualized by indirect immunofluorescence. Similar to VP22 in BHV-1 (Fig. 6A), the mutant VP22 in vYNull (Fig. 6B) localized in infected cell nuclei. In transfected D17 cells, mutant VP22 in vYNull also localized in cell nuclei similar to VP22 in the wild type (data not shown). Virus growth was also tested by plaque assay. vY38F and vYNull mutant viruses yielded plaque sizes similar to those of the wild type, while vdUL49 had a significantly decreased plaque size (data not shown). The vY38F and vYNull mutant viruses yielded virus titers comparable to those of the wild type as shown in Fig. 6C. The vdUL49 mutant virus produced a 1-log-unit decrease in virus titer, similar to the observation of others (23).
FIG. 6.
The loss of tyrosine phosphorylation does not affect the subcellular localization of VP22 or mutant virus replication in vitro. MDBK cells were infected at an MOI of 0.01 with BHV-1 (A) or vYNull (B) for 18 h and then fixed with 4% paraformaldehyde, labeled with anti-VP22 antibody, and analyzed by indirect immunofluorescence microscopy. Both forms of VP22 localized in cell nuclei. (C) Levels of virus growth in vitro were compared using a single-step growth curve. The dephosphorylation of VP22 resulted in only a slight decrease of virus titer.
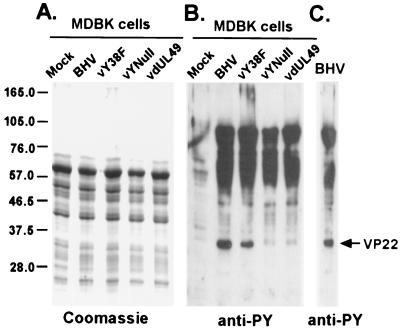
Major tyrosine-phosphorylated proteins in BHV-1-infected MDBK cells are likely of viral origin.
The difference in the levels of tyrosine phosphorylation of the VP22 Y38F protein between transfected cells and infected cells suggests that there might be a difference in the kinase activities responsible for VP22 tyrosine phosphorylation. To gain insight into virus-induced tyrosine phosphorylation, the levels of tyrosine phosphorylation in total cell lysates from infected and mock-infected cells were compared. MDBK cells were infected with BHV-1 or VP22 mutant virus at an MOI of 1 for 18 h. Then, cell lysates were analyzed by SDS-PAGE by using Coomassie blue staining for total proteins and immunoblot analysis with an antiphosphotyrosine antibody to detect tyrosine phosphorylation. Following 18 h of BHV-1 infection, total protein levels were comparable in both infected and mock-infected cell lysates (Fig. 7A). No additional bands were detected in the infected cell lysates compared to those in mock-infected cell lysates by Coomassie blue staining, suggesting that the amount of viral proteins did not constitute the major species of proteins in the total cell lysates. However, when the same protein samples were analyzed by antiphosphotyrosine blotting, a dramatic difference was observed between the levels of tyrosine phosphorylation in infected and uninfected cells. In uninfected MDBK cells, the cellular protein had minimal tyrosine phosphorylation without additional stimulation (Fig. 7B). Interestingly, the tyrosine phosphorylation level was significantly higher on some proteins in the BHV-1-infected cells (Fig. 7B). When compared with purified BHV-1 virus (Fig. 7C), the majority of the tyrosine-phosphorylated protein bands in BHV-1-infected cells (Fig. 7B) had patterns and molecular masses very similar to those of tyrosine-phosphorylated virus structural proteins. Most prominent were protein bands at 90, 50 to 70, and 32 kDa (VP22). These data suggest that, during BHV-1 infection, increased tyrosine kinase activities were induced and that viral proteins were preferentially phosphorylated compared to cellular proteins.
FIG. 7.
Comparison of levels of tyrosine phosphorylation in infected and uninfected cells. Equal amounts of protein from total cell lysates of mock-infected and virus-infected MDBK cells were analyzed by SDS-PAGE and Coomassie blue staining (A) or immunoblotted and probed with polyclonal antiphosphotyrosine antibody (B). Note that, in infected cells (B), tyrosine phosphorylation increased and that the phosphorylated protein bands have patterns and molecular masses similar to those of the purified BHV-1 phosphorylated structural proteins (C). Molecular mass markers are indicated at the left in kilodaltons.
DISCUSSION
To determine the role of tyrosine phosphorylation in BHV VP22, we have used site-directed mutagenesis to substitute phenylalanine for all the tyrosine sites. Five plasmids containing selected VP22 tyrosine mutations and two viruses containing VP22 with the Y38F mutation or a mutation of all VP22 tyrosines were constructed. Our results demonstrate that several viral structural proteins are tyrosine phosphorylated; one is tegument protein VP22 (Fig. 1). In transfected cells, tyrosine 38 is a major phosphorylation site (Fig. 4A). However, in the VP22 tyrosine 38-to-phenylalanine mutant virus, mutant VP22 remains tyrosine phosphorylated but at a reduced level (Fig. 4B). In vYNull mutant virus, where all tyrosines were mutated to phenylalanines, the loss of VP22 tyrosine phosphorylation correlated with reduced VP22 incorporation into virions (Fig. 5).
Why VP22 tyrosine phosphorylation correlates with the amount of VP22 incorporated into virions is uncertain. However, the decrease in mutant VP22 incorporation does not result from decreased VP22 availability during virus replication (Fig. 5D). Conceivably, tyrosine phosphorylation may change the conformation of VP22 or provide a tag for molecular interaction (29). However, the possibility that the mutated tyrosines or tyrosine-containing motifs, such as the YXXL motif involving tyrosine 38, are important for the structure or function of VP22 cannot be ruled out, since tyrosine and phenylalanine are structurally similar but not identical. Nonetheless, since VP22 is tyrosine phosphorylated during infection (Fig. 1) (34), our data support the concept that tyrosine phosphorylation of VP22 plays a role in determining the incorporation of VP22 into the virion.
The tegument is an amorphous and unique structure of herpesviruses. Most tegument proteins are still poorly defined, and little is known regarding tegument structure and assembly. Also not known is why phosphorylation of a tegument protein affects its abundance in the virions. A tyrosine-to-phenylalanine substitution mutation is not likely to alter the transcription or translation of VP22. Similar to wild-type BHV-1 and HSV (10), where VP22 protein localizes in the cell nucleus, the VP22 tyrosine-to-phenylalanine mutant also localizes in the cell nucleus during infection or transfection. Therefore, phosphorylation does not affect the cellular localization of VP22 but does influence the incorporation of VP22 into virions. The abundance of VP22 in HSV-1 virions can be regulated by the VP22 expression levels in the infected cells (21). In contrast, incorporation of HSV-1 tegument protein UL37 into the virions is tightly controlled (25).
Interestingly, in virus-infected cells, major tyrosine-phosphorylated proteins have molecular masses similar to those of the tyrosine-phosphorylated BHV-1 structural proteins. In BHV-1-infected cells, most cellular protein synthesis is shut off; thus, phosphorylation occurs predominantly on viral proteins. To date, no virally encoded tyrosine kinase has been reported for herpesviruses, but BHV-1 gE is tyrosine phosphorylated during viral replication and viral titer is proportional to phosphorylation of this envelope protein (39). However, the exact role that tyrosine phosphorylation plays during herpesvirus infection remains to be determined. It is noteworthy that, although the incorporation of VP22 into vYNull mutant virus is severely impaired, vYNull replicates in a manner similar to that of wild-type virus, indicating that VP22 is dispensable for virus replication in vitro (23). Nonetheless, the VP22 deletion mutant virus is avirulent and asymptomatic in infected cattle (22), suggesting that VP22 is an important virulence factor in vivo.
ACKNOWLEDGMENTS
We thank Lorne Babiuk for providing the vdUL49 mutant virus and the 114 peptide antibody.
This work was supported by USDA grant 99-35204-7933 and NIH grant R01GM/AI 60986.
REFERENCES
- 1.Baird A, Florkiewicz R Z, Maher P A, Kaner R J, Hajjar D P. Mediation of virion penetration into vascular cells by association of basic fibroblast growth factor with herpes simplex virus type 1. Nature. 1990;348:344–346. doi: 10.1038/348344a0. [DOI] [PubMed] [Google Scholar]
- 2.Blaho J A, Mitchell C, Roizman B. An amino acid sequence shared by the herpes simplex virus 1 alpha regulatory proteins 0, 4, 22, and 27 predicts the nucleotidylylation of the UL21, UL31, UL47, and UL49 gene products. J Biol Chem. 1994;269:17401–17410. [PubMed] [Google Scholar]
- 3.Blaho J A, Zong C S, Mortimer K A. Tyrosine phosphorylation of the herpes simplex virus type 1 regulatory protein ICP22 and a cellular protein which shares antigenic determinants with ICP22. J Virol. 1997;71:9828–9832. doi: 10.1128/jvi.71.12.9828-9832.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brewis N, Phelan A, Webb J, Drew J, Elliott G, O'Hare P. Evaluation of VP22 spread in tissue culture. J Virol. 2000;74:1051–1056. doi: 10.1128/jvi.74.2.1051-1056.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coulter L J, Moss H W, Lang J, McGeoch D J. A mutant of herpes simplex virus type 1 in which the UL13 protein kinase gene is disrupted. J Gen Virol. 1993;74:387–395. doi: 10.1099/0022-1317-74-3-387. [DOI] [PubMed] [Google Scholar]
- 6.Dilber M S, Phelan A, Aints A, Mohamed A J, Elliott G, Smith C I, O'Hare P. Intercellular delivery of thymidine kinase prodrug activating enzyme by the herpes simplex virus protein VP22. Gene Ther. 1999;6:12–21. doi: 10.1038/sj.gt.3300838. [DOI] [PubMed] [Google Scholar]
- 7.Elliott G, Mouzakitis G, O'Hare P. VP16 interacts via its activation domain with VP22, a tegument protein of herpes simplex virus, and is relocated to a novel macromolecular assembly in coexpressing cells. J Virol. 1995;69:7932–7941. doi: 10.1128/jvi.69.12.7932-7941.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott G, O'Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 9.Elliott G, O'Hare P. Herpes simplex virus type 1 tegument protein VP22 induces the stabilization and hyperacetylation of microtubules. J Virol. 1998;72:6448–6455. doi: 10.1128/jvi.72.8.6448-6455.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott G, O'Hare P. Cytoplasm-to-nucleus translocation of a herpesvirus tegument protein during cell division. J Virol. 2000;74:2131–2141. doi: 10.1128/jvi.74.5.2131-2141.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott G, O'Reilly D, O'Hare P. Phosphorylation of the herpes simplex virus type 1 tegument protein VP22. Virology. 1996;226:140–145. doi: 10.1006/viro.1996.0638. [DOI] [PubMed] [Google Scholar]
- 12.Elliott G, O'Reilly D, O'Hare P. Identification of phosphorylation sites within the herpes simplex virus tegument protein VP22. J Virol. 1999;73:6203–6206. doi: 10.1128/jvi.73.7.6203-6206.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Favoreel H W, Nauwynck H J, Pensaert M B. Role of the cytoplasmic tail of gE in antibody-induced redistribution of viral glycoproteins expressed on pseudorabies-virus-infected cells. Virology. 1999;259:141–147. doi: 10.1006/viro.1999.9749. [DOI] [PubMed] [Google Scholar]
- 14.Granzow H, Weiland F, Jons A, Klupp B G, Karger A, Mettenleiter T C. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J Virol. 1997;71:2072–2082. doi: 10.1128/jvi.71.3.2072-2082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grose C. Glycoproteins encoded by varicella-zoster virus: biosynthesis, phosphorylation, and intracellular trafficking. Annu Rev Microbiol. 1990;44:59–80. doi: 10.1146/annurev.mi.44.100190.000423. [DOI] [PubMed] [Google Scholar]
- 16.Harms J S, Ren X, Oliveira S C, Splitter G A. Distinctions between bovine herpesvirus 1 and herpes simplex virus type 1 VP22 tegument protein subcellular associations. J Virol. 2000;74:3301–3312. doi: 10.1128/jvi.74.7.3301-3312.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 18.Kasamatsu H, Nakanishi A. How do animal DNA viruses get to the nucleus? Annu Rev Microbiol. 1998;52:627–686. doi: 10.1146/annurev.micro.52.1.627. [DOI] [PubMed] [Google Scholar]
- 19.Knopf K W, Kaerner H C. Virus-specific basic phosphoproteins associated with herpes simplex virus type a (HSV-1) particles and the chromatin of HSV-1-infected cells. J Gen Virol. 1980;46:405–414. doi: 10.1099/0022-1317-46-2-405. [DOI] [PubMed] [Google Scholar]
- 20.Lemaster S, Roizman B. Herpes simplex virus phosphoproteins. II. Characterization of the virion protein kinase and of the polypeptides phosphorylated in the virion. J Virol. 1980;35:798–811. doi: 10.1128/jvi.35.3.798-811.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leslie J, Rixon F J, McLauchlan J. Overexpression of the herpes simplex virus type 1 tegument protein VP22 increases its incorporation into virus particles. Virology. 1996;220:60–68. doi: 10.1006/viro.1996.0286. [DOI] [PubMed] [Google Scholar]
- 22.Liang X, Chow B, Babiuk L A. Study of immunogenicity and virulence of bovine herpesvirus 1 mutants deficient in the UL49 homolog, UL49.5 homolog and dUTPase genes in cattle. Vaccine. 1997;15:1057–1064. doi: 10.1016/s0264-410x(97)00008-x. [DOI] [PubMed] [Google Scholar]
- 23.Liang X, Chow B, Li Y, Raggo C, Yoo D, Attah-Poku S, Babiuk L A. Characterization of bovine herpesvirus 1 UL49 homolog gene and product: bovine herpesvirus 1 UL49 homolog is dispensable for virus growth. J Virol. 1995;69:3863–3867. doi: 10.1128/jvi.69.6.3863-3867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayfield J E, Good P J, VanOort H J, Campbell A R, Reed D E. Cloning and cleavage site mapping of DNA from bovine herpesvirus 1 (Cooper strain) J Virol. 1983;47:259–264. doi: 10.1128/jvi.47.1.259-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLauchlan J. The abundance of the herpes simplex virus type 1 UL37 tegument protein in virus particles is closely controlled. J Gen Virol. 1997;78:189–194. doi: 10.1099/0022-1317-78-1-189. [DOI] [PubMed] [Google Scholar]
- 26.McLauchlan J, Rixon F J. Characterization of enveloped tegument structures (L particles) produced by alphaherpesviruses: integrity of the tegument does not depend on the presence of capsid or envelope. J Gen Virol. 1992;73:269–276. doi: 10.1099/0022-1317-73-2-269. [DOI] [PubMed] [Google Scholar]
- 27.Meredith D M, Lindsay J A, Halliburton I W, Whittaker G R. Post-translational modification of the tegument proteins (VP13 and VP14) of herpes simplex virus type 1 by glycosylation and phosphorylation. J Gen Virol. 1991;72:2771–2775. doi: 10.1099/0022-1317-72-11-2771. [DOI] [PubMed] [Google Scholar]
- 28.Morrison E E, Stevenson A J, Wang Y F, Meredith D M. Differences in the intracellular localization and fate of herpes simplex virus tegument proteins early in the infection of Vero cells. J Gen Virol. 1998;79:2517–2528. doi: 10.1099/0022-1317-79-10-2517. [DOI] [PubMed] [Google Scholar]
- 29.Morrison E E, Wang Y F, Meredith D M. Phosphorylation of structural components promotes dissociation of the herpes simplex virus type 1 tegument. J Virol. 1998;72:7108–7114. doi: 10.1128/jvi.72.9.7108-7114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson J K, Bishop G A, Grose C. Varicella-zoster virus Fc receptor gE glycoprotein: serine/threonine and tyrosine phosphorylation of monomeric and dimeric forms. J Virol. 1997;71:110–119. doi: 10.1128/jvi.71.1.110-119.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phelan A, Elliott G, O'Hare P. Intercellular delivery of functional p53 by the herpesvirus protein VP22. Nat Biotechnol. 1998;16:440–443. doi: 10.1038/nbt0598-440. [DOI] [PubMed] [Google Scholar]
- 32.Pinard M F, Simard R, Bibor-Hardy V. DNA-binding proteins of herpes simplex virus type 1-infected BHK cell nuclear matrices. J Gen Virol. 1987;68:727–735. doi: 10.1099/0022-1317-68-3-727. [DOI] [PubMed] [Google Scholar]
- 33.Pomeranz L E, Blaho J A. Modified VP22 localizes to the cell nucleus during synchronized herpes simplex virus type 1 infection. J Virol. 1999;73:6769–6781. doi: 10.1128/jvi.73.8.6769-6781.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pomeranz L E, Blaho J A. Assembly of infectious herpes simplex virus type 1 virions in the absence of full-length VP22. J Virol. 2000;74:10041–10054. doi: 10.1128/jvi.74.21.10041-10054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qie L, Marcellino D, Herold B C. Herpes simplex virus entry is associated with tyrosine phosphorylation of cellular proteins. Virology. 1999;256:220–227. doi: 10.1006/viro.1999.9673. [DOI] [PubMed] [Google Scholar]
- 36.Rixon F J, Addison C, McLauchlan J. Assembly of enveloped tegument structures (L particles) can occur independently of virion maturation in herpes simplex virus type 1-infected cells. J Gen Virol. 1992;73:277–284. doi: 10.1099/0022-1317-73-2-277. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez V, Angeletti P C, Engler J A, Britt W J. Localization of human cytomegalovirus structural proteins to the nuclear matrix of infected human fibroblasts. J Virol. 1998;72:3321–3329. doi: 10.1128/jvi.72.4.3321-3329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez V, Greis K D, Sztul E, Britt W J. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J Virol. 2000;74:975–986. doi: 10.1128/jvi.74.2.975-986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaw A M, Braun L, Frew T, Hurley D J, Rowland R R, Chase C C. A role for bovine herpesvirus 1 (BHV-1) glycoprotein E (gE) tyrosine phosphorylation in replication of BHV-1 wild-type virus but not BHV-1 gE deletion mutant virus. Virology. 2000;268:159–166. doi: 10.1006/viro.1999.0164. [DOI] [PubMed] [Google Scholar]
- 40.Smibert C A, Popova B, Xiao P, Capone J P, Smiley J R. Herpes simplex virus VP16 forms a complex with the virion host shutoff protein vhs. J Virol. 1994;68:2339–2346. doi: 10.1128/jvi.68.4.2339-2346.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steven A C, Spear P G. Herpesvrus capsid assembly and envelopment. In: Chiu W, Burnet R M, Garcea R L, editors. Structural biology of viruses. New York, N.Y: Oxford University Press; 1997. pp. 312–351. [Google Scholar]
- 42.Trus B L, Gibson W, Cheng N, Steven A C. Capsid structure of simian cytomegalovirus from cryoelectron microscopy: evidence for tegument attachment sites. J Virol. 1999;73:2181–2192. doi: 10.1128/jvi.73.3.2181-2192.1999. . (Erratum, 73:4530.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward P L, Ogle W O, Roizman B. Assemblons: nuclear structures defined by aggregation of immature capsids and some tegument proteins of herpes simplex virus 1. J Virol. 1996;70:4623–4631. doi: 10.1128/jvi.70.7.4623-4631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yura Y, Kusaka J, Kondo Y, Tsujimoto H, Yoshida H, Sato M. Inhibitory effect of tyrphostin on the replication of herpes simplex virus type 1. Arch Virol. 1995;140:1181–1194. doi: 10.1007/BF01322745. [DOI] [PubMed] [Google Scholar]
- 45.Yura Y, Kusaka J, Tsujimoto H, Yoshioka Y, Yoshida H, Sato M. Effects of protein tyrosine kinase inhibitors on the replication of herpes simplex virus and the phosphorylation of viral proteins. Intervirology. 1997;40:7–14. doi: 10.1159/000150515. [DOI] [PubMed] [Google Scholar]
- 46.Yura Y, Yoshida H, Sato M. Inhibition of herpes simplex virus replication by genistein, an inhibitor of protein-tyrosine kinase. Arch Virol. 1993;132:451–461. doi: 10.1007/BF01309554. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Z H, Chen D H, Jakana J, Rixon F J, Chiu W. Visualization of tegument-capsid interactions and DNA in intact herpes simplex virus type 1 virions. J Virol. 1999;73:3210–3218. doi: 10.1128/jvi.73.4.3210-3218.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Z H, Dougherty M, Jakana J, He J, Rixon F J, Chiu W. Seeing the herpesvirus capsid at 8.5 A. Science. 2000;288:877–880. doi: 10.1126/science.288.5467.877. [DOI] [PubMed] [Google Scholar]