Abstract
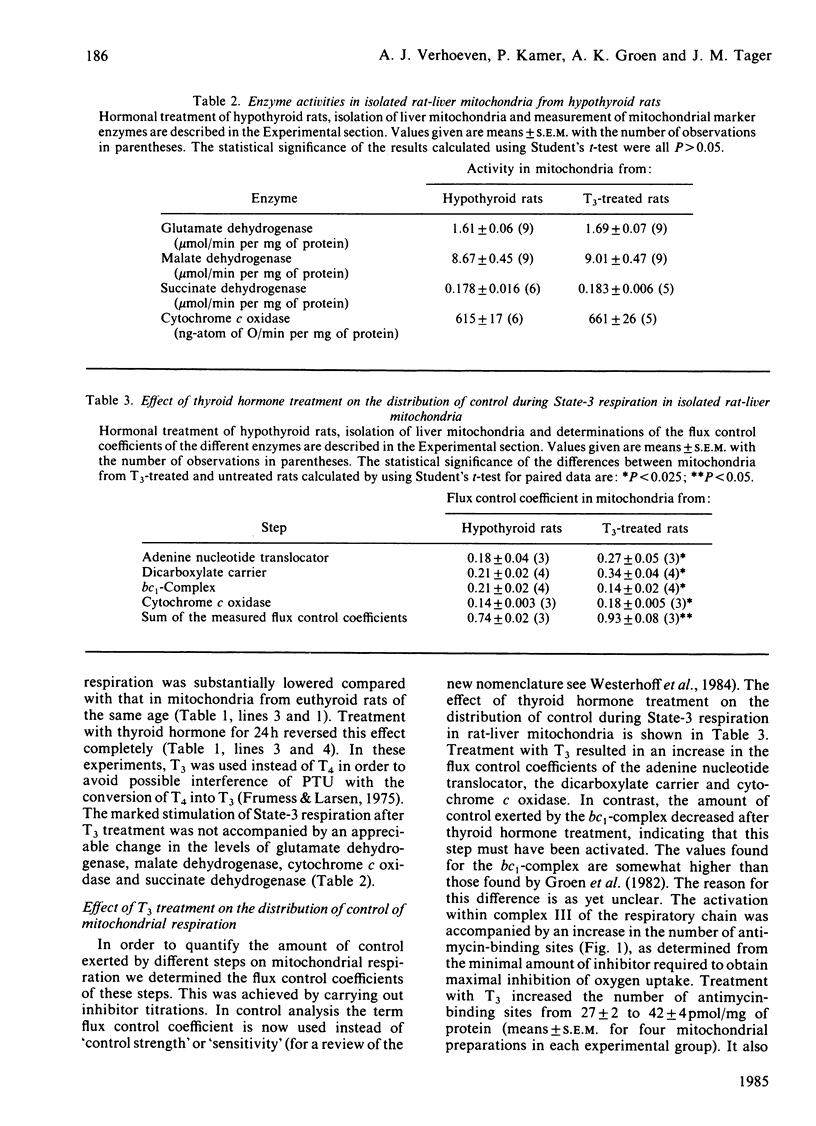
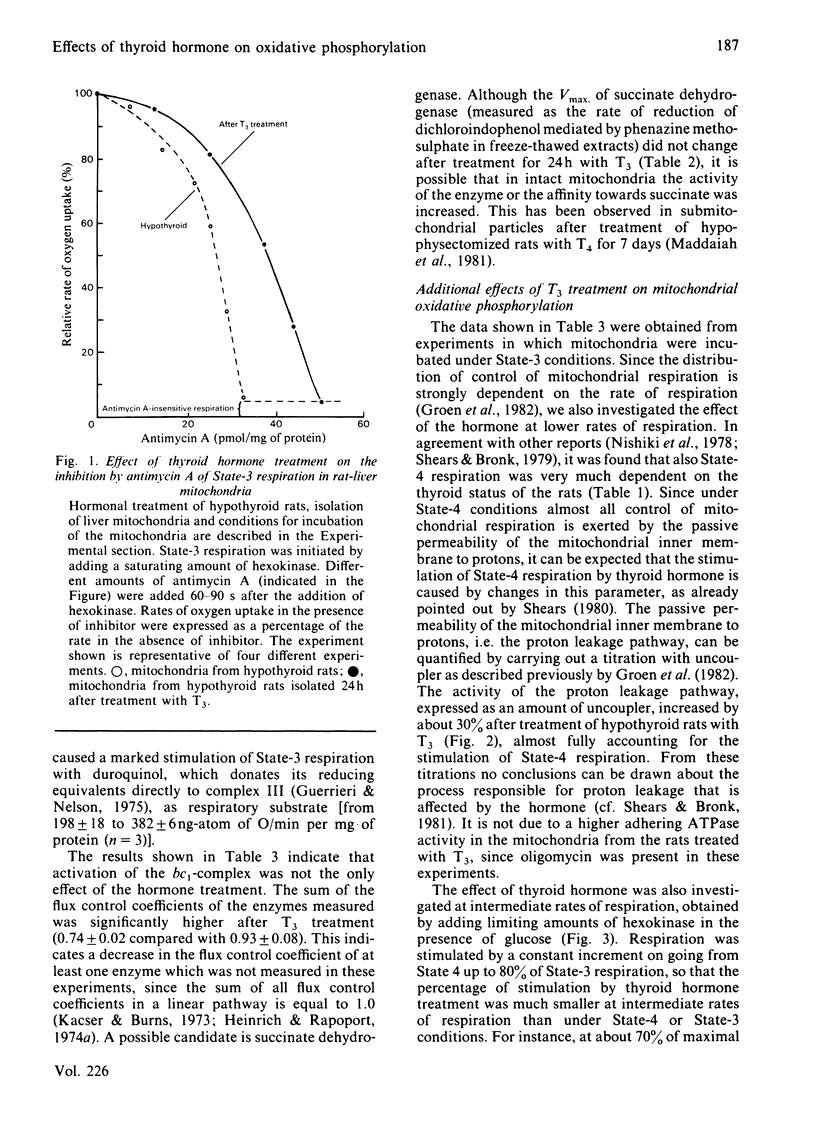
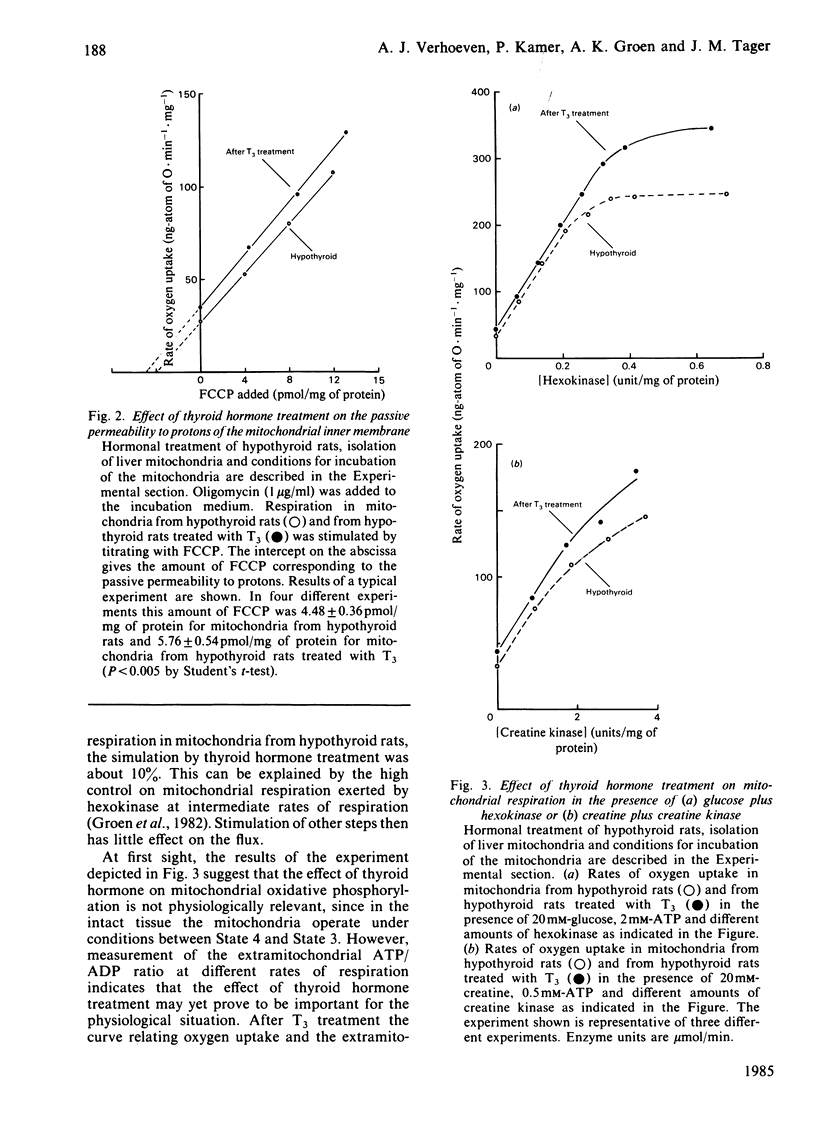
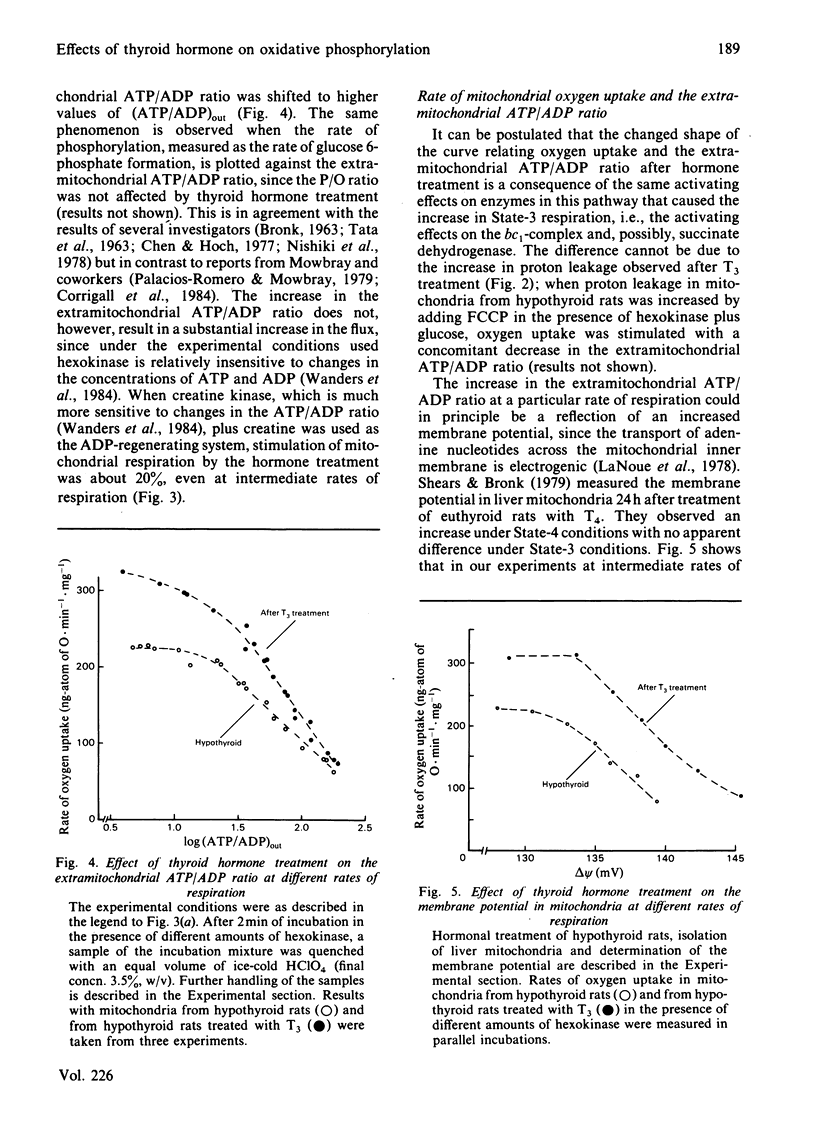
In order to locate sites of action of thyroid hormone on mitochondrial oxidative phosphorylation we have used an experimental application of control analysis as previously described [Groen, Wanders, Westerhoff, Van der Meer & Tager (1982) J. Biol. Chem. 257, 2754-2757]. Rat-liver mitochondria were isolated from hypothyroid rats or from hypothyroid rats 24 h after treatment with a single dose of 3,3',5-triiodothyronine (T3). The amount of control exerted by four different steps on State-3 respiration with succinate as respiratory substrate was quantified by using specific inhibitors. The hormone treatment resulted in an increase in the flux control coefficient of the adenine nucleotide translocator, the dicarboxylate carrier and cytochrome c oxidase and a decrease in the flux control coefficient of the bc1-complex. The results of this analysis indicate that thyroid hormone treatment results in an activation of the bc1-complex and of at least one other enzyme, possibly succinate dehydrogenase. Measurement of the extramitochondrial ATP/ADP ratio at different rates of respiration (induced by addition of different amounts of hexokinase in the presence of glucose and ATP) showed that the adenine nucleotide translocator operates at a higher (ATP/ADP)out after T3 treatment, which supports previous reports on stimulation of this step by thyroid hormone.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
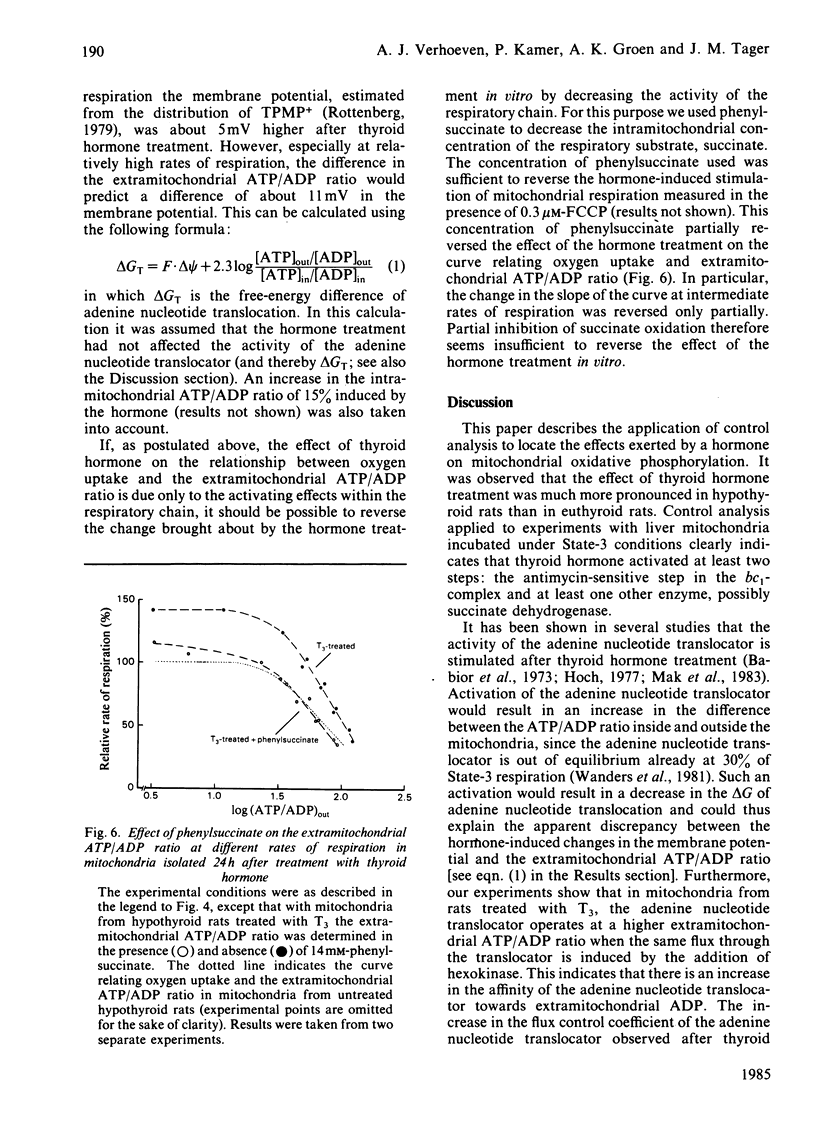
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- Allan E. H., Chisholm A. B., Titheradge M. A. The stimulation of hepatic oxidative phosphorylation following dexamethasone treatment of rats. Biochim Biophys Acta. 1983 Oct 31;725(1):71–76. doi: 10.1016/0005-2728(83)90225-6. [DOI] [PubMed] [Google Scholar]
- BRONK J. R. THYROID HORMONES: CONTROL OF TERMINAL OXIDATION. Science. 1963 Aug 30;141(3583):816–818. doi: 10.1126/science.141.3583.816. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Creagan S., Ingbar S. H., Kipnes R. S. Stimulation of mitochondrial adenosine diphosphate uptake by thyroid hormones. Proc Natl Acad Sci U S A. 1973 Jan;70(1):98–102. doi: 10.1073/pnas.70.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berden J. A., Slater E. C. The allosteric binding of antimycin to cytochrome b in the mitochondrial membrane. Biochim Biophys Acta. 1972 Feb 28;256(2):199–215. doi: 10.1016/0005-2728(72)90053-9. [DOI] [PubMed] [Google Scholar]
- Bronk J. R. Thyroid hormone: effects on electron transport. Science. 1966 Aug 5;153(3736):638–639. doi: 10.1126/science.153.3736.638. [DOI] [PubMed] [Google Scholar]
- Bryla J., Harris E. J., Plumb J. A. The stimulatory effect of glucagon and dibutyryl cyclic AMP on ureogenesis and gluconeogenesis in relation to the mitochondrial ATP content. FEBS Lett. 1977 Aug 15;80(2):443–448. doi: 10.1016/0014-5793(77)80494-8. [DOI] [PubMed] [Google Scholar]
- CLELAND K. W., SLATER E. C. Respiratory granules of heart muscle. Biochem J. 1953 Mar;53(4):547–556. doi: 10.1042/bj0530547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall J., Tselentis B. S., Mowbray J. The efficiency of oxidative phosphorylation and the rapid control by thyroid hormone of nicotinamide nucleotide reduction and transhydrogenation in intact rat liver mitochondria. Eur J Biochem. 1984 Jun 1;141(2):435–440. doi: 10.1111/j.1432-1033.1984.tb08210.x. [DOI] [PubMed] [Google Scholar]
- Frumess R. D., Larsen P. R. Correlation of serum triiodothyronine (T3) and thyroxine (T4) with biologic effects of thyroid hormone replacement in propylthiouracil-treated rats. Metabolism. 1975 Apr;24(4):547–554. doi: 10.1016/0026-0495(75)90079-7. [DOI] [PubMed] [Google Scholar]
- Groen A. K., Wanders R. J., Westerhoff H. V., van der Meer R., Tager J. M. Quantification of the contribution of various steps to the control of mitochondrial respiration. J Biol Chem. 1982 Mar 25;257(6):2754–2757. [PubMed] [Google Scholar]
- Guerrieri F., Nelson B. D. Studies on the characteristics of a proton pump in phospholipid vesicles inlayed with purified complex III from beef heart mitochondria. FEBS Lett. 1975 Jul 1;54(3):339–342. doi: 10.1016/0014-5793(75)80935-5. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P. Stimulation of pyruvate transport in metabolizing mitochondria through changes in the transmembrane pH gradient induced by glucagon treatment of rats. Biochem J. 1978 Jun 15;172(3):389–398. doi: 10.1042/bj1720389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P. The nature of the stimulation of the respiratory chain of rat liver mitochondria by glucagon pretreatment of animals. Biochem J. 1982 Apr 15;204(1):37–47. doi: 10.1042/bj2040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P. The nature of the stimulation of the respiratory chain of rat liver mitochondria by glucagon pretreatment of animals. Biochem J. 1982 Apr 15;204(1):37–47. doi: 10.1042/bj2040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich R., Rapoport T. A. A linear steady-state treatment of enzymatic chains. Critique of the crossover theorem and a general procedure to identify interaction sites with an effector. Eur J Biochem. 1974 Feb 15;42(1):97–105. doi: 10.1111/j.1432-1033.1974.tb03319.x. [DOI] [PubMed] [Google Scholar]
- Heinrich R., Rapoport T. A. A linear steady-state treatment of enzymatic chains. General properties, control and effector strength. Eur J Biochem. 1974 Feb 15;42(1):89–95. doi: 10.1111/j.1432-1033.1974.tb03318.x. [DOI] [PubMed] [Google Scholar]
- Hinkle P. C., Yu M. L. The phosphorus/oxygen ratio of mitochondrial oxidative phosphorylation. J Biol Chem. 1979 Apr 10;254(7):2450–2455. [PubMed] [Google Scholar]
- Hoch F. L. Adenine nucleotide translocation in liver mitochondria of hypothyroid rats. Arch Biochem Biophys. 1977 Jan 30;178(2):535–545. doi: 10.1016/0003-9861(77)90224-7. [DOI] [PubMed] [Google Scholar]
- Hoch F. L. Thyroid control over biomembranes. VII. Heart muscle mitochondria from L-triiodothyronine-injected rats. J Mol Cell Cardiol. 1982 Feb;14(2):81–90. doi: 10.1016/0022-2828(82)90196-1. [DOI] [PubMed] [Google Scholar]
- Hulbert A. J., Augee M. L., Raison J. K. The influence of thyroid hormones on the structure and function of mitochondrial membranes. Biochim Biophys Acta. 1976 Dec 2;455(2):597–601. doi: 10.1016/0005-2736(76)90328-x. [DOI] [PubMed] [Google Scholar]
- Hulbert A. J. The thyroid hormones: a thesis concerning their action. J Theor Biol. 1978 Jul 6;73(1):81–100. doi: 10.1016/0022-5193(78)90181-9. [DOI] [PubMed] [Google Scholar]
- Ida Chen Y. D., Hoch F. L. Thryoid control over biomembranes. Rat liver mitochondrial inner membranes. Arch Biochem Biophys. 1977 Jun;181(2):470–483. doi: 10.1016/0003-9861(77)90253-3. [DOI] [PubMed] [Google Scholar]
- Jakovcic S., Swift H. H., Gross N. J., Rabinowitz M. Biochemical and stereological analysis of rat liver mitochondria in different thyroid states. J Cell Biol. 1978 Jun;77(3):887–901. doi: 10.1083/jcb.77.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacser H., Burns J. A. The control of flux. Symp Soc Exp Biol. 1973;27:65–104. [PubMed] [Google Scholar]
- LaNoue K., Mizani S. M., Klingenberg M. Electrical imbalance of adenine nucleotide transport across the mitochondrial membrane. J Biol Chem. 1978 Jan 10;253(1):191–198. [PubMed] [Google Scholar]
- Laker M. E., Mayes P. A. Effect of hyperthyroidism and hypothyroidism on lipid and carbohydrate metabolism of the perfused rat liver. Biochem J. 1981 Apr 15;196(1):247–255. doi: 10.1042/bj1960247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddaiah V. T., Clejan S., Palekar A. G., Collipp P. J. Hormones and liver mitochondria: effects of growth hormone and thyroxine on respiration, fluorescence of 1-anilino-8-naphthalene sulfonate and enzyme activities of complex I and II of submitochondrial particles. Arch Biochem Biophys. 1981 Sep;210(2):666–677. doi: 10.1016/0003-9861(81)90234-4. [DOI] [PubMed] [Google Scholar]
- Mak I. T., Shrago E., Elson C. E. Effect of thyroidectomy on the kinetics of ADP-ATP translocation in liver mitochondria. Arch Biochem Biophys. 1983 Oct 1;226(1):317–323. doi: 10.1016/0003-9861(83)90298-9. [DOI] [PubMed] [Google Scholar]
- Martin B. R., Denton R. M. The intracellular localization of enzymes in white-adipose-tissue fat-cells and permeability properties of fat-cell mitochondria. Transfer of acetyl units and reducing power between mitochondria and cytoplasm. Biochem J. 1970 May;117(5):861–877. doi: 10.1042/bj1170861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiki K., Erecińska M., Wilson D. F., Cooper S. Evaluation of oxidative phosphorylation in hearts from euthyroid, hypothyroid, and hyperthyroid rats. Am J Physiol. 1978 Nov;235(5):C212–C219. doi: 10.1152/ajpcell.1978.235.5.C212. [DOI] [PubMed] [Google Scholar]
- Palacios-Romero R., Mowbray J. Evidence for the rapid direct control both in vivo and in vitro of the efficiency of oxidative phosphorylation by 3,5,3'-tri-iodo-L-thyronine in rats. Biochem J. 1979 Dec 15;184(3):527–538. doi: 10.1042/bj1840527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROODYN D. B., FREEMAN K. B., TATA J. R. THE STIMULATION BY TREATMENT IN VIVO WITH TRI-IODOTHYRONINE OF AMINO ACID INCORPORATION INTO PROTEIN BY ISOLATED RAT-LIVER MITOCHONDRIA. Biochem J. 1965 Mar;94:628–641. doi: 10.1042/bj0940628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Shears S. B., Bronk J. R. The effects of thyroxine treatment, in vivo and in vitro, on Ca2+ efflux from rat liver mitochondria. FEBS Lett. 1981 Apr 6;126(1):9–12. doi: 10.1016/0014-5793(81)81020-4. [DOI] [PubMed] [Google Scholar]
- Shears S. B., Bronk J. R. The influence of thyroxine administered in vivo on the transmembrane protonic electrochemical potential difference in rat liver mitochondria. Biochem J. 1979 Feb 15;178(2):505–507. doi: 10.1042/bj1780505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears S. B. The thyroid gland and the liver mitochondrial protonic electrochemical potential difference: a novel hormone action? J Theor Biol. 1980 Jan 7;82(1):1–13. doi: 10.1016/0022-5193(80)90087-9. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Wieland O. H. Glucagon-induced stimulation of 2-oxoglutarate metabolism in mitochondria from rat liver. FEBS Lett. 1978 Sep 15;93(2):301–306. doi: 10.1016/0014-5793(78)81126-0. [DOI] [PubMed] [Google Scholar]
- TATA J. R., ERNSTER L., LINDBERG O., ARRHENIUS E., PEDERSEN S., HEDMAN R. The action of thyroid hormones at the cell level. Biochem J. 1963 Mar;86:408–428. doi: 10.1042/bj0860408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TATA J. R., ERNSTER L., LINDBERG O. Control of basal metabolic rate by thyroid hormones and cellular function. Nature. 1962 Mar 17;193:1058–1060. doi: 10.1038/1931058a0. [DOI] [PubMed] [Google Scholar]
- Titheradge M. A., Coore H. G. Hormonal regulation of liver mitochondrial pyruvate carrier in relation to gluconeogenesis and lipogenesis. FEBS Lett. 1976 Nov 15;72(1):73–78. doi: 10.1016/0014-5793(76)80901-5. [DOI] [PubMed] [Google Scholar]
- Titheradge M. A., Haynes R. C., Jr Glucagon treatment stimulates the oxidation of durohydroquinone by rat liver mitochondria. FEBS Lett. 1979 Oct 15;106(2):330–334. doi: 10.1016/0014-5793(79)80526-8. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., Groen A. K., Meijer A. J., Tager J. M. Determination of the free-energy difference of the adenine nucleotide translocator reaction in rat-liver mitochondria using intra- and extramitochondrial ATP-utilizing reactions. FEBS Lett. 1981 Sep 28;132(2):201–206. doi: 10.1016/0014-5793(81)81160-x. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., Groen A. K., Van Roermund C. W., Tager J. M. Factors determining the relative contribution of the adenine-nucleotide translocator and the ADP-regenerating system to the control of oxidative phosphorylation in isolated rat-liver mitochondria. Eur J Biochem. 1984 Jul 16;142(2):417–424. doi: 10.1111/j.1432-1033.1984.tb08303.x. [DOI] [PubMed] [Google Scholar]
- Westerhoff H. V., Groen A. K., Wanders R. J. Modern theories of metabolic control and their applications (review). Biosci Rep. 1984 Jan;4(1):1–22. doi: 10.1007/BF01120819. [DOI] [PubMed] [Google Scholar]
- Yamazaki R. K. Glucagon stimulation of mitochondrial respiration. J Biol Chem. 1975 Oct 10;250(19):7924–7930. [PubMed] [Google Scholar]


