Abstract
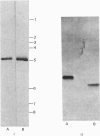
A procedure was developed for the purification of shikimate dehydrogenase from Escherichia coli. Homogeneous enzyme with specific activity 1100 units/mg of protein was obtained in 21% overall yield. The subunit Mr estimated by polyacrylamide-gel electrophoresis in the presence of sodium dodecyl sulphate was 32 000. The native Mr, estimated by gel-permeation chromatography on a TSK G2000SW column, was also 32 000. E. coli shikimate dehydrogenase is therefore a monomeric NADP-linked dehydrogenase.
Full text
PDF

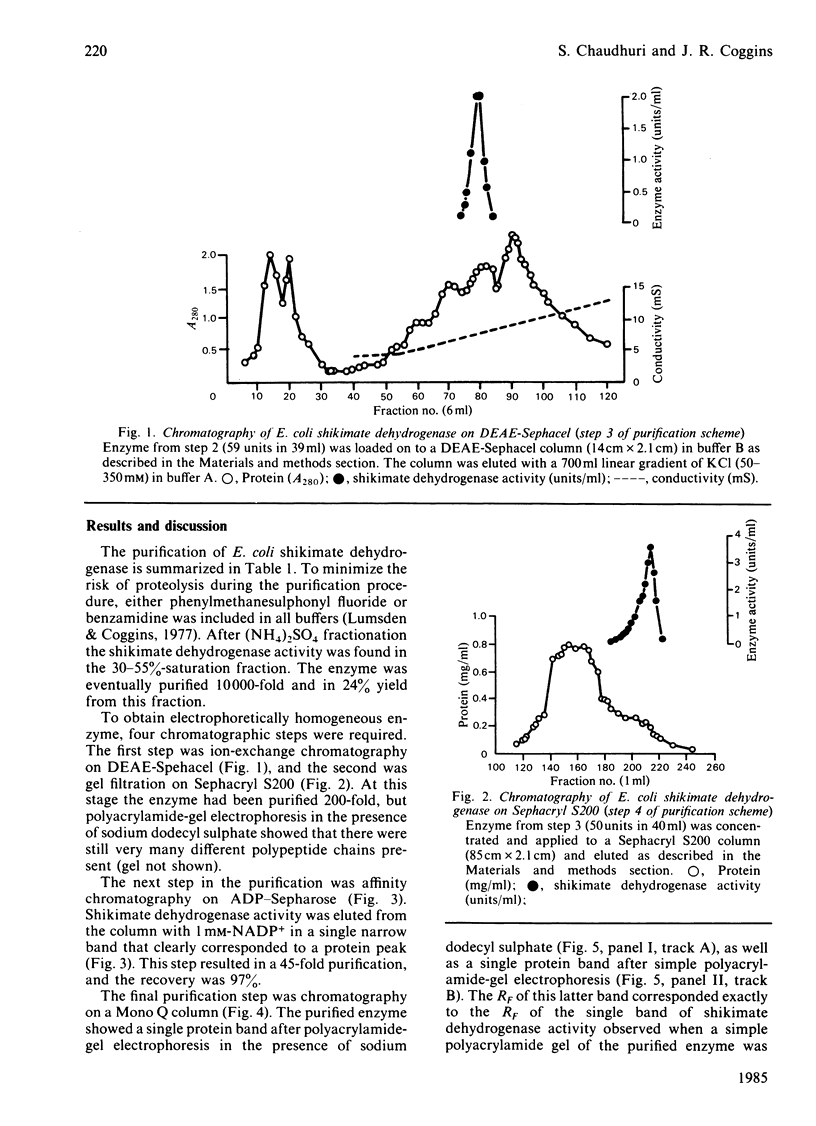
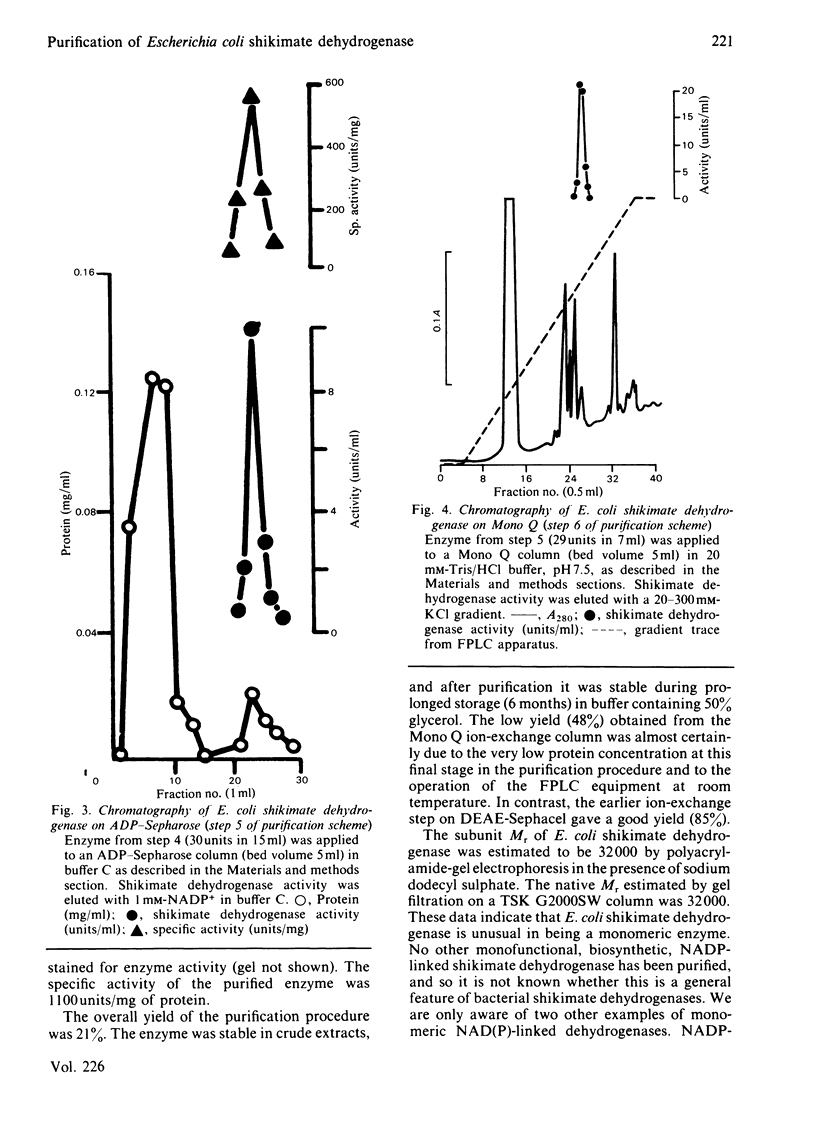

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barea J. L., Giles N. H. Purification and characterization of quinate (shikimate) dehydrogenase, an enzyme in the inducible quinic acid catabolic pathway of Neurospora crassa. Biochim Biophys Acta. 1978 May 11;524(1):1–14. doi: 10.1016/0005-2744(78)90097-9. [DOI] [PubMed] [Google Scholar]
- Berlyn M. B., Giles N. H. Organization of enzymes in the polyaromatic synthetic pathway: separability in bacteria. J Bacteriol. 1969 Jul;99(1):222–230. doi: 10.1128/jb.99.1.222-230.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Ely B., Pittard J. Aromatic amino acid biosynthesis: regulation of shikimate kinase in Escherichia coli K-12. J Bacteriol. 1979 Jun;138(3):933–943. doi: 10.1128/jb.138.3.933-943.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenbruch M., Bürk R. R. Experimentally improved reliability of ultrasensitive silver staining of protein in polyacrylamide gels. Anal Biochem. 1982 Sep 1;125(1):96–99. doi: 10.1016/0003-2697(82)90387-6. [DOI] [PubMed] [Google Scholar]
- Frost J. W., Bender J. L., Kadonaga J. T., Knowles J. R. Dehydroquinate synthase from Escherichia coli: purification, cloning, and construction of overproducers of the enzyme. Biochemistry. 1984 Sep 11;23(19):4470–4475. doi: 10.1021/bi00314a036. [DOI] [PubMed] [Google Scholar]
- Gaertner F. H., Cole K. W. A cluster-gene: evidence for one gene, one polypeptide, five enzymes. Biochem Biophys Res Commun. 1977 Mar 21;75(2):259–264. doi: 10.1016/0006-291x(77)91037-3. [DOI] [PubMed] [Google Scholar]
- Gibson F., Pittard J. Pathways of biosynthesis of aromatic amino acids and vitamins and their control in microorganisms. Bacteriol Rev. 1968 Dec;32(4 Pt 2):465–492. [PMC free article] [PubMed] [Google Scholar]
- Hautala J. A., Jacobson J. W., Case M. E., Giles N. H. Purification and characterization of catabolic dehydroquinase, an enzyme in the inducible quinic acid catabolic pathway of Neurospora crassa. J Biol Chem. 1975 Aug 10;250(15):6008–6014. [PubMed] [Google Scholar]
- Hawkins A. R., Reinert W. R., Giles N. H. Characterization of Neurospora crassa catabolic dehydroquinase purified from N. crassa and Escherichia coli. Biochem J. 1982 Jun 1;203(3):769–773. doi: 10.1042/bj2030769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T. Purification of two forms of the associated 3-dehydroquinate hydro-lyase and shikimate:NADP+ oxidoreductase in Phaseolus mungo seedlings. Biochim Biophys Acta. 1978 Jan 12;522(1):10–18. doi: 10.1016/0005-2744(78)90317-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larimer F. W., Morse C. C., Beck A. K., Cole K. W., Gaertner F. H. Isolation of the ARO1 cluster gene of Saccharomyces cerevisiae. Mol Cell Biol. 1983 Sep;3(9):1609–1614. doi: 10.1128/mcb.3.9.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewendon A., Coggins J. R. Purification of 5-enolpyruvylshikimate 3-phosphate synthase from Escherichia coli. Biochem J. 1983 Jul 1;213(1):187–191. doi: 10.1042/bj2130187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden J., Coggins J. R. The subunit structure of the arom multienzyme complex of Neurospora crassa. A possible pentafunctional polypeptide chain. Biochem J. 1977 Mar 1;161(3):599–607. doi: 10.1042/bj1610599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden J., Coggins J. R. The subunit structure of the arom multienzyme complex of Neurospora crassa. Evidence from peptide 'maps' for the identity of the subunits. Biochem J. 1978 Feb 1;169(2):441–444. doi: 10.1042/bj1690441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra U. S., Sprinson D. B. 5-Dehydro-3-deoxy-D-arabino-heptulosonic acid 7-phosphate. An intermediate in the 3-dehydroquinate synthase reaction. J Biol Chem. 1978 Aug 10;253(15):5426–5430. [PubMed] [Google Scholar]
- Miozzari G. F., Yanofsky C. Gene fusion during the evolution of the tryptophan operon in enterobacteriaceae. Nature. 1979 Feb 8;277(5696):486–489. doi: 10.1038/277486a0. [DOI] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley L. D. Purification and characterization of 3-dehydroquinate hydrolase and shikmate oxidoreductase. Evidence for a bifunctional enzyme. Biochim Biophys Acta. 1978 Sep 11;526(1):259–266. doi: 10.1016/0005-2744(78)90310-8. [DOI] [PubMed] [Google Scholar]
- Smith D. D., Coggins J. R. Isolation of a bifunctional domain from the pentafunctional arom enzyme complex of Neurospora crassa. Biochem J. 1983 Aug 1;213(2):405–415. doi: 10.1042/bj2130405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz K. W., Matthews D. A., Alden R. A., Freer S. T., Hansch C., Kaufman B. T., Kraut J. Crystal structure of avian dihydrofolate reductase containing phenyltriazine and NADPH. J Biol Chem. 1982 Mar 10;257(5):2528–2536. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YANIV H., GILVARG C. Aromatic biosynthesis. XIV. 5-Dehydroshikimic reductase. J Biol Chem. 1955 Apr;213(2):787–795. [PubMed] [Google Scholar]
- Zalkin H., Yanofsky C. Yeast gene TRP5: structure, function, regulation. J Biol Chem. 1982 Feb 10;257(3):1491–1500. [PubMed] [Google Scholar]