Abstract
Background
Compositional data analysis (CoDA) techniques are well suited for examining associations between 24-h movement behaviors (i.e., sleep, sedentary behavior, physical activity) and indicators of health given they recognize these behaviors are co-dependent, representing relative parts that make up a whole day. Accordingly, CoDA techniques have seen increased adoption in the past decade, however, heterogeneity in research reporting practices may hinder efforts to synthesize and quantify these relationships via meta-analysis. This systematic review described reporting practices in studies that used CoDA techniques to investigate associations between 24-h movement behaviors and indicators of health.
Methods
A systematic search of eight databases was conducted, in addition to supplementary searches (e.g., forward/backward citations, expert consultation). Observational studies that used CoDA techniques involving log-ratio transformation of behavioral data to examine associations between time-based estimates of 24-h movement behaviors and indicators of health were included. Reporting practices were extracted and classified into seven areas: (1) methodological justification, (2) behavioral measurement and data handling strategies, (3) composition construction, (4) analytic plan, (5) composition-specific descriptive statistics, (6) model results, and (7) auxiliary information. Study quality and risk of bias were assessed by the National Institutes of Health Quality Assessment Tool for Observational Cohort and Cross-sectional Studies.
Results
102 studies met our inclusion criteria. Reporting practices varied considerably across areas, with most achieving high standards in methodological justification, but inconsistent reporting across all other domains. Some items were reported in all studies (e.g., how many parts the daily composition was partitioned into), whereas others seldom reported (e.g., definition of a day: midnight-to-midnight versus wake-to-wake). Study quality and risk of bias was fair in most studies (85%).
Conclusions
Current studies generally demonstrate inconsistent reporting practices. Consistent, clear and detailed reporting practices are evidently needed moving forward as the field of time-use epidemiology aims to accurately capture and analyze movement behavior data in relation to health outcomes, facilitate comparisons across studies, and inform public health interventions and policy decisions. Achieving consensus regarding reporting recommendations is a key next step.
Supplementary Information
The online version contains supplementary material available at 10.1186/s44167-024-00062-8.
Keywords: Physical activity, Sedentary behavior, Screen time, Sleep
Introduction
Previous research has established independent associations between time spent engaging in physical activity [1, 2], sedentary behavior [3, 4], and sleep [5, 6] in relation to several indicators of health and health outcomes. However, studies have generally examined these behaviors in isolation, neglecting the fact that these behaviors are co-dependent and mutually exclusive over the course of a full 24-h day [7–9]. That is, time spent engaging in one behavior (e.g., physical activity) reduces time during the day available for other behaviors (e.g., sedentary behavior, sleep). Researchers have begun to acknowledge the limitation of solely focusing on individual behaviors in the past decade, sparking the shift to a novel 24-h paradigm that emphasizes the need to consider the collective influence of physical activity, sedentary behavior and sleep over the course of a whole day for health [10–12]. While consensus terminology for referring to these behaviors as a collective has yet to be established [13], they are most commonly referred to as 24-h movement behaviors, the 24-h activity cycle, physical behaviors, time-use behaviors or time-use activity behaviors.
The proliferation of research investigating how combinations of physical activity, sedentary behavior and sleep is associated with indicators of health was sparked by the release of the 24-Hour Movement Guidelines for Children and Youth in Canada in 2016 [14]. Recommendations tailored for several other segments of the population are also now available, including young children (0 to 4 years of age) [15], adults, and older adults [16], and have been adopted by several countries globally [17–21]. At present, the literature examining associations between 24-h movement behaviors and health has focused largely on guideline adherence [22–28]. Such work is important from a behavioral surveillance standpoint, but classifying individuals into groups based on whether or not they meet threshold-based behavioral recommendations [e.g., ≥ 60 min/day of moderate-to-vigorous physical activity (MVPA) for children and youth] may oversimplify these relationships as it fails to consider the full range of time-use estimates over the course of a whole day. This may be partly attributable to the current 24-h movement behavior data available to assess these relationships. That is, many of these studies used data from behavioral surveillance systems (e.g., U.S. National Survey of Children’s Health; Korea National Health and Nutrition Examination Survey; Australian National Secondary Students’ Diet and Activity Survey) that employ crude self- or proxy-reported instruments to assess physical activity, sedentary behavior and sleep in line with recommendations for each movement behavior irrespective of whether they have adopted integrated 24-h guidelines or not. However, it is becoming increasingly feasible to capture higher resolution estimates of movement behaviors within a 24-h day with recent advances and improvements in accelerometry (e.g., improved data processing, wearable device integration, cost reduction) as well as the availability of whole day recall instruments such as the Activities Completed over Time in 24-h (ACT-24) [29], Multimedia Activity Recall for Children and Adults (MARCA) [30], and 24-h Physical Activity Recall (24PAR) [31]. These advances in assessment open the door for alternative analytic approaches that can provide more nuanced insights into the integrative influence of 24-h movement behaviors on health.
Compositional data analysis (CoDA) is a statistical approach that is well suited for quantifying associations between movement behaviors and indicators of health given its ability to model the relative nature of 24-h time use data [7–9]. Specifically, CoDA considers that each movement behavior represents a mutually exclusive part of a finite period (i.e., whole day) [32]. This is done through transforming absolute values (i.e., 600 min of sleep) into relative proportions (i.e., 41.6% of a 24-h day) via sets of log-ratios (e.g., isometric, additive, centered). This relative proportion approach adjusts for the co-dependency of these behaviors, helping to address their multi-collinearity, and thus, overcoming a major challenge of traditional statistical approaches that use absolute values [7]. These properties of CoDA make this approach appropriate for analyzing time-use data with multiple components that comprise a whole day, and several techniques are available to examine different research questions related to time-use such as compositional isotemporal substitution (i.e., the influence of replacing time spent in one behavior with time spent in another behavior) [33], the Goldilocks approach (i.e., the optimal distribution of time spent in different movement behaviors for optimal health benefit) [34], and the Many Different Roads Lead to Rome approach (i.e., different movement behavior distributions associated with equivalent health benefits) [35]. Further, CoDA can be implemented in several commonly used analytic frameworks (e.g., general linear modeling, structural equation modeling, mixture modeling). This flexibility has allowed adoption of CoDA to proliferate in the field of time-use epidemiology. An example of this growth can be seen with the literature examining 24-h movement behaviors in relation to indicators of mental health. Only two CoDA studies were included in the first systematic review of 24-h movement behaviors among children and youth published in 2020 [27], whereas eight CoDA studies examining children and youth were included in a subsequent systematic review published 4 years later [23]. This point is further underscored by a total of 61 CoDA studies (employing 24-h measurement) that were captured in an October 2022 systematic search of the literature investigating the influence of reallocating time across movement behaviors on health [36].
Despite the rapid increase in studies using CoDA, only two systematic reviews specific to this literature focused on how 24-h movement behaviors relate to indicators of health exist, with one review focused on adults [37] and the other review focused on early childhood [38]. The landscape regarding reporting practices for CoDA studies has yet to be explored, however, representing a key knowledge gap considering there is currently no formal guidance regarding research reporting practices in this area. Such issues have been recognized in other areas of research such as ecological momentary assessments (EMA) of diet and physical activity, which resulted in the creation of the Checklist for Reporting EMA Studies (CREMAS) after characterizing methodological practices in prior studies [39]. Akin to what has been established for EMA studies, investigating existing reporting practices in the CoDA literature may help to inform uniform reporting procedures in the future. Therefore, the purpose of this study was to describe research reporting practices in observational studies that have examined associations between 24-h movement behavior compositions and indicators of health using CoDA.
Methods
Protocol and registration
This review was preregistered with the International Prospective Register of Systematic Reviews (PROSPERO; submitted September 23, 2023; ID: CRD42023456880). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed [40], and items are reported using the PRISMA Checklist (see Supplemental Materials Table 1).
Inclusion criteria
We included studies that met the following nine criteria: (a) published in the English language, (b) published in peer-reviewed journals, (c) included human participants, (d) original empirical investigation, (e) observational design, (f) used CoDA techniques, (g) included time-based estimates of all three behaviors: physical activity, sedentary behavior, and sleep, (h) focused on the whole day (i.e., 24-h movement compositions), and (i) examined associations with health outcomes or indicators of health as the dependent variable. Self- or proxy-reported and device-assessed estimates of movement behaviors were included given that CoDA can be used to investigate associations between movement behaviors and indicators of health with both types of measures, including a combination of both types of measures (e.g., device-assessed sedentary behavior and physical activity, self-reported sleep). Indicators of health and health outcomes were operationalized as any indicator of physical, cognitive or mental health (e.g., working memory, bone density, physical functioning) or health outcome (e.g., mortality, overweight/obesity status, depression).
Studies were excluded for the respective nine major reasons: (a) published in a language other than English, (b) not a peer-reviewed article (i.e., Masters thesis, PhD dissertations, conference abstracts), (c) reviews, case studies, qualitative studies, protocol papers, commentaries/opinions, or book chapters, (d) used an experimental approach to assess the relationship with a health outcome, (e.g., randomized controlled trial), (e) did not use CoDA techniques (e.g., isotemporal substitution studies that did not involve log-ratio transformations), (f) examined associations with other health behaviors (e.g., diet, smoking), (g) specified the 24-h movement composition as the dependent variable, (h) did not include human participants (e.g., animal models), and (i) methods focused papers (e.g., [7, 41, 42]). Methods focused papers were excluded given that demonstrating the implementation of a CoDA technique may have had different reporting standards for the methods and results as would be expected in subsequent studies using established analytic techniques. Studies using experimental designs were excluded based on the premise that research reporting recommendations for experimental designs (Consolidated Standards of Reporting Trials guidelines [43]) differ from those that exist for observational designs [Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [44]]. However, studies that involved cross-sectional analysis of baseline data from experimental studies were included. It is worth noting that much of the existing body of literature investigating 24-h movement behaviors in relation to health outcomes stems from secondary data analysis of baseline experimental data and behavioral surveillance studies [22, 23, 37].
Search strategy, data extraction, and data synthesis
In consultation with a research librarian, we conducted an electronic search of eight databases. The MEDLINE EBSCO, PsycINFO, SPORTDiscus, Web of Science, CINAHL and Scopus databases were searched from inception to October 12th, 2023. The Cochrane Library, and EMBASE/Ovid databases were searched from inception to October 23rd, 2023, and October 31st, 2023, respectively. These databases were searched based on their relevance to the review topic and for consistency with previous reviews examining associations between 24-h movement behaviors and health outcomes that also searched these databases [22, 23, 27, 28]. Search terms can be found in Supplemental Materials File 1. A manual search of the Journal of Activity, Sedentary, and Sleep Behaviors, which at the time of conducting this review had yet to be indexed, was also performed on February 16th, 2024, given the relevance of its scope. Further, a manual search of the International Network of Time-Use Epidemiologists (INTUE) publications list was performed on March 11th, 2024, given the relevance of the Network’s mission. Additional supplemental search strategies included forward (searching citationss of included papers) and backward (reviewing references within included studies) citation searches, in addition to contacting experts in the field. Forward and backward citation searches were also performed for CoDA methods papers identified by the research team [7, 33, 41, 42, 45–49].
Retrieved references were imported into Covidence (Evidence Partners, Ottawa, ON, Canada), where duplicates were removed and titles/abstracts were reviewed by two independent reviewers (DB, SB, CG, GMB, CP, CSL, CK) for initial inclusion. After initial screening, full texts were retrieved and independently examined by two reviewers from the same group for final inclusion. A pre-piloted protocol was created for both stages prior to study selection, in which reviewers had to achieve ≥ 80% accuracy prior to completing both stages. Any conflicts during each stage were resolved by a third independent reviewer. Data extraction was performed independently by two reviewers (SB, CG, GMB, CP, CP, CSL, CK) and a third reviewer examined the data for consensus (DB). Extracted data included general article characteristics (e.g., publication year, sample, study design). Data extraction for items relevant to CoDA studies were classified into seven areas, which included: (a) methodological justification; (b) behavioral measurement and data handling strategies; (c) composition construction; (d) analytic plan; (i) composition-specific descriptive statistics; (e) model results; and (f) auxiliary information (i.e., limitations of CoDA, clinical implications, funding, conflicts of interest). These categories were created based on past reporting checklists of behavioral approaches (e.g., CREMAS, STROBE), and the data extraction spreadsheet was reviewed by 11 international experts in this area to ensure all pertinent items were addressed.
Considering the focus of this review is to characterize reporting practices, data analysis focused on a numerical presentation (i.e., central tendencies and percentages) of whether items were reported (or not). Distributions of reporting practices for each item of interest are presented (yes/no/unclear) within their respective seven major reporting categories, and narratively synthesized.
Methodological quality and risk of bias assessment
All included studies used an observational design, and thus, an adaptation of the National Institutes of Health Quality Assessment Tool for Observational Cohort and Cross-sectional Studies (QATOCCS [50]) was used to assess methodological quality and validity of each study as well as their risk of bias. Study quality and risk of bias was assessed independently by two reviewers on the 14 criteria assessing clarity in reporting (e.g., research question, population details), justification of methodological choices (e.g., reliability and validity of measurement tools, sample size), and use of best practices (e.g., repeated assessments, adjusting for confounders). Each study received a “yes”, “no”, or “other” response to each question to then be rated as “poor”, “fair”, or “good” based on these considerations as concerned with the exposure (i.e., 24-h movement behaviors) and outcomes of interest (i.e., indicators of health). The responses are intended to be used as a guide for assessing the quality and risk of bias rating, however, in line with previous work that has used ranges of scores to provide quantitative evaluations [51, 52], we considered studies with a score of ≤ 4 to be “poor”, between 5 to 9 to be “fair”, and > 9 to be “good” study quality.
Results
Included studies
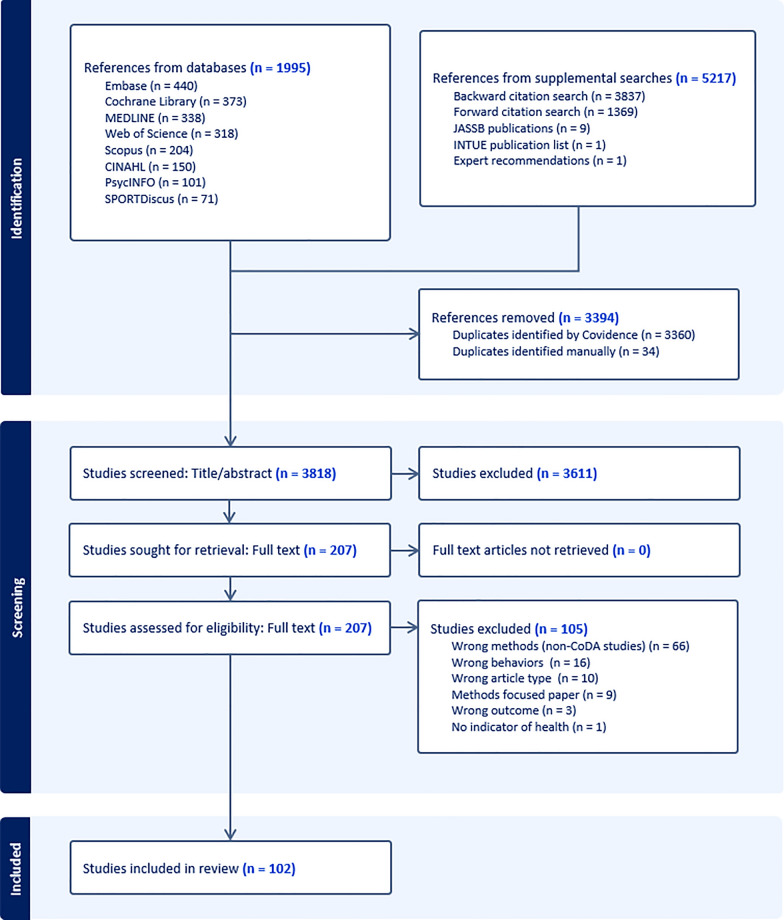
The initial search identified 1995 records, which was reduced to 872 after de-duplication. Supplementary search strategies identified an additional nine articles in the Journal of Activity, Sedentary and Sleep Behaviors, and one article was found in the INTUE publication list. Forward (n = 1369) and backward citation (n = 3837) searches and articles from expert consultation (n = 1) identified an additional 5217 records. In total, 7212 records were identified by the search strategies, and reduced to 3818 after de-duplication. These articles were reduced to 207 for full-text review, which resulted in 102 studies that met our inclusion criteria. Full-text articles were excluded for three main reasons: (1) wrong methods (i.e., did not use CoDA techniques; n = 66), (2) wrong behaviors (e.g., missing sleep, sedentary behavior, or physical activity within the 24-h composition; n = 16), and (3) wrong article type (n = 10). A PRISMA flow diagram is presented in Fig. 1. A list of studies excluded at full-text and reasons are presented in Supplementary Table 2. Funding and conflict of interest of included studies are included in Supplementary Table 3.
Fig. 1.
PRISMA flow chart
Description of studies
Study characteristics are presented in Table 1. Studies included were published between 2016 and 2024 with samples from 29 different countries: Australia: (n = 24), Belgium (n = 2), Brazil (n = 7), Bulgaria (n = 1), Canada (n = 11), China (n = 7), Colombia (n = 1), Czech Republic (n = 1), Denmark (n = 6), England (n = 6), Finland (n = 7), India (n = 1), Iran (n = 1), Ireland (n = 1), Japan (n = 3), Kenya (n = 1), Luxembourg (n = 2), Netherlands (n = 4), New Zealand (n = 4), Portugal (n = 1), Scotland (n = 1), Singapore (n = 2), Slovenia (n = 1), South Africa (n = 2), Spain (n = 5), Sweden (n = 4), United Kingdom (n = 10), USA (n = 13), and Wales (n = 3). Eight studies involved multi-country samples ranging from two to eight countries. A total of 79 studies used cross-sectional designs, 18 used longitudinal designs, and five included both. Analytic sample sizes ranged from 28 [53] to 130,239 [54] participants, with participants ranging in age from 1 [55] to 75.8 [56] years on average.
Table 1.
Study characteristics
| Study | Analytic sample size | Age in years: 1Mean and/or range | Sex/gender | Country | Indicator(s) of health | Type of Study | MVPA Measure | LPA Measure | SB Measure | SL Measure | What parts was the day partitioned into? | CoDA Associations Examined |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asano et al. [96] | 113 | 75.2 (4.6) | 40.7% female | Japan | Phase angle | Cross-sectional | Device-based | Device-based | Device-based | Questionnaire (self-reported) | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM; Proportional ISM |
| Bezerra et al. [97] | 123 | 55.2 (9.2) months | 50.4% female | Brazil | Executive function | Cross-sectional | Device-based | Device-based | Device-based | Questionnaire (proxy-reported) | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
| Bianchim et al. [98] | Children: 86; Adults: 43 |
Children: 13.6 (2.8); Adults: 24.6 (4.7) |
Total: 48% female; Child: 47% female; Adults: 48% female |
Australia, South Wales | FEV1% | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
| Biddle et al. [99] | 435 | 66.9 (7.4) | 61.7% male | UK | Glucose regulation, Insulin sensitivity | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SL; Sitting; Standing; Stepping |
Individual; 1-to-1 ISM |
| Blodgett et al. [100] | 4738 | 46 | 52.3% female | England, Scotland, Wales | Depression | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA |
Individual; 1-to-1 ISM |
| Blodgett et al. [101] | 15,253 | 53.7 (9.7) | 54.7% female | Netherlands, UK, Australia, Denmark, Finland | BMI, Waist circumference, HDL cholesterol, Total: HDL cholesterol ratio, Triglycerides, HbA1c | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA; Standing |
Individual; 1-to-1 ISM |
| Booker et al. [60] | 2805 | 60.7 (11.7) | 62% female | USA | High-sensitivity c-reactive protein | Cross-sectional | Questionnaire (self-reported) | Questionnaire (self-reported) | Other: Computed from the subtraction of time spent in PA and sleep from 1440 min | Questionnaire (self-reported) | SB; SL; Lying down; Total PA | 1-to-1 ISM |
| Brakenridge et al. [61] | 648 | Low risk: 56.0 (9.8); High risk: 60.2 (9.3) |
Lower Diabetes Risk: 52.7% female; Higher Diabetes Risk: 60.7% female |
Australia | Glycaemic measures (HbA1c, FPG, 2hPLG) | Cross-sectional | Device-based | Device-based | Device-based | Device-based; Questionnaire (self-reported) | SL; Sitting; Standing; Stepping |
Individual; 1-to-1 ISM |
| Brayton et al. [64] | 33 | 15.8 (1.2) | 52% female | USA | Concussion recovery | Longitudinal | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Individual |
| Cabanas-Sánchez et al. [102] | X: 2,489; L: 1,679 |
X: 71.7 (4.3) L: 71.4 (4.2) |
X: 53.1% female; L: 51.7% female |
Spain | Depression, Loneliness, Happiness, Global mental health | Cross-sectional; Longitudinal | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
| Carson et al. [103] | 4169 | 11.4 (0.1) | 51.3% male | Canada | BMI, Waist circumference, Systolic BP, Diastolic BP, Behavioral strengths and difficulties, Triglycerides, Cholesterol, C-reactive protein, Insulin, Aerobic fitness | Cross-sectional | Device-based | Device-based | Device-based | Questionnaire (self-reported); Questionnaire (proxy-reported) | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
| Carson et al. [104] | 552 | 3.5 (0.0) | 49.2% female | Canada | Waist circumference, BMIz | Cross-sectional | Device-based | Device-based | Device-based | Questionnaire (proxy-reported) | SB; SL; LPA; MVPA | Overall; Individual |
| Chao et al. [105] | 1475 | 20.7 (1.6) | 68.0% female | China | Anxiety symptoms | Cross-sectional | Questionnaire (self-reported) | Questionnaire (self-reported) | Questionnaire (self-reported) | Questionnaire (self-reported) | SB; SL; LPA; MVPA | Overall; 1-to-1 ISM |
| Chastin et al. [54] | 130,239 |
ABC: 52.8 NHANES 2003–2006: 63.6 REGARDS: 63.4 UK Biobank: 62.3 Whitehall II: 69.4 Women's Health Study: 72.0 |
ABC: 44% female NHANES 2003–2006: 49.3% female REGARDS: 54% female UK Biobank: 56.2% female Whitehall II: 25.9% female Women's Health Study: 100% female |
Sweden, USA, UK | All-cause mortality | Longitudinal | Device-based | Device-based | Device-based | Device-based; Questionnaire (self-reported) | SB; SL; LPA; MVPA | Individual; 1-to-1 ISM |
| Chen et al. [62] | 8045 |
2018: 3.8 (1.3) 2019: 3.8 (1.3) 2020: 3.9 (1.3) 2021: 3.8 (1.3) |
2018: 51.3% female 2019: 48.6% female 2020: 50.0% female 2021: 51.1% female |
Singapore | Quality of life | Cross-sectional | Questionnaire (proxy-reported) | Questionnaire (proxy-reported) | Questionnaire (proxy-reported) | SB; SL; Total PA | Overall; Individual; 1-to-1 ISM | |
| Chen et al. [106] | 389 | 11.9 (2.1) | 50.1% female | China | BMI | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Individual; 1-to-1 ISM; Proportional ISM |
| Chong et al. [107] |
X: 127 L: 88 |
X: 11.7 (0.5) L: 11.8 (0.4) |
Cross-sectional: 57.5% female Longitudinal: 59.1% female |
Australia | Psychosocial health: Internalizing problems, Externalizing problems, Total difficulties, Prosocial behavior, Psychological distress | Cross-sectional; Longitudinal | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual |
| Chong et al. [108] | 909 | 10.4 (0.5) | 53.1% female | Australia |
Psychosocial health: Emotional symptoms, Conduct problems, Hyperactivity, Peer relationship problems, Prosocial behavior |
Longitudinal | Questionnaire (self-reported) | Questionnaire (self-reported) | Questionnaire (self-reported) | Questionnaire (self-reported) |
Screen time; SL; Other: Self-care/ Domestic activities, PA, Social, Education, Recreational screen use, Quiet time, Passive transport |
Overall; Individual; Proportional ISM |
| Clarke et al. [109] | 2838 | 46.4 | 51.8% female | USA | Mortality | Longitudinal | Device-based | Device-based | Device-based | Questionnaire (self-reported) | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM; Proportional ISM |
| Collings et al. [110]a | 1046 | 51.2 (12.2) | 53.5% female | Luxembourg | Waist circumference, Total body fat, Systolic blood pressure, Diastolic blood pressure, Fasting glucose, Triglycerides, HDL-c, Fasting insulin, APoB/A, Cardiometabolic risk score | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; 1-to-1 ISM |
| Collings et al. [111]a | 1001 | 50.6 (12.2) | 53.4% female | Luxembourg | Arterial stiffness | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | 1-to-1 ISM |
| Curtis et al. [112] | 430 | 41.3 (11.7) | 74% female | Australia | BMI, HRQoL, Anxiety, Depression, Stress | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; 1-to-1 ISM |
| Curtis et al. [113] | 322 | 40.4 (5.9) | 58.1% female | Australia | Symptoms of depression, anxiety, and stress | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | 1-to-1 ISM |
| de Faria et al. [114] | 217 | 16.0 | 49.4% female | Brazil | Symptoms of depression and anxiety | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
| Del Pozo Cruz et al. [115] | 3233 | 47.4 (19.5) | 52.1% female | USA | Depressive symptoms | Cross-sectional | Device-based | Device-based | Device-based | Questionnaire (self-reported) | SB; SL; LPA; MVPA | Individual; 1-to-1 ISM |
| Domingues et al. [116] | 185 | 16.0 (1.0) | 49.2% female | Brazil | BMIz | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; 1-to-1 ISM |
| Dumuid et al. [117] | 1728 | 9–11 | 56% female | Australia, Canada, UK, Finland | Body fat % | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | 1-to-1 ISM |
| Dumuid et al. [118] | 122 | 65.4 (2.9) | 61% female | Australia | Cardio-respiratory fitness (VO2max), BMI, Total cholesterol; Blood pressure (systolic and diastolic); Fasting blood glucose; Waist-to-hip ratio | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; 1-to-1 ISM |
| Dumuid et al. [119] | 5855 | 9–11 | 45% female | Australia, England, Canada, Finland, Portugal, USA, Brazil, Colombia, China, India, South Africa, Kenya | HRQoL | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual; Proportional ISM |
| Dumuid et al. [120] | 971 | 11.9 (0.4) | 50% female | Australia | Body composition | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; 1-to-1 ISM; Proportional ISM |
| Dumuid et al. [121] | 804 | 11.9 (0.4) | 49.6% female | Australia | Bone density, Bone geometry, Bone strength | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual; Goldilocks |
| Dumuid et al. [122] | 1182–1137 | 12 (0.4) | 49% female | Australia | Fitness, Adiposity | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Goldilocks |
| Dumuid et al. [123] | 82 |
APOE E4 non-carrier: 65.1 (7.7) APOE E4 carrier: 67.1 (7.1) |
APOE E4 non carrier: 58% female APOE E4 carrier: 64% female |
Australia | Cognitive function | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Proportional ISM |
| Dumuid et al. [35] | 2123 | 14.4 (0.5) | 50% female | Australia | Physical functioning | Cross-sectional | Questionnaire (self-reported) | Questionnaire (self-reported) | Questionnaire (self-reported) | Screen time; SL; Other: Self-Care, School-Related, Quiet Time, PA, Domestic/Social Activities | Overall; Many Different Roads | |
| Fairclough et al. [124] | 169 | 10.3 (0.3) | 50.3% female | England | BMI, Percentage of waist circumference-to-height ratio, Cardiorespiratory fitness, Peak oxygen uptake (VO2 peak) | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; 1-to-1 ISM |
| Fairclough et al. [125] | 359 | 11.5 (1.4) | 50.7% female | England | Self-esteem, Depression, Emotional and behavioral problems, Executive function | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
| Fairclough et al. [65] | 301 | 11.1 (1.6) | 60.1% female | England | Mental health | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MPA; VPA | Overall; Individual; Goldilocks |
| Farrahi et al. [126] | 3443 | 46.6 (0.5) | 55.5% female | Finland | Cardiometabolic health markers: Plasma glucose, Serum insulin, Total cholesterol, HDL cholesterol, LDL cholesterol, Triglycerides | Cross-sectional | Device-based | Device-based | Device-based | Questionnaire (self-reported) | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM; Proportional ISM |
| Feter et al. [127] | 8608 | 58.9 (8.6) | 55.9% female | Brazil | Cognitive function | Cross-sectional | Device-based | Device-based | Device-based | Questionnaire (self-reported) | SB; SL; LPA; MVPA | 1-to-1 ISM; Proportional ISM |
| Franssen et al. [128] | 61 | 33.6 (10.7) | 33% female | Belgium | Cardiovascular health: Systolic and diastolic BP, Mean arterial pressure, Resting heart rate, HDL cholesterol, LDL cholesterol, Triglycerides, Clustered cardiometabolic risk score; Glucose tolerance: Fasting glucose, Fasting insulin, glucose 120 min, Insulin 120 min, Matsuda index, Insulinogenic index, Homeostatic model assessment of insulin resistance, Homeostatic model assessment of insulin sensitivity, Muscle insulin sensitivity index; Waist circumference, Fat mass percentage, Cardiorespiratory fitness | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA; Standing | Individual |
| Gupta et al. [129] | 827 | 45 (10) | 46% female | Denmark | Blood pressure | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual; Proportional ISM |
| Gupta et al. [130] | 669 | 45.1 (9.9) | 45% female | Denmark | Systolic and diastolic blood pressure | Cross-sectional | Device-based | Device-based | Device-based | Questionnaire (self-reported) | SB; SL; LPA; MVPA | Overall; Proportional ISM |
| Gupta et al. [131] | 929 | 44.9 (9.7) | 45% female | Denmark | Long-term sickness absence | Longitudinal | Device-based | Device-based | Device-based | Questionnaire (self-reported) | SB; SL; LPA; MVPA; Standing | Overall; Individual; Proportional ISM |
| Gupta et al. [132] | 807 | 45.1 (9.7) | 54.4% male | Denmark | Obesity: Waist circumference, BMI, Fat % | Cross-sectional | Device-based | Device-based | Device-based | Questionnaire (self-reported) | SB; SL; LPA; MVPA; Standing | Other: Latent profile analysis |
| Healy et al. [53] | 28 | 13.7 (3.0) | 76.7% male | USA | BMI | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | 1-to-1 ISM; Proportional ISM |
| Hofman et al. [133] | 1943 | 70.9 (9.3) | 51.6% female | Netherlands | Depressive symptoms, Anxiety symptoms | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | 1-to-1 ISM |
| Hyodo et al. [56] | 76 | 75.8 | 63% female | Japan | Executive function | Cross-sectional | Device-based | Device-based | Device-based | Questionnaire (self-reported) | SB; SL; LPA; MPA |
Individual; 1-to-1 ISM |
| Kandola et al. [134] | 60,235 | 55.9 (7.7) | 56% female | England, Scotland, Wales | Depressive symptoms, Anxiety symptoms | Longitudinal | Device-based | Device-based | Device-based | Questionnaire (self-reported) | SB; SL; LPA; MVPA |
Individual; 1-to-1 ISM |
| Kastelic et al. [135] | 2333 | 18–44; 45–64; 65+ | 74% female | Slovenia | Lower back pain frequency, Lower back pain intensity | Cross-sectional | Questionnaire (self-reported) | Questionnaire (self-reported) | Questionnaire (self-reported) | Questionnaire (self-reported) | SB; SL; LPA; MVPA | Overall; 1-to-1 ISM |
| Kim et al. [59] | 1247 | 50.1 (12.5) | 57.3% female | USA | BMI | Cross-sectional | Device-based; Questionnaire (self-reported) | Device-based; Questionnaire (self-reported) | Device-based; Questionnaire (self-reported) | Device-based; Questionnaire (self-reported) | SB; SL; LPA; MVPA | Individual; 1-to-1 ISM |
| Kitano et al. [136] | 1095 | 50.2 (9.5) | 68.6% female | Japan | Psychological distress | Cross-sectional | Device-based | Device-based | Device-based | Questionnaire (self-reported) | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
| Kuzik et al. [137] | 95 | 45 (0.7) | 30.5% female | Canada |
Physical development: Motor skills, Adiposity, Growth; Cognitive development: Response inhibition, Visual-spatial working memory, language; Social-emotional development: Sociability, Externalizing problems, internalizing problems, Prosocial behaviour, Cognitive self-regulation, Emotional self-regulation, Behavioural self-regulation |
Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
| Larisch et al. [138] | 348–370 | 41 (9) | 68% female | Sweden | Depression, Anxiety, Wellbeing | Cross-sectional | Device-based | Device-based | Device-based | Questionnaire (self-reported) | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
| Lau et al. [139] | 426 | 3.8 (0.6) | 45.8% female | China | Executive function | Cross-sectional | Device-based | Device-based | Device-based | Questionnaire (proxy-reported) | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
| Le et al. [140] | 361 | 22.6 (5.3) | 72.5% female | Australia | Daily affect | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA; Other: Time awake in bed | Individual; 1-to-1 ISM |
| Lee et al. [141] | 136 | 73 (2) | 100% female | USA | Metabolic syndrome | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
| Lemos et al. [142] | 270 | 4.0 (0.8) | 51% female | Brazil | Physical fitness: Cardiorespiratory fitness, Speed-agility, Lower body muscular strength | Cross-sectional | Device-based | Device-based | Device-based | Questionnaire (proxy-reported) | SB; SL; LPA; MVPA | Overall; 1-to-1 ISM |
| Lewthwaite et al. [63] | 95 | 70.5 (6.8) | 37% female | Australia | Breathlessness, Anxiety and depressive symptoms, HRQoL | Longitudinal | Questionnaire (self-reported) | Questionnaire (self-reported) | Questionnaire (self-reported) | Questionnaire (self-reported) | SB; SL; LPA; MVPA | Overall; 1-to-1 ISM; Proportional ISM |
| Lin et al. [143] | 2375 | 20 | 50% female | USA | Depressive symptoms | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Individual |
| Lu et al. [144] | 135 | 4.6 (0.5) | 49.63% male | China | Executive function | Cross-sectional | Device-based | Device-based | Device-based | Questionnaire (proxy-reported) | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
| Lund Rasmussen et al. [145] | 659 | 13.9 (2.8) | 48.5% female | Czech Republic | BMI, Adiposity | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Goldilocks |
| Madden et al. [146] | 54 | 71.4 (0.6) | 56% female | Canada | Waist circumference, Triglycerides, HDL, Systolic blood pressure, Fasting glucose, Continuous metabolic syndrome risk score | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual |
| Marshall et al. [147] | 37 | 11.9 (1.6) | 57% male | Wales | Anthropometrics, Arterial stiffness, Cardiac autonomic activity | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
| Marshall et al. [148] | 101 | 12.4 (1.6) | 45% female | Wales | Arterial stiffness | Cross-sectional | Device-based | Device-based | Device-based | Questionnaire (self-reported) | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
| Matricciani et al. [149] | Children:1073; Adults: 1378 |
Children: 12.0 (0.4) Parents: 44.0 (5.1) |
Children: 50% males Parents: 13% males |
Australia | BMI, Systolic blood pressure, Diastolic blood pressure, Metabolic syndrome severity score, Glycoprotein acetyls, Apolipoprotein B/A1 | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM; Proportional ISM |
| McGee et al. [150] | 119 | 5.7 (0.2) | 47% female | Canada | Body composition | Cross-sectional | Device-based | Device-based | Device-based | Questionnaire (proxy-reported) | SB; SL; LPA; MVPA | Individual; 1-to-1 ISM |
| McGregor et al. [151] | 7776 |
Adults: 41.3 (0.2) Older Adults: 69.3 (0.3) |
Adults: 50.4% male Older Adults: 47.6% male |
Canada | BMI, Waist circumference, Aerobic fitness, Resting heart rate, HDL cholesterol, Triglycerides, Blood glucose, Insulin levels | Cross-sectional | Device-based | Device-based | Device-based | Questionnaire (self-reported) | SB; SL; LPA; MVPA | Individual |
| McGregor et al. [152] | 1468 | 63.1 (0.2) | 48.3% female | USA | Mortality | Longitudinal | Device-based | Device-based | Device-based | Questionnaire (self-reported) | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
| Mellow et al. [153] | 384 | 65.5 (3.0) | 68.5% female | Australia | Cognitive function | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual |
| Mellow et al. [154] | 378 | 65.6 (3.0) | 67.5% female | Australia |
Cognitive function: Long-term memory, Executive function, Processing speed; Brain gray matter: Total, lateral ventricle, bilateral frontal lobe, bilateral temporal lobe and bilateral hippocampus volumes |
Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; 1-to-1 ISM; Proportional ISM |
| Migueles et al. [155] | 93 | 10 (1) | 40% female | Spain | Gray matter volume (left and right hippocampus) | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Individual; 1-to-1 ISM |
| Migueles et al. [156] | X: 315; L: 201 | 4.5 (0.1), 9.6 (0.1) | N/R | Sweden | Body composition, BMI, Cardiorespiratory fitness, Motor skills, Muscular fitness | Longitudinal | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual |
| Mitchell et al. [157] | 4481 | 47 (0.6) | 52% female | England, Scotland, Wales | Cognition | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Individual; 1-to-1 ISM |
| Mota et al. [158] | 204 | 4.5 (0.8) | 50.5% female | Brazil | Fundamental movement skills | Cross-sectional | Device-based | Device-based | Device-based | Questionnaire (proxy-reported) | SB; SL; LPA; MVPA | Overall; 1-to-1 ISM |
| Murray et al. [159] | 770 | 20.4 (0.7) | 55% female | Canada | Depressive symptoms, Self-rated mental health | Cross-sectional | Questionnaire (self-reported) | Questionnaire (self-reported) | Questionnaire (self-reported) | Questionnaire (self-reported) | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
| Ng et al. [160] | 1,179 | 12.0 (0.4) | 49% female | Australia | Adiposity, HRQoL | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Individual; 1-to-1 ISM; Proportional ISM |
| Niemelä et al. [161] | 4147 | 53 | 58% female | Finland | Major adverse cardiac events | Longitudinal | Device-based | Device-based | Device-based | Questionnaire (self-reported) | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
| Olds et al. [58] | 105 | 62.3 (4.3) | 51.4% female | Australia | Mental health: Depression, Anxiety, Stress, Well-being, Life satisfaction, Self-esteem | Longitudinal | Questionnaire (self-reported) | Questionnaire (self-reported) | Questionnaire (self-reported) | Questionnaire (self-reported) | Screen time; SL; Other: Transport, Work, PA, Chores, Self-care, Quiet time, Social/Domestic Activities | Overall; 1-to-1 ISM |
| Oviedo-Caro et al. [162] | 130 | 32.8 (4.5) | 100% female | Spain | Adiposity, Cardiorespiratory fitness | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; 1-to-1 ISM |
| Pina et al. [163] | Scotland: 150; South Africa: 138 | 60–85 | 78% & 82% female | Scotland, South Africa | Musculoskeletal health: Muscle strength, Muscle mass, Physical performance, and Bone mineral density | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual |
| Powell et al. [164] | 366 | 4.6 (5.3) | 46% female | Ireland | Cardiometabolic health markers | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA; Standing | Individual; 1-to-1 ISM; Proportional ISM |
| Rees-Punia et al. [165] | 549 | 30–65 | 58% female | USA | Weight change | Longitudinal | Device-based | Device-based | Device-based | Questionnaire (self-reported) | SB; SL; LPA; MPA; VPA | Overall; Individual; 1-to-1 ISM |
| Runacres et al. [166] | 176 | 13.8 (1.8) | 47.7% female | UK | Aerobic fitness (VO2 max) | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MPA; VPA | Individual; 1-to-1 ISM |
| Sampasa-Kanyinga et al. [57] | 14,620 | 14.9 (1.2) | 65.1% female | Canada | Depressive symptoms | Longitudinal | Questionnaire (self-reported) | Questionnaire (self-reported) | Questionnaire (self-reported) | Screen time; SL; MVPA | Individual; 1-to-1 ISM; Proportional ISM | |
| Sandborg et al. [167] |
X: 273 L: 242 |
31 (4) | 100% female | Sweden | Cardiometabolic health, Body composition | Cross-sectional; Longitudinal | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Individual; 1-to-1 ISM; Proportional ISM |
| Segura-Jiménez et al. [168] | 296 | 12.8 (2.4) | 49% female | Spain | Inflammatory markers: CRP, C3, C4, Leptin, TNF-α, IL-6, Adiponectin | Longitudinal | Device-based | Device-based | Device-based | Questionnaire (self-reported) | SB; SL; LPA; MVPA | 1-to-1 ISM |
| Smith et al. [169] | 258 | 9.7 (0.5) | 48.4% male | Iran, England | Fundamental movement skills | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
| Smith et al. [170] | 34 | 66.9 (4.5) | 56% male | Australia | Neuroplasticity | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual |
| St Laurent et al. [171] | 388 | 51.5 (9.5) months | 44.6% female | USA | Cognition, Social-emotional health | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Goldilocks |
| Su et al. [172] | 1475 | 20.7 (1.60) | 68% female | China | Depression symptoms | Cross-sectional | Questionnaire (self-reported) | Questionnaire (self-reported) | Questionnaire (self-reported) | Questionnaire (self-reported) | SB; SL; LPA; MVPA | Overall; 1-to-1 ISM |
| Suorsa et al. [173] | 213 | 63.5 (1.1) | 82% female | Finland | BMI, Waist circumference | Longitudinal | Device-based | Device-based | Device-based | Questionnaire (self-reported) | SB; SL; LPA; MVPA | Individual; 1-to-1 ISM |
| Swindell et al. [174] | 1462 | 52.8 (11.1) | 66% female | Denmark, Finland, The Netherlands, UK, Spain, Bulgaria, Australia, New Zealand | Cardiometabolic risk factors: BMI, Waist circumference, Body fat %, Triglycerides, Glucose fasting, Glucose 2 h, Insulin, HOMA-IR, HDL-C, LDL-C, Total cholesterol, hs-CRP, HbA1c, Systolic BP, Diastolic BP | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; 1-to-1 ISM |
| Talarico et al. [175] | 434 | 10–13 | 50.2% female | Canada | BMIz, Waist circumference, Log fat mass index | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual; Proportional ISM |
| Tan et al. [176] | 370 | 8–10 | 50.5% female | Singapore | HRQoL | Cross-sectional; Longitudinal | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Individual; 1-to-1 ISM |
| Taylor et al. [55] | 380 | 1, 2, 3.5, 5 | 48.5, 43.7, 46.3, and 49.2% female | New Zealand | Body composition, Bone health | Cross-sectional; Longitudinal | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Individual; Proportional ISM |
| Taylor et al. [177] | 690 | 7.9 (1.1) | 51.5% female | New Zealand | BMI | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA; Other: WASO | Proportional ISM |
| Taylor et al. [178] | 392 | 3.5, 5 | 50% female | New Zealand | Psychosocial and mental health | Longitudinal | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Proportional ISM |
| Tyler et al. [179] | 359 | 11.5 (1.4) | 49.3% male | England | Motor competence | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
| Vanderlinden et al. [180] | 410 | 71.3 (6.3) | 71% female | Belgium | Mental well-being | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
| Verhoog et al. [181] | 1934 | 70.9 (9.3) | 51.5% female | Netherlands | HRQoL | Cross-sectional | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | 1-to-1 ISM |
| Walmsley et al. [182] | 87498 | 40–79 | 58% female | UK | Cardiovascular disease | Longitudinal | Device-based | Device-based | Device-based | Device-based | SB; SL; LPA; MVPA | 1-to-1 ISM; Proportional ISM |
| Wang et al. [183] | 437 | 20.1 (1.7) | 51.7% female | China | Depression, Anxiety, Stress | Longitudinal | Questionnaire (self-reported) | Questionnaire (self-reported) | Questionnaire (self-reported) | Questionnaire (self-reported) | SB; Screen time; SL; LPA; MVPA | Overall; Individual; 1-to-1 ISM |
1Age in months indicated if not reported in years
aAlso examined 5-part composition with prolonged and non-prolonged sedentary behavior
N/R not reported, X cross-sectional, L longitudinal, HRQoL Health related quality of life, LPA light physical activity, MVPA moderate-to-vigorous physical activity, SB Sedentary behavior, SL sleep, WASO wake after sleep onset
The majority of studies (82/102) partitioned the 24-h movement composition into four parts consisting of sleep, sedentary behavior, light physical activity (LPA) and MVPA. A three-part composition represented the fewest number of time-use categories (e.g., screen time, sleep, MVPA) [57], whereas a nine-part composition represented the greatest number of time-use categories (e.g., screen time; sleep; other: transport, work, general physical activity, chores, self-care, quiet time, social) [58]. Of the 102 studies that assessed MVPA,1 the majority (89/102) used device-based estimates, with 12 using self- or proxy-reports, and one that used a combination of both methods [59]. Of the 99 studies that assessed LPA, the majority (89/99) used device-based estimates, with nine using self- or proxy-reports, and one study that used a combination of both methods [59]. A majority of studies (89/102) used device-based estimates to assess sedentary behavior, with only 11 studies using self- or proxy-reports. One study used a combination of both methods [59], and one study inferred daily sedentary time by subtracting sleep and physical activity estimates from 1440 min [60]. A majority of studies (60/102) used device-based estimates to assess sleep duration, with 39 studies using self- or proxy-reports, and only three studies using a combination [54, 59, 61].
Regarding analytic approaches, 64 studies examined the overall composition in relation to indicators of health, 67 studies examined associations between each behavior (relative to others) in relation to indicators of health, and 84 studies involved compositional isotemporal substitution modeling, of which 59 studies used 1-to-1 substitutions, 10 used proportional substitutions, and 15 used both approaches. A total of six studies examined optimal behavioral compositions for indicators of health, of which five of these studies used the Goldilocks approach and one study used the Many Different Roads approach.
Reporting practices
A summary of reporting practices by area and item is presented in Table 2, whereas detailed reporting practices for each study can be found in Supplementary File 2.
Table 2.
Summary statistics for reporting practices by item
| Area | Item | n/N (%) |
|---|---|---|
| Methodological justification | Was CoDA mentioned in Title/Abstract? | 100/102 (98%) |
| Was the concept of CoDA introduced and reasons provided for utilizing CoDA to examine associations between 24 h movement behaviors and indicators of health? | 94/102 (92%) | |
| Behavioral measurement and data handling strategies | Were the scoring/processing procedures for sleep described? | 90/102 (88%) |
| If device-based sleep assessment, was a sleep log used to aid in data processing? | 25/63 (40%) | |
| If device-based sleep assessment, was it clear how wake bouts were addressed? | 15/63 (24%) | |
| Was sleep clearly conceptualized (i.e., 24-h, nocturnal only, day only)? | 50/102 (49%) | |
| Was sleep clearly defined (i.e., sleep duration, time in bed)? | 37/102 (36%) | |
| Were naps included in the composition? |
Yes: 17/102 (17%) Unclear: 44/102 (43%) |
|
| Were the scoring/processing decisions for sedentary behavior clearly described? | 93/102 (91%) | |
| Were the scoring/processing decisions for LPA clearly described? | 92/99 (93%) | |
| Were the scoring/processing decisions for MVPA clearly described? | 94/102 (92%) | |
| If device-based measurement, was device placement described? | 87/90 (97%) | |
| If device-based measurement, was how many minutes of wear time was considered a valid day described? | 80/90 (89%) | |
| If device-based measurement, was how many valid days was needed to be considered a valid sample described? | 85/90 (94%) | |
| If device-based measurement, was which valid days were used for analysis described? | 44/90 (49%) | |
| Was how non-wear (if device-assessed) or time not accounted for (if questionnaire) described? | 61/100 (61%) | |
| Composition construction | Was how many parts (i.e., behaviors) the day was partitioned into clearly described? | 102/102 (100%) |
| Was how the behaviors were transformed (e.g., isometric log-ratio) described? | 101/102 (99%) | |
| Was the definition of a day (e.g., Midnight to Midnight/Wake to Wake) described? | 6/102 (6%) | |
| Was the time-bound window that the composition was closed to (e.g., exactly 24 h, mean wear time of sample) described? | 67/102 (66%) | |
| Was how zeros in the behavioral data were handled clearly described? | 27/102 (26%) | |
| Analytic plan | Was the analytic technique after compositional transformation reported clearly? | 101/102 (99%) |
| Were covariates adjusted for within CoDA models clearly outlined? | 99/102 (97%) | |
| Was how missing data was handled in the full dataset described? | 64/102 (63%) | |
| Was a comparison between those included in the analytic sample vs the full sample performed? | 29/101 (29%) | |
| Was a power analysis reported? | 15/102 (15%) | |
| Composition-specific descriptive statistics | Were the geometric means (% of time) for each behavior reported? | 74/102 (73%) |
| Were the arithmetic and/or geometric compositional means reported? | 97/102 (95%) | |
| Was the compositional variation matrix reported? | 51/102 (50%) | |
| Model results | Were the overall composition model statistics in relation to the outcome reported? | 64/102 (63%) |
| If associations between each behavior (relative to the other behaviors) in relation to the outcome were examined, were model statistics including standardized effect sizes (e.g., standardized beta) for each individual behavior reported? | 25/67 (37%) | |
| If isotemporal substitution was used, was the overall composition significantly associated with the outcome? | 43/84 (51%) | |
| If isotemporal substitution was used, was it clearly reported whether 1 to 1 or proportional replacement was computed? | 83/84 (99%) | |
| If isotemporal substitution was used, were substitutions across all behaviors reported? | 69/84 (82%) | |
| If isotemporal substitution was used, were the model statistics for replacing each behavior with time spent in the other behaviors reported, including effect sizes? | 72/84 (86%) | |
| If an optimal behavioral composition model was reported, was the overall composition significantly associated with the outcome? | 6/6 (100%) | |
| If an optimal behavior model was reported, were the estimates for optimal time spent in each behavior reported (in text, Table or a Figure), including a range (Goldilocks) or different options (Many Different Roads) associated with an optimal % of the outcome? | 5/6 (83%) | |
| If an optimal behavior model was reported, was the range associated with an optimal % of the outcome clearly described (e.g., 5%)? | 6/6 (100%) | |
| Auxiliary reporting | Were limitations of compositional data analysis discussed? | 16/102 (16%) |
| Was clinical meaningfulness of the effects discussed? | 22/102 (22%) | |
| Were study funding sources reported? | 100/102 (98%) | |
| Were conflicts of interest reported (including no COI)? | 95/102 (93%) |
Methodological justification
This reporting area included two items, with the majority of studies providing sufficient information (100/102 studies; 94/102 studies, respectively). Specifically, nearly all studies mentioned CoDA in the title and/or abstract (100/102) and introduced the concept of CoDA and provided reasons for utilizing CoDA to examine associations between 24-h movement behaviors and indicators of health (94/102).
Behavioral measurement and data handling strategies
This reporting area included 14 items. The majority of studies (≥ 75%) provided sufficient information for seven items. Most studies reported the scoring/processing procedures for MVPA2 (94/102), LPA (92/99), sedentary behavior (93/102), and sleep (90/102). Among the studies that used device-based assessments of 24-h movement behaviors, the device placement (87/90), how many minutes of wear time was considered a valid day (80/90), and how many valid days was needed to be considered a valid sample (85/90) were clearly described in most studies, but far fewer studies (44/90) clearly described which valid days were used for analysis (e.g., average across all days, four random days, proportion of weekdays and weekend). Across all studies, slightly more than half (61/100) clearly described how non-wear (if device-assessed) or time not accounted for (if self- or proxy-reported) was handled. Two of the 102 studies used a 24-h recall instrument that did not allow for time to be left unaccounted for.
Regarding sleep, roughly half (50/102) of the studies clearly conceptualized sleep (e.g., 24-h, nocturnal only, day only), but fewer (37/102) clearly defined sleep (e.g., sleep variable of interest and how it was calculated and/or defined). Very few studies included naps in the composition (17/102), although it was unclear in 44 studies. For studies with device-based sleep estimates, less than half (25/63) used sleep diaries to aid with data processing, and few (15/63) clearly reported how wake bouts were addressed.
Composition construction
This reporting area included five items. The majority of studies (≥ 75%) provided sufficient information for two items. That is, all 102 studies described how many parts (i.e., behaviors) the day was partitioned into and nearly all studies (101/102) reported how the behaviors were transformed via sets of log ratios. Very few studies (6/102) clearly described the definition of a day (e.g., midnight to midnight/wake to wake), although most studies (67/102) clearly described the time-bound window that the composition was closed to (e.g., exactly 24 h, mean wear time of sample). Further, few studies (27/102) clearly described how zeros in the behavioral data were handled. Among these studies, eight did not find cases with zeros during inspection, whereas 19 observed zeros and reported how they were handled.
Analytic plan
This reporting area included five items. The majority of studies (≥ 75%) provided sufficient information for two items. Specifically, nearly all studies (101/102) clearly reported the analytic technique (e.g., linear regression, latent profile analysis) used after compositional transformation, and whether covariates were adjusted for (99/102). We were able to infer how missing data was handled in more than half of the studies (64/102). In many instances the techniques used to handle missing data were not outright stated (e.g., listwise deletion, mean substitution, multiple imputation), but enough information was provided to determine which procedures were implemented. Less than a third of studies (29/101) compared the analytic sample to the total sample,3 and very few (15/102) studies provided a power analysis.
Composition-specific descriptive statistics
This reporting area included three items. The majority of studies (≥ 75%) provided sufficient information for one item. Most studies (74/102) reported the geometric means of the parts that comprised the 24-h movement composition (i.e., relative percentages of time spent in each behavior), whereas nearly all studies (97/102) reported the arithmetic and/or compositional means for each behavior. Of these studies, roughly one third (34/97) reported both the arithmetic (i.e., absolute) and compositional (i.e., adjusted to 24 h) means, nearly two thirds (60/97) reported only the compositional means, two studies reported only the arithmetic means [62, 63], and in one study it was unclear which means were reported [64]. Roughly half of studies (51/102) reported the compositional variation matrix, which shows the correlation between compositional parts.
Model results
This reporting area included nine items. The majority of studies (≥ 75%) provided sufficient information for six items. Over half of studies (64/102) reported overall model statistics regarding whether the 24-h movement behavior composition was significantly associated with the health outcome, of which only 25 studies included a standardized effect size (e.g., R2). Four studies reported a standardized effect size for the relationship between the 24-h movement behavior composition and indicator of health despite not reporting whether a significant association was observed.
Of the 67 studies that examined associations between each behavior (relative to others) in relation to indicators of health, less than half (25/67) reported associations for each behavior only if the overall model was significant, but four of these studies did not report statistical information that could be used to determine effect sizes for all behaviors (e.g., beta coefficient without standard error). 36 studies reported associations for each behavior regardless of whether the overall model was significant. Six out of 67 studies reported associations for only some behaviors (relative to others), regardless of whether the overall model was significant.
For studies that computed compositional isotemporal substitution models, roughly half (43/84) only examined time reallocations for indicators of health significantly associated with the overall 24-h movement behavior composition. In contrast, very few studies (9/84) computed isotemporal substitution models regardless of whether the overall 24-h movement behavior composition was significantly associated with the health outcome. In the remaining 32 studies, it was not reported whether the overall 24-h movement behavior composition was significantly associated with indicators of health. In all but one study (83/84) [54], it was clearly reported whether 1-to-1 (e.g., 10 min in MVPA replaced with 10 min in sleep) or proportional replacement (e.g., 10 min in MVPA proportionally reallocated across all other behaviors) was used. Most studies (69/84) reported substitutions across all behaviors, whereas some studies (15/84) only reported substitutions for select behaviors. Most studies (72/84) reported model statistics for reallocating time across behaviors, including effect sizes (e.g., beta coefficients with standard error or 95% confidence intervals).
Among the six studies that examined optimal behavioral compositions for indicators of health, all studies (6/6) clearly described the optimal % of the health outcome (e.g., best 5%, 85th percentile), and nearly all studies (5/6) reported estimates for the optimal amount of time spent in each behavior, including a range (Goldilocks approach) or different options (Many Different Roads approach) associated with the optimal % of the outcome. One study using the Goldilocks Approach only reported the exact optimal time-use estimates, but not the associated ranges of optimal time spent in each behavior [65].
Auxiliary reporting
This reporting area included four items and focused on placing CoDA findings within context, and general article reporting practices. The majority of studies (≥ 75%) provided sufficient information for two items, in that nearly all studies reported funding sources (100/102) and conflicts of interest (95/102). Very few (16/102) studies acknowledged potential limitations of using CoDA, or the clinical meaningfulness of the effect sizes observed (22/102).
Methodological quality and risk of bias assessment
The study quality and risk of bias results are presented in Table 3. The majority of studies (81/102) were considered to be fair quality, with only 19 considered to be good quality and two considered poor quality.
Table 3.
Study quality and risk of bias assessment by study
| Asano et al. [96], | Bezerra et al. [97], | Biddle et al. [99], | Blodgett et al. [100], | Blodgett et al. [101], | Booker et al. [60], | Brakenridge et al. [61], | Brayton et al. [64] | Cabanas- Sánchez et al. [102] | Carson et al. [103] | Carson et al. [104] | Chao et al. [105] | Chastin et al. [54] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Was the research question or objective in this paper clearly stated? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. Was the study population clearly specified and defined? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
| 3. Was the participation rate of eligible persons at least 50%? | No | No | Other | Yes | Other | Yes | Yes | Yes | Yes | Yes | Yes | Other | Other |
| 4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Other | No |
| 5. Was a sample size justification, power description, or variance and effect estimates provided? | No | No | No | No | No | No | No | Yes | No | No | No | No | No |
| 6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | No | No | No | No | No | No | No | Yes | Yes | No | No | No | Yes |
| 7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | No | No | No | No | No | No | No | Yes | Yes | No | No | No | Yes |
| 8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | No | Yes | Yes | No | No | Yes | No | Yes | No | No | Yes | No | No |
| 10. Was the exposure(s) assessed more than once over time? | No | Other | No | No | No | No | Other | No | Yes | No | No | No | No |
| 11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 12. Were the outcome assessors blinded to the exposure status of participants? | Other | Other | Other | Other | Other | No | Other | Other | Other | Other | Other | Other | Other |
| 13. Was loss to follow-up after baseline 20% or less? | Other | Other | Other | Other | Other | Other | Other | Yes | No | Other | Other | Other | No |
| 14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Totals | 6 | 7 | 7 | 7 | 5 | 7 | 7 | 11 | 10 | 6 | 8 | 4 | 6 |
| Chen et al. [62] | Chen et al. [106] | Chong et al. [107] | Chong et al. [108] | Clarke et al. [109] | Collings et al. [110]. D&MS | Collings et al. [111] Atherosclerosis | Curtis et al. [112] | Curtis et al. [113] | de Faria et al. [114] | del Pozo- Cruz et al. [115] | Domingues et al. [116] | Dumuid et al. [117] Qual Life Res | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Was the research question or objective in this paper clearly stated? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. Was the study population clearly specified and defined? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3. Was the participation rate of eligible persons at least 50%? | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Other | No | Other |
| 4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | No | Other | Yes | Yes | Yes | Yes | Yes | Other | Yes | Yes | Yes | Yes | Yes |
| 5. Was a sample size justification, power description, or variance and effect estimates provided? | No | No | No | No | No | No | No | No | No | Yes | No | Yes | No |
| 6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | No | No | Yes | Yes | Yes | No | No | No | No | No | No | No | No |
| 7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | No | No | Yes | Yes | Yes | No | No | No | No | No | No | No | No |
| 8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | No | No | Yes | No | No | Yes | Yes | No | No | Yes | No | No | Yes |
| 10. Was the exposure(s) assessed more than once over time? | No | No | Yes | Yes | No | No | No | No | No | No | No | No | No |
| 11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 12. Were the outcome assessors blinded to the exposure status of participants? | Other | Other | No | Other | Yes | No | Other | Other | Other | Other | Other | Other | Other |
| 13. Was loss to follow-up after baseline 20% or less? | Other | Other | No | Yes | Yes | Other | Other | Other | Other | Other | Other | Other | Other |
| 14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Totals | 6 | 6 | 10 | 10 | 11 | 8 | 8 | 6 | 7 | 9 | 6 | 6 | 7 |
| Dumuid et al. [118] Maturitas | Dumuid et al. [119] BMC Pub Health | Dumuid et al. [120] | Dumuid et al. [121] | Dumuid et al. [122] | Dumuid et al. [123] | Dumuid et al. [35] JAD | Faircloug h et al. [124] | Fairclough et al. [125] | Fairclough et al. [65] | Farrahi et al. [126] | Feter et al. [127] | Franssen et al. [128] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Was the research question or objective in this paper clearly stated? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. Was the study population clearly specified and defined? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3. Was the participation rate of eligible persons at least 50%? | Yes | Yes | No | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| 4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| 5. Was a sample size justification, power description, or variance and effect estimates provided? | Yes | Yes | No | No | No | No | No | No | No | No | No | No | Yes |
| 6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | No | No | No | No | No | No | No | No | No | No | No | No | No |
| 7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | No | No | No | No | No | No | No | No | No | No | No | No | No |
| 8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| 9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | No | Yes | No | Yes | Yes | No | No | Yes | Yes | No | Yes | No | No |
| 10. Was the exposure(s) assessed more than once over time? | No | No | Other | No | No | No | Other | No | No | No | No | Other | No |
| 11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 12. Were the outcome assessors blinded to the exposure status of participants? | No | Other | Other | Other | Other | No | Other | Yes | Other | No | No | Other | Other |
| 13. Was loss to follow-up after baseline 20% or less? | Other | Other | Other | Other | Other | Other | Other | Other | Other | Other | No | Other | Other |
| 14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Totals | 8 | 9 | 6 | 7 | 8 | 7 | 7 | 8 | 8 | 5 | 8 | 7 | 8 |
| Gupta et al. [129] | Gupta et al. [130] | Gupta et al. [131]. IJoO | Gupta et al. [132] IJBNPA | Healy et al. [53] | Hofman et al. [133] | Hyodo et al. [56] | Kastelic et al. [135], | Kandola et al. [134] | Kim et al. [59] | Kitano et al. [136] | Kuzik et al. [137] | Larisch et al. [138] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Was the research question or objective in this paper clearly stated? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. Was the study population clearly specified and defined? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| 3. Was the participation rate of eligible persons at least 50%? | No | Yes | Yes | No | Other | Other | Other | Other | No | Yes | Other | No | No |
| 4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 5. Was a sample size justification, power description, or variance and effect estimates provided? | No | No | No | No | No | No | No | No | Yes | No | No | No | No |
| 6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | No | No | No | Yes | No | No | No | No | Yes | No | No | No | No |
| 7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | No | No | No | Yes | No | No | No | No | Yes | No | No | No | No |
| 8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | Yes | No | No | Yes | No | Yes | No | Yes | Yes | No | No | No |
| 10. Was the exposure(s) assessed more than once over time? | No | No | No | No | No | Other | No | No | No | No | No | No | No |
| 11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 12. Were the outcome assessors blinded to the exposure status of participants? | Other | Other | Other | Other | Other | Other | Other | Other | Other | Other | Other | Other | Other |
| 13. Was loss to follow-up after baseline 20% or less? | Other | Other | Other | No | Other | Other | Other | Other | No | Other | Other | Other | Other |
| 14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Totals | 7 | 8 | 7 | 8 | 7 | 6 | 7 | 6 | 10 | 8 | 6 | 6 | 5 |
| Lau et al. [139] | Le et al. [140] | Lee et al. [141] | Lemons et al. [142] | Lewthwaite et al. [63] | Lin et al. [143] | Lu et al. [144] | Lund Rasmussen et al. [145] | Madden et al. [146] | Marshall et al. [147] | Marshall et al. [148] | Matricciani et al. [149] | McGee et al. [150] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Was the research question or objective in this paper clearly stated? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. Was the study population clearly specified and defined? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3. Was the participation rate of eligible persons at least 50%? | No | No | Yes | Yes | No | Other | Yes | Yes | Yes | Other | Other | Yes | No |
| 4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 5. Was a sample size justification, power description, or variance and effect estimates provided? | No | No | No | No | Yes | No | Yes | No | No | No | No | No | Yes |
| 6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | No | No | No | No | Yes | No | No | No | No | No | No | No | No |
| 7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | No | No | No | No | Yes | No | No | No | No | No | No | No | No |
| 8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| 9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | No | No | No | No | Yes | Yes | Yes | No | No | No | No | No |
| 10. Was the exposure(s) assessed more than once over time? | Other | No | No | Other | Yes | No | Other | No | No | Other | Other | No | No |
| 11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 12. Were the outcome assessors blinded to the exposure status of participants? | Other | Other | Other | Other | Other | Other | Other | Other | Other | Other | Other | Other | Other |
| 13. Was loss to follow-up after baseline 20% or less? | Other | Other | Other | Other | No | Other | Other | Other | Other | Other | Other | Other | Other |
| 14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Totals | 7 | 5 | 7 | 7 | 10 | 7 | 9 | 8 | 6 | 6 | 6 | 7 | 7 |
| McGregor et al. [151] | McGregor et al. [152] | Mellow et al. [153] | Mellow et al. [154] | Migueles et al. [155] | Migueles et al. [156] | Mitchell et al. [157] | Mota et al. [158] | Murray et al. [159] | Ng et al. [160] | Niemelä et al. [161] | Olds et al. [58] | Oviedo-Caro et al. [162] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Was the research question or objective in this paper clearly stated? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. Was the study population clearly specified and defined? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3. Was the participation rate of eligible persons at least 50%? | Yes | Yes | Other | Yes | Yes | Other | Yes | No | Yes | No | No | Other | Other |
| 4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 5. Was a sample size justification, power description, or variance and effect estimates provided? | No | No | Yes | Yes | No | No | No | Yes | No | No | No | Yes | No |
| 6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | No | Yes | No | No | No | Yes | No | Other | No | No | Yes | Yes | No |
| 7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | No | Yes | No | No | No | Yes | No | No | No | No | Yes | No | No |
| 8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | No | Yes | No | No | No | No | No | No | Yes | No | No | No | Yes |
| 10. Was the exposure(s) assessed more than once over time? | No | No | Other | No | Other | Yes | No | Other | Other | No | No | Yes | Other |
| 11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 12. Were the outcome assessors blinded to the exposure status of participants? | Other | Other | Other | Other | Other | Other | Other | Other | Other | Other | Other | Other | Other |
| 13. Was loss to follow-up after baseline 20% or less? | Other | Yes | Other | Other | Other | No | Other | Other | Other | Other | No | Yes | Other |
| 14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Totals | 7 | 11 | 7 | 8 | 7 | 9 | 7 | 7 | 8 | 6 | 8 | 10 | 7 |
| Pina et al. [163], | Powell et al. [164] | Rees-Punia et al. [165] | Runacres et al. [166] | Sampasa- Kanyinga et al. [57] | Sandborg et al. [167] | Segura-Jiménez et al. [168] | Smith et al. [169] SJMSS | Smith et al. [170], Clin Neurophysiol | St. Laurent et al. [171] | Su et al. [172], | Suorsa et al. [173] | Swindell et al. [174] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Was the research question or objective in this paper clearly stated? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. Was the study population clearly specified and defined? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | No | Yes | Yes |
| 3. Was the participation rate of eligible persons at least 50%? | Yes | No | No | Yes | Other | Yes | No | Other | Yes | No | Yes | No | Yes |
| 4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | Other | Yes | Yes | Other | Yes | Yes | Yes | Other | No | Yes | No | Yes | Yes |
| 5. Was a sample size justification, power description, or variance and effect estimates provided? | Yes | No | No | No | No | Yes | No | No | No | No | No | No | No |
| 6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | No | No | Yes | No | Yes | Yes | Yes | No | No | No | No | Yes | No |
| 7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | No | No | Yes | No | Yes | Yes | Yes | No | No | No | No | Yes | No |
| 8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | No | No | Yes | No | No | No | Yes | No | No | Yes | No | No | No |
| 10. Was the exposure(s) assessed more than once over time? | Other | Other | No | No | Yes | Yes | Yes | No | No | No | No | Yes | No |
| 11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 12. Were the outcome assessors blinded to the exposure status of participants? | Other | Other | Other | Other | Other | Other | Other | Other | Other | Other | Other | Other | Other |
| 13. Was loss to follow-up after baseline 20% or less? | Other | Other | No | Other | No | No | No | Other | Other | Other | Other | Yes | Other |
| 14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Totals | 7 | 6 | 9 | 6 | 9 | 11 | 10 | 4 | 5 | 7 | 5 | 10 | 7 |
| Talarico et al. [175] | Tan et al. [176] | Taylor et al. [55] | Taylor et al. [177], | Taylor et al. [178], | Tyler et al. [179], | Vanderlinden et al. [180] | Verhoog et al. [181] | Walmsley et al. [182] | Wang et al. [183] | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Was the research question or objective in this paper clearly stated? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||
| 2. Was the study population clearly specified and defined? | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | |||
| 3. Was the participation rate of eligible persons at least 50%? | Yes | No | Yes | Other | Yes | Yes | Other | Yes | Yes | Yes | |||
| 4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | Yes | Yes | Yes | Yes | Yes | Other | Yes | Yes | Yes | Yes | |||
| 5. Was a sample size justification, power description, or variance and effect estimates provided? | No | No | No | No | No | No | No | No | No | Yes | |||
| 6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | No | Yes | Yes | No | Yes | No | No | No | Yes | Yes | |||
| 7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | No | Yes | Yes | No | Yes | No | No | No | Yes | Yes | |||
| 8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||
| 9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | No | Yes | No | Yes | Yes | No | No | No | No | Yes | |||
| 10. Was the exposure(s) assessed more than once over time? | No | Yes | Yes | No | Yes | Other | No | No | No | No | |||
| 11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||
| 12. Were the outcome assessors blinded to the exposure status of participants? | Other | Other | Yes | Other | Yes | Other | Other | Other | Other | Other | |||
| 13. Was loss to follow-up after baseline 20% or less? | Other | No | No | Other | No | Other | Other | Other | Yes | Yes | |||
| 14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||
| Totals | 7 | 10 | 11 | 7 | 12 | 5 | 6 | 7 | 10 | 12 |
Discussion
The purpose of this systematic review was to describe research reporting practices in observational studies that have examined associations between 24-h movement behaviors and indicators of health using CoDA techniques. There has been considerable growth in this body of literature since Chastin and colleagues’ [41] first applied these technique to examine associations between 24-h movement behaviors and indicators of health in 2015, as evidenced by the inclusion of 102 observational studies with compositional exposures. While there was consistency across studies in reporting items specific to methodological justification, other areas had considerably more variation. Nevertheless, our results for study quality and risk of bias suggest that, to date, most published CoDA studies rely on rigorously collected data from quality samples, giving support for the utility of this approach as an impactful technique for public health researchers and policy makers. This rapidly growing body of literature has clear opportunities to improve reporting practices to provide further rigorous evidence surrounding the associations between 24-h movement behaviors and indicators of health. Doing so may help future systematic reviews and meta-analyses, and thus should be a top priority. The following sections provide detailed descriptions of our results by reporting areas.
Methodological justification
Given the recent shift from studying physical activity, sedentary behavior and sleep independently to the novel 24-h movement paradigm, it is encouraging to see that most studies (92%) introduced the statistical concept of CoDA and explained why CoDA is particularly well-suited for examining associations between 24-h movement behaviors and indicators of health. Despite receiving increased attention and adoption in recent years, the 24-h movement paradigm may still be novel to the audience of many journals depending on where authors submit manuscripts, thus requiring methodological justification to help readers understand the rationale behind adopting a compositional approach. It is also promising that nearly every study (98%) mentioned CoDA (or “compositions”) in the title and/or abstract, which will help with discovering CoDA studies in systematic searches of the literature.
Behavioral measurement and data handling strategies
It was encouraging that the majority of studies provided sufficient detail for half of the 14 items, but considerable improvements can be made in this area. While most studies (88–93%) described their data scoring/processing procedures for each behavior, reporting could be more clearly articulated to ensure the procedures are reproducible. For instance, authors who use the open-source 24-h accelerometry processing package GGIR [66] in R could include their data processing parameters in the supplemental materials as these are typically customized to each study and variations will influence the output. It is also worth noting that commercial wearables may pose challenges for transparency and reproducibility as many manufacturers use propriety algorithms to process data, although the availability of device-agnostic metrics derived from consumer wearable data may help to overcome this issue [67]. Other aspects of data handling were also consistently reported such as device placement and inclusion criteria that inform whether an accelerometry sample is considered valid (i.e., minimum daily wear time, number of valid days). This may be attributable to existing recommendations for reporting accelerometry methods in physical activity studies [68, 69]. However, greater consistency in reporting which valid days were selected for analysis (e.g., average across all days, four random days) is needed. This is particularly relevant in light of recent work demonstrating how different data handling strategies influence associations between 24-h movement guideline adherence and overweight/obesity status [70]. With consistent reporting of accelerometry data handling strategies, readers would be better able to evaluate the generalizability of the data to all days of the week. Despite the advantages of accelerometry, thorough and transparent reporting practices regarding the data handling are warranted regardless of the instrument used to assess 24-h movement behaviors.
Special attention should also be given to reporting practices surrounding sleep as a behavioral component in CoDA studies. Although sleep is a more recent addition to CoDA time-use analyses, researchers have been using accelerometers to measure sleep (i.e., actigraphy) since the early 1970s [71]. Similar to wake-time movement behaviors, there are recommendations and various approaches to processing and reporting sleep metrics measured via actigraphy [72–74] and questionnaires [75, 76]. However, there are notable gaps among included studies with adequately reporting sleep measures. Measurement periods of sleep (e.g., 24-h, nocturnal, daytime sleep, or naps) were often undefined or only overnight sleep was addressed or measured even though daytime sleep could be present in the sample. For example, daytime sleep was often not mentioned or studies using accelerometry did not use a sleep log to help with scoring additional sleep periods. Thus, in such cases, daytime sleep could be misclassified as sedentary behavior or, when processed with GGIR (without a sleep log), only the single longest sustained inactivity bout (i.e., sleep period) could be estimated. Further, like physical activity, sleep ‘time’ can be categorized into various metrics (e.g., time in bed, sleep duration), but most studies did not clearly identify the variable that was used or how it was defined. Additionally, whether wake after sleep onset (WASO) at night were included in their sleep measure was commonly unreported. Altogether, there is room to improve reporting practices of sleep in time-use studies and the adoption of common terms and recommendations that have been promoted among sleep science research is recommended [73, 74, 77, 78].
Composition construction
Studies had variable reporting in this area, with consistent reporting for two areas, and low reporting in the remaining three. First, the most consistent reporting across studies (100%) related to describing how many parts the day was partitioned into and how the behaviors were transformed via log ratios (99%). Although we were able to infer how many parts the day was partitioned into in all studies, researchers are encouraged to more clearly articulate such information in their data analysis section. Constructing the 24-h movement behavior composition is a crucial step before any CoDA approach can be undertaken. Beyond these two items, information pertaining to the time-bound window and composition closure was less consistently reported. One potential explanation for the former is that researchers may assume the reader interprets the composition having been closed to exactly 24 h despite alternative options (e.g., mean wear time of the sample). Nevertheless, this should be made clear. The latter finding was somewhat surprising given how many studies used GGIR to process their accelerometry data and its requirement to specify what constitutes a day as part of the code. Such reporting may be particularly valuable for studies investigating within-person effects regarding how daily movement compositions relate to health outcomes also measured daily (e.g., HDL and LDL cholesterol, stress). Finally, it was concerning that only a quarter of studies acknowledged zeroes given the challenges that zeros present in the behavioral data can pose for CoDA. Of the 19 studies that observed zeros, the most common procedures to address this issue were imputation using the log-ratio expectation maximization algorithm or multiplicative replacement using a fixed value in the dataset (e.g., 65% of the smallest possible non-zero value). This is promising as recent work has shown that these methods are preferred over simple replacement for preserving the data structure [79]. Researchers are advised to clearly indicate that behavioral data was inspected for zeros and report the procedures used to address this issue if such values are present in the dataset.
Analytic plan
Existing studies have consistently reported the analytic technique(s) used after compositional transformation (101/102) as well as whether covariates were adjusted for (99/102). In fact, acknowledging that key confounding variables were adjusted for was one of the items that contributed to the positive study quality and risk of bias scores observed. However, far fewer reported how missing data was handled, compared the analytic sample to the total sample or provided a power calculation. Understanding how missing data was handled has implications for generalizability and can influence the magnitude of the effects observed [80]. Similarly, contrasting the analytic and total sample can help identify any systematic differences that may introduce bias into the results. The low number of studies that computed a power analysis is likely attributable to most CoDA studies involving secondary data analysis, but as the 24-h movement paradigm sees greater adoption, it is reasonable to expect reporting of sample size estimates to become more common as studies are designed with the primary goal of examining how 24-h movement behaviors relate to indicators of health. Further, power calculations for 24-h movement behavior studies may not be as straightforward as more traditional analytic approaches and formal guidance has yet to be published. The lack of sample size justifications was one of the primary items that was a detriment to study quality and risk of bias scores.
Composition-specific descriptive statistics
Compositional descriptive statistics describing centrality were presented more than those for dispersion as evidenced by most studies reporting the compositional (92%) or geometric means (73%) and roughly half reporting the compositional variation matrix. The arithmetic means for each behavior (35%) were reported much less often than the compositional means. While reporting all these metrics within a manuscript may introduce some redundancy, researchers should consider allocating those not reported to the electronic supplemental materials.
Model results
Model results reporting was perhaps the most inconsistent among the various sections of the manuscripts that we examined, which may largely be attributed to selective reporting due to the lack of general standards in the field. Only 64 studies indicated whether the overall 24-h movement behavior composition was significantly associated with the outcome of interest, with even fewer reporting standardized effect sizes (e.g., R2). To our knowledge, there is currently no formal guidance regarding whether researchers should proceed to conduct subsequent analyses only if the overall composition is significant, but this topic deserves clarification. We raise this point because we found inconsistency in procedures adopted to date in that some studies moved on to subsequent modeling techniques (e.g., individual behaviors, isotemporal substitution) only if the overall model was significant, whereas others computed subsequent models regardless. Other studies even proceeded to subsequent modeling techniques without acknowledging whether the overall composition was related to the outcome. In these cases, it is possible that such analyses were only conducted if the overall model was significant, but insufficient reporting precludes such conclusions.
Another issue pertains to selective reporting of certain behaviors that comprise the composition. That is, several studies examining associations for individual behaviors or reallocation across behaviors did not report the results for every component of the composition. Such missing data poses an issue for quantifying effects across behaviors via meta-analysis. One reason for this may be the specificity of the research questions that have been investigated (e.g., What is the effect of reallocating time away from sedentary behavior on depression). In such instances, authors may view reporting certain estimates as irrelevant (e.g., replacing sleep with MVPA), but this information could be provided in supplementary materials. Doing so may also limit the practice of dividing a single substantial piece of research into several smaller, separate publications, otherwise known as salami slicing [81]. Finally, the field could also benefit from more consistently reporting statistical information that can be used to compute effect sizes. For instance, only reporting an unstandardized beta coefficient and indicating significance via a p value is insufficient. Researchers are strongly encouraged to report adequate statistical information (e.g., standardized estimates, 95% confidence intervals, standard errors) that can be used to perform meta-analysis as post-publication data requests often go unanswered [82].
While isotemporal substitution has received the most attention, other CoDA approaches are quickly emerging. Models that identify optimal behavioral compositions (i.e., Goldilocks approach; [34]) or similar behavioral compositions that result in equivalent outcomes (i.e., Many Different Roads approach; [35]) have been introduced in recent years. Perhaps due to their recency, but balanced against the limited number of studies, consistent reporting was observed across these six studies in that each study clearly reported the optimal value of the indicator of health used as a referent (e.g., best 5%) and nearly all studies provided estimates for the optimal range or different behavioral combinations associated with the optimal outcome.
Auxiliary reporting
Despite most studies introducing the concept of CoDA, few acknowledged its limitations and utility amongst clinical evidence. Inherently, one difficulty when using a compositional approach is the ability to translate the relative values (compositional components) back to values that may be more relevant in the public health sector (minutes of activity). As for utility, one potential explanation may be the availability of such metrics for the indicators of health examined. In future, authors are encouraged to describe if a meaningful difference has been established for their outcome of interest as a starting point. This also ties back to the importance of reporting of effect sizes and/or confidence intervals [83] in that if these statistics are not presented, it is challenging to determine how close to a meaningful effect there may be. Another reason relates to the scope of the journals that authors direct their manuscripts to in that clinical meaningfulness may be more commonly reported in clinically oriented journals than those focused more broadly on public health. In future, authors are encouraged to describe if a meaningful difference has been established for their outcome of interest as a starting point. In contrast, conflicts of interest (95/102) and funding sources (100/102) were consistently reported across studies, which may be driven by the requirements of journals (i.e., using the STROBE checklist).
Future directions
We found that a significant amount of information was not reported in the studies included in this review, which underscores the need for a harmonized approach and subsequent reporting checklist for observational studies utilizing CoDA to examine associations between 24-h movement behaviors and indicators of health. Unifying reporting in this rapidly growing body of literature is a key next step to be able to precisely quantify associations between 24-h movement behaviors and indicators of health. While it may be unreasonable to expect that every item is applicable when reporting individual studies given the growing variety of analytic approaches available, we believe that this review provides some initial guidance and recommendations to inform the creation of an observational CoDA checklist. The development of the CREMAS [39], another common methodology in 24-h movement behaviors research, followed similar procedures as those adopted in the present study, however, additional procedures can be adopted to achieve consensus regarding best practices for reporting moving forward. Specifically, Delphi methodology represents a useful tool to systematically reach consensus through an iterative and interactive process involving experts in the field and end-users [84]. Delphi methodology has been used often in the field of physical activity research [85–88], including checklist development (e.g., [89–91]). The present review represents a key first step in developing a reporting checklist for movement behavior studies that use CoDA, to be followed by adopting a Delphi process to achieve consensus on best practices in the field. While there are inherent limitations of such checklists, establishing reporting practices in this area stands to be an informative and succinct way of improving transparency and reproducibility. To circumvent journal-imposed word count and table limits, the checklist could be included in manuscript submissions as an electronic supplemental material, which are encouraged and available in most journals. Finally, this review only examined observational studies in which 24-h movement behaviors were specified as the exposure, failing to consider reporting practices in studies that employ experimental designs or those specifying 24-h movement behaviors as the outcome. Examining reporting practices in other study designs may be worthwhile as the body of literature evolves and may ultimately help to inform a more comprehensive reporting checklist for all studies employing CoDA.
Strengths and limitations
Strengths of the current review include assessment of a novel and timely topic (CoDA) in the 24-h movement realm, following systematic review best practice recommendations, including multiple supplementary strategies, and a comprehensive approach to examine reporting practices using this suite of analytic techniques, which was informed by existing evidence and expert guidance. A final strength relates to the inclusion of studies that used self-reported instruments and/or device-based measures, which allowed for more studies to be included to better understand reporting practices in the field to date—an important consideration given most of the current literature involved secondary analysis of existing data sources. This inclusive approach is an important consideration given the resources available to researchers differ vastly across institutions and countries. While there are inherent differences in what is reported when using self-reported versus device-based instruments, the development and implementation of a single reporting standards checklist may be beneficial for promoting consistency in the field regardless of the instruments used to assess 24-h movement behaviors.
While the findings of this review provide valuable insights, five major limitations must be acknowledged. First, we focused on studies that used CoDA to examine associations between 24-h movement behaviors and indicators of health, but studies that only examine wake-time movement compositions are also common (i.e., no sleep) [92–94]. It is reasonable to posit that reporting practices may be similar in CoDA studies that do not include sleep, but this review also revealed a clear need to further our understanding of sleep assessment in CoDA. Second, we appreciate the overall quality of included studies was fair, which may have also translated to their mediocre reporting practices. This review also highlighted multiple areas that could be improved in future studies to enhance to rigor of reporting and general study conduct. Third, CoDA techniques applied in the field of time-use epidemiology are still emerging, and therefore, we were only able to examine reporting practices based on what exists in the literature today. As novel techniques are developed and implemented, such as a “Movement Index” score [95], reporting practices will need to be revisited. At the same time, some items we examined may not remain relevant in the future and could be removed from reporting standards. For instance, the concept of CoDA has received considerable attention as evidenced by over 100 studies included in the present review, which brings into question whether introduction and justification of using CoDA is required as audiences of this literature become more familiar with the concept. Given that reporting checklists are often updated, there exists an opportunity to update guidance as CoDA sees more widespread adoption. Fourth, we took a conservative approach when classifying items as not reported if they were not explicitly acknowledged, which may have led to underestimating of reporting in some areas. For example, only 27 studies mentioned whether the behavioral data was examined for zero values, and if present, how they were handled, but it is possible that authors felt it was implied that no zeros were present and therefore did not report this item. Such issues would be resolved with standardized reporting guidance. Finally, despite the comprehensiveness of our literature search, some studies may have been overlooked, such as those that have been published in languages other than English.
Conclusion
This review described research reporting practices in 102 observational studies that have used CoDA to investigate associations between 24-h movement behaviors and indicators of health. Study quality and risk of bias was average for this relatively new area of inquiry, which was also demonstrated in the considerable variability in CoDA research reporting practices. Consistent, clear, and detailed reporting practices are needed as the field of time-use epidemiology aims to accurately capture and analyze movement behavior data, facilitate comparisons across studies, and inform public health interventions and policy decisions. Achieving consensus regarding reporting recommendations is a key next step.
Supplementary Information
Acknowledgements
We would like to thank Matt Hayward for assistance with developing the search strategy. We would also like to thank Drs. Dot Dumuid, Sebastian Chastin, Valarie Carson, Corneel Vandelanotte, Željko Pedišić, Simone Verswiejen, Ashleigh Smith, Scott Duncan, Timothy Olds, Stuart Fairclough and Maddison Mellow for their expert consultation.
Author contributions
CRediT author statement: DB: conceptualization, methodology, investigation, data curation, formal analysis, writing—original draft; project administration; SB: methodology, investigation, writing—original draft; CG: methodology, investigation, writing—review & editing; GB: methodology, investigation, writing—review & editing; CP: methodology, investigation, writing—original draft; CP: investigation, writing—review & editing; CSL: methodology, investigation, writing—original draft; EJ: methodology; CK: methodology, investigation, writing—original draft; project administration.
Funding
(1) CLK was supported by K99HD107158 (PI: Kracht) and P20GM144269 (PIs: Donnelly, Thyfault, Weinman). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. (2) Research reported in this publication was supported in part by the NIGMS under Award Number P20GM130420 (Burkart). (3) GMB is jointly funded by the Canadian Institutes of Health Research and Michael Smith Health Research BC postdoctoral fellowships.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
DB is an Editorial Board Member of the Journal of Activity, Sedentary and Sleep Behaviors. All other authors have no conflicts of interest to disclose.
Footnotes
For reporting purposes, compositions that included stepping or total physical activity were inferred as MVPA.
For reporting purposes, compositions that included stepping or total physical activity were inferred as MVPA.
Reporting in one study did not allow for comparison as we were unable to determine whether the analytic sample or total sample was reported, resulting in this item being reported out of 101 instead of 102 studies.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32(5):541–56. [DOI] [PubMed] [Google Scholar]
- 2.Posadzki P, Pieper D, Bajpai R, Makaruk H, Könsgen N, Neuhaus AL, et al. Exercise/physical activity and health outcomes: an overview of Cochrane systematic reviews. BMC Public Health. 2020;20(1):1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Rezende LFM, Lopes MR, Rey-López JP, Matsudo VKR, Luiz OC. Sedentary behavior and health outcomes: an overview of systematic reviews. PLoS ONE. 2014;9(8): e105620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saunders TJ, McIsaac T, Douillette K, Gaulton N, Hunter S, Rhodes RE, et al. Sedentary behaviour and health in adults: an overview of systematic reviews. Appl Physiol Nutr Metab. 2020;45(10 Suppl 2):S197-217. [DOI] [PubMed] [Google Scholar]
- 5.Firth J, Solmi M, Wootton RE, Vancampfort D, Schuch FB, Hoare E, et al. A meta-review of “lifestyle psychiatry”: the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry. 2020;19(3):360–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaput JP, Dutil C, Featherstone R, Ross R, Giangregorio L, Saunders TJ, et al. Sleep duration and health in adults: an overview of systematic reviews. Appl Physiol Nutr Metab. 2020;45(10 Suppl 2):S218–31. [DOI] [PubMed] [Google Scholar]
- 7.Dumuid D, Stanford TE, Martin-Fernández JA, Pedišić Ž, Maher CA, Lewis LK, et al. Compositional data analysis for physical activity, sedentary time and sleep research. Stat Methods Med Res. 2018;27(12):3726–38. [DOI] [PubMed] [Google Scholar]
- 8.Dumuid D, Pedišić Ž, Palarea-Albaladejo J, Martín-Fernández JA, Hron K, Olds T. Compositional data analysis in time-use epidemiology: what, why, how. Int J Environ Res Public Health. 2020;17(7):2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumuid D, Pedišić Ž, Palarea-Albaladejo J, Martín-Fernández JA, Hron K, Olds T. Compositional data analysis in time-use epidemiology. In: Filzmoser P, Hron K, Martín-Fernández JA, Palarea-Albaladejo J, editors. Advances in compositional data analysis. Springer International Publishing; 2021. p. 383–404. 10.1007/978-3-030-71175-7_20. [Google Scholar]
- 10.Chaput JP, Carson V, Gray CE, Tremblay MS. Importance of all movement behaviors in a 24 hour period for overall health. Int J Environ Res Public Health. 2014;11(12):12575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tremblay MS, Ross R. How should we move for health? The case for the 24-hour movement paradigm. CMAJ. 2020;192(49):E1728–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberger ME, Fulton JE, Buman MP, Troiano RP, Grandner MA, Buchner DM, et al. The 24-hour activity cycle: a new paradigm for physical activity. Med Sci Sports Exerc. 2019;51(3):454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falck RS, Davis JC, Li L, Stamatakis E, Liu-Ambrose T. Preventing the ‘24-hour Babel’: the need for a consensus on a consistent terminology scheme for physical activity, sedentary behaviour and sleep. Br J Sports Med. 2021;56:367–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tremblay MS, Carson V, Chaput JP, Connor Gorber S, Dinh T, Duggan M, et al. Canadian 24-hour movement guidelines for children and youth: an integration of physical activity, sedentary behaviour, and sleep. Appl Physiol Nutr Metab. 2016;41(6 Suppl 3):S311–27. [DOI] [PubMed] [Google Scholar]
- 15.Tremblay MS, Chaput JP, Adamo KB, Aubert S, Barnes JD, Choquette L, et al. Canadian 24-hour movement guidelines for the early years (0–4 years): an integration of physical activity, sedentary behaviour, and sleep. BMC Public Health. 2017;17(Suppl 5):874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross R, Chaput JP, Giangregorio LM, Janssen I, Saunders TJ, Kho ME, et al. Canadian 24-hour movement guidelines for adults aged 18–64 years and adults aged 65 years or older: an integration of physical activity, sedentary behaviour, and sleep. Appl Physiol Nutr Metab. 2020;45(10 Suppl 2):S57-102. [DOI] [PubMed] [Google Scholar]
- 17.Draper CE, Tomaz SA, Biersteker L, Cook CJ, Couper J, de Milander M, et al. The South African 24-hour movement guidelines for birth to 5 years: an integration of physical activity, sitting behavior, screen time, and sleep. J Phys Act Health. 2020;17(1):109–19. [DOI] [PubMed] [Google Scholar]
- 18.Okely AD, Ghersi D, Hesketh KD, Santos R, Loughran SP, Cliff DP, et al. A collaborative approach to adopting/adapting guidelines—the Australian 24-hour movement guidelines for the early years (Birth to 5 years): an integration of physical activity, sedentary behavior, and sleep. BMC Public Health. 2017;17(Suppl 5):869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reilly JJ, Hughes AR, Janssen X, Hesketh KR, Livingstone S, Hill C, et al. GRADE-ADOLOPMENT process to develop 24-hour movement behavior recommendations and physical activity guidelines for the under 5s in the United Kingdom, 2019. J Phys Act Health. 2020;17(1):101–8. [DOI] [PubMed] [Google Scholar]
- 20.Loo BKG, Okely AD, Pulungan A, Jalaludin MY. Asia-Pacific Consensus Statement on integrated 24-hour activity guidelines for children and adolescents. Br J Sports Med. 2022;56(10):539–45. [DOI] [PubMed] [Google Scholar]
- 21.Australian Government Department of Health. Australian 24-hour movement guidelines for children and young people (5–17 years)—an integration of physical activity, sedentary behaviour and sleep. Australian Government Department of Health and Ageing; 2019. https://www1.health.gov.au/internet/main/publishing.nsf/Content/health-24-hours-phys-act-guidelines
- 22.Rollo S, Antsygina O, Tremblay MS. The whole day matters: understanding 24-hour movement guideline adherence and relationships with health indicators across the lifespan. J Sport Health Sci. 2020;9:493–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groves CI, Huong C, Porter CD, Summerville B, Swafford I, Witham B, et al. Associations between 24-h movement behaviors and indicators of mental health and well-being across the lifespan: a systematic review. J Act Sedentary Sleep Behav. 2024;3(1):9. [Google Scholar]
- 24.Feng J, Zheng C, Sit CHP, Reilly JJ, Huang WY. Associations between meeting 24-hour movement guidelines and health in the early years: a systematic review and meta-analysis. J Sports Sci. 2021;39:1–13. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Li X, Li G, Haegele JA, Zou L, Chen S, et al. Prevalence of meeting 24-hour movement guidelines and its associations with health indicators in people with disabilities: a systematic review and meta-analysis. Disabil Health J. 2024;17: 101616. [DOI] [PubMed] [Google Scholar]
- 26.Wilhite K, Booker B, Huang BH, Antczak D, Corbett L, Parker P, et al. Combinations of physical activity, sedentary behavior, and sleep and their associations With physical, psychological, and educational outcomes in children and adolescents: a systematic review. Am J Epidemiol. 2022;192:665–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sampasa-Kanyinga H, Colman I, Goldfield GS, Janssen I, Wang J, Podinic I, et al. Combinations of physical activity, sedentary time, and sleep duration and their associations with depressive symptoms and other mental health problems in children and adolescents: a systematic review. Int J Behav Nutr Phys Act. 2020;17:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Lannoy L, Barbeau K, Vanderloo LM, Goldfield G, Lang JJ, MacLeod O, et al. Evidence supporting a combined movement behavior approach for children and youth’s mental health—a scoping review and environmental scan. Ment Health Phys Act. 2023;24: 100511. [Google Scholar]
- 29.Matthews CE, Keadle SK, Moore SC, Schoeller DS, Carroll RJ, Troiano RP, et al. Measurement of active and sedentary behavior in context of large epidemiologic studies. Med Sci Sports Exerc. 2018;50(2):266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ridley K, Olds TS, Hill A. The Multimedia activity recall for children and adolescents (MARCA): development and evaluation. Int J Behav Nutr Phys Act. 2006;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calabro MA, Welk GJ, Carriquiry AL, Nusser SM, Beyler NK, Mathews CE. Validation of a computerized 24-hour physical activity recall (24PAR) instrument with pattern-recognition activity monitors. J Phys Act Health. 2009;6(2):211–20. [DOI] [PubMed] [Google Scholar]
- 32.Aitchison J. The statistical analysis of compositional data. J R Stat Soc Ser B Methodol. 1982;44(2):139–77. [Google Scholar]
- 33.Dumuid D, Pedišić Ž, Stanford TE, Martín-Fernández JA, Hron K, Maher CA, et al. The compositional isotemporal substitution model: a method for estimating changes in a health outcome for reallocation of time between sleep, physical activity and sedentary behaviour. Stat Methods Med Res. 2019;28(3):846–57. [DOI] [PubMed] [Google Scholar]
- 34.Dumuid D, Olds T, Lange K, Edwards B, Lycett K, Burgner DP, et al. Goldilocks Days: optimising children’s time use for health and well-being. J Epidemiol Community Health. 2022;76(3):301–8. [DOI] [PubMed] [Google Scholar]
- 35.Dumuid D, Mellow ML, Stanford TE, Chong KH, Sawyer SM, Smith AE, et al. Many different roads lead to Rome: equivalence of time-use for activity, sedentary and sleep behaviours and dietary intake profiles among adolescents. J Act Sedentary Sleep Behav. 2022;1(1):6. [Google Scholar]
- 36.Miatke A, Olds T, Maher C, Fraysse F, Mellow ML, Smith AE, et al. The association between reallocations of time and health using compositional data analysis: a systematic scoping review with an interactive data exploration interface. Int J Behav Nutr Phys Act. 2023;20(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janssen I, Clarke AE, Carson V, Chaput JP, Giangregorio LM, Kho ME, et al. A systematic review of compositional data analysis studies examining associations between sleep, sedentary behaviour, and physical activity with health outcomes in adults1. Appl Physiol Nutr Metab. 2020. 10.1139/apnm-2020-0160. [DOI] [PubMed] [Google Scholar]
- 38.Zahran S, Visser C, Ross-White A, Janssen I. A systematic review of compositional analysis studies examining the associations between sleep, sedentary behaviour, and physical activity with health indicators in early childhood. J Act Sedentary Sleep Behav. 2023;2(1):1. [Google Scholar]
- 39.Liao Y, Skelton K, Dunton G, Bruening M. A systematic review of methods and procedures used in ecological momentary assessments of diet and physical activity research in youth: An adapted STROBE checklist for reporting EMA studies (CREMAS). J Med Internet Res. 2016;18(6): e4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chastin SFM, Palarea-Albaladejo J, Dontje ML, Skelton DA. Combined effects of time spent in physical activity, sedentary behaviors and sleep on obesity and cardio-metabolic health markers: a novel compositional data analysis approach. PLoS ONE. 2015;10: e0139984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Y, Rosenberg DE, Greenwood-Hickman MA, McCurry SM, Proust-Lima C, Nelson JC, et al. Analysis of the 24-h activity cycle: an illustration examining the association with cognitive function in the Adult Changes in Thought study. Front Psychol. 2023;14:1083344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother. 2010;1(2):100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9. [DOI] [PubMed] [Google Scholar]
- 45.Mcgregor DE, Dall PM, Palarea-Albaladejo J, Chastin SFM. Compositional data analysis in physical activity and health research. Looking for the right balance. In: Filzmoser P, Hron K, Martín-Fernández JA, Palarea-Albaladejo J, editors. Advances in compositional data analysis. Springer International Publishing; 2021. p. 363–82. 10.1007/978-3-030-71175-7_19. [Google Scholar]
- 46.Haszard JJ, Meredith-Jones K, Farmer V, Williams S, Galland B, Taylor R. Non-wear time and presentation of compositional 24-hour time-use analyses influence conclusions about sleep and body mass index in children. J Meas Phys Behav. 2020;3(3):204–10. [Google Scholar]
- 47.Dumuid D, Olds T, Wake M, Rasmussen CL, Pedišić Ž, Hughes JH, et al. Your best day: An interactive app to translate how time reallocations within a 24-hour day are associated with health measures. PLoS ONE. 2022;17(9): e0272343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGregor D, Palarea-Albaladejo J, Dall P, Hron K, Chastin S. Cox regression survival analysis with compositional covariates: application to modelling mortality risk from 24-h physical activity patterns. Stat Methods Med Res. 2020;29(5):1447–65. [DOI] [PubMed] [Google Scholar]
- 49.Jašková P, Palarea-Albaladejo J, Gába A, Dumuid D, Pedišić Ž, Pelclová J, et al. Compositional functional regression and isotemporal substitution analysis: methods and application in time-use epidemiology. Stat Methods Med Res. 2023;32(10):2064–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Institutes of Health. National Institutes of Health quality assessment tool for observational cohort and cross-sectional studies. Bethesda: NIH; 2016. [Google Scholar]
- 51.Vizheh M, Qorbani M, Arzaghi SM, Muhidin S, Javanmard Z, Esmaeili M. The mental health of healthcare workers in the COVID-19 pandemic: a systematic review. J Diabetes Metab Disord. 2020;19(2):1967–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Madigan S, Prime H, Graham SA, Rodrigues M, Anderson N, Khoury J, et al. Parenting behavior and child language: a meta-analysis. Pediatrics. 2019;144(4): e20183556. [DOI] [PubMed] [Google Scholar]
- 53.Healy S, Brewer B, Garcia J, Daly J, Patterson F. Sweat, sit, sleep: a compositional analysis of 24-hr movement behaviors and body mass index among children with Autism Spectrum Disorder. Autism Res Off J Int Soc Autism Res. 2021;14(3):545–50. [DOI] [PubMed] [Google Scholar]
- 54.Chastin S, McGregor D, Palarea-Albaladejo J, Diaz KM, Hagströmer M, Hallal PC, et al. Joint association between accelerometry-measured daily combination of time spent in physical activity, sedentary behaviour and sleep and all-cause mortality: a pooled analysis of six prospective cohorts using compositional analysis. Br J Sports Med. 2021;55(22):1277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor RW, Haszard JJ, Meredith-Jones KA, Galland BC, Heath ALM, Lawrence J, et al. 24-h movement behaviors from infancy to preschool: cross-sectional and longitudinal relationships with body composition and bone health. Int J Behav Nutr Phys Act. 2018;15(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hyodo K, Kitano N, Ueno A, Yamaguchi D, Watanabe Y, Noda T, et al. Association between intensity or accumulating pattern of physical activity and executive function in community-dwelling older adults: a cross-sectional study with compositional data analysis. Front Hum Neurosci. 2023;16:1018087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sampasa-Kanyinga H, Colman I, Dumuid D, Janssen I, Goldfield GS, Wang JL, et al. Longitudinal association between movement behaviours and depressive symptoms among adolescents using compositional data analysis. PLoS ONE. 2021;16(9): e0256867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olds T, Burton NW, Sprod J, Maher C, Ferrar K, Brown WJ, et al. One day you’ll wake up and won’t have to go to work: the impact of changes in time use on mental health following retirement. PLoS ONE. 2018;13(6): e0199605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim Y, Burns R, Lee D, Welk G. Associations of movement behaviors and body mass index: comparison between a report-based and monitor-based method using Compositional Data Analysis. Int J Obes 2005. 2021;45(1):266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Booker R, Holmes ME, Newton RL, Norris KC, Thorpe RJ, Carnethon MR. Compositional analysis of movement behaviors’ association on high-sensitivity c-reactive protein: the Jackson heart study. Ann Epidemiol. 2022;76:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brakenridge CJ, Healy GN, Sethi P, Carver A, Bellettiere J, Salim A, et al. Contrasting compositions of sitting, standing, stepping, and sleeping time: associations with glycaemic outcome by diabetes risk. Int J Behav Nutr Phys Act. 2021;18(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen M, Chua T, Shen Z, Tay LY, Wang X, Chia M. The associations between 24-hour movement behaviours and quality of life in preschoolers: a compositional analysis of cross-sectional data from 2018–2021. Int J Environ Res Public Health. 2022;19(22):14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lewthwaite H, Olds T, Williams M, Effing T, Dumuid D. Use of time in chronic obstructive pulmonary disease: longitudinal associations with symptoms and quality of life using a compositional analysis approach. PLoS ONE. 2019;14(3): e0214058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brayton RP, Price AM, Jones C, Ellis C, Burkhart S, Knell G. Prospective evaluation of 24-hour movement behaviors among adolescents recovering from a sport-related concussion. Appl Neuropsychol Child. 2023. 10.1080/21622965.2023.2181082. [DOI] [PubMed] [Google Scholar]
- 65.Fairclough SJ, Clifford L, Brown D, Tyler R. Characteristics of 24-hour movement behaviours and their associations with mental health in children and adolescents. J Act Sedentary Sleep Behav. 2023;2(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Migueles JH, Rowlands AV, Huber F, Sabia S, van Hees VT. GGIR: A research community–driven open source R package for generating physical activity and sleep outcomes from multi-day raw accelerometer data. J Meas Phys Behav. 2019;2(3):188–96. [Google Scholar]
- 67.Weaver RG, White J, Finnegan O, Nelakuditi S, Zhu X, Burkart S, et al. A device agnostic approach to predict children’s activity from consumer wearable accelerometer data: a proof-of-concept study. Med Sci Sports Exerc. 2024;56(2):370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montoye AHK, Moore RW, Bowles HR, Korycinski R, Pfeiffer KA. Reporting accelerometer methods in physical activity intervention studies: a systematic review and recommendations for authors. Br J Sports Med. 2018;52(23):1507–16. [DOI] [PubMed] [Google Scholar]
- 69.Migueles JH, Cadenas-Sanchez C, Ekelund U, Delisle Nyström C, Mora-Gonzalez J, Löf M, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med Auckl NZ. 2017;47(9):1821–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pfledderer CD, Burkart S, Dugger R, Parker H, von Klinggraeff L, Okely AD, et al. The impact of different data handling strategies on the proportion of children classified as meeting 24-h movement guidelines and associations with overweight and obesity. J Act Sedentary Sleep Behav. 2024;3(1):1. [Google Scholar]
- 71.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–92. [DOI] [PubMed] [Google Scholar]
- 72.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15(5):461–9. [DOI] [PubMed] [Google Scholar]
- 73.de Zambotti M, Cellini N, Goldstone A, Colrain IM, Baker FC. Wearable sleep technology in clinical and research settings. Med Sci Sports Exerc. 2019;51(7):1538–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Depner CM, Cheng PC, Devine JK, Khosla S, de Zambotti M, Robillard R, et al. Wearable technologies for developing sleep and circadian biomarkers: a summary of workshop discussions. Sleep. 2020;43(2):zsz254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robbins R, Quan SF, Barger LK, Czeisler CA, Fray-Witzer M, Weaver MD, et al. Self-reported sleep duration and timing: a methodological review of event definitions, context, and timeframe of related questions. Sleep Epidemiol. 2021;1: 100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nascimento-Ferreira MV, Collese TS, de Moraes ACF, Rendo-Urteaga T, Moreno LA, Carvalho HB. Validity and reliability of sleep time questionnaires in children and adolescents: a systematic review and meta-analysis. Sleep Med Rev. 2016;30:85–96. [DOI] [PubMed] [Google Scholar]
- 77.Quante M, Kaplan ER, Rueschman M, Cailler M, Buxton OM, Redline S. Practical considerations in using accelerometers to assess physical activity, sedentary behavior, and sleep. Sleep Health. 2015;1(4):275–84. [DOI] [PubMed] [Google Scholar]
- 78.Mazzotti DR, Haendel MA, McMurry JA, Smith CJ, Buysse DJ, Roenneberg T, et al. Sleep and circadian informatics data harmonization: a workshop report from the Sleep Research Society and Sleep Research Network. Sleep. 2022;45(6):zsac002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lund Rasmussen C, Palarea-Albaladejo J, Johansson MS, Crowley P, Stevens ML, Gupta N, et al. Zero problems with compositional data of physical behaviors: a comparison of three zero replacement methods. Int J Behav Nutr Phys Act. 2020;17(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Woods AD, Gerasimova D, Van Dusen B, Nissen J, Bainter S, Uzdavines A, et al. Best practices for addressing missing data through multiple imputation. Infant Child Dev. 2023;33: e2407. [Google Scholar]
- 81.Norman G. Data dredging, salami-slicing, and other successful strategies to ensure rejection: twelve tips on how to not get your paper published. Adv Health Sci Educ. 2014;19(1):1–5. [DOI] [PubMed] [Google Scholar]
- 82.Savage CJ, Vickers AJ. Empirical study of data sharing by authors publishing in PLoS journals. PLoS ONE. 2009;4(9): e7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kallogjeri D, Spitznagel EL Jr, Piccirillo JF. Importance of defining and interpreting a clinically meaningful difference in clinical research. JAMA Otolaryngol Neck Surg. 2020;146(2):101–2. [DOI] [PubMed] [Google Scholar]
- 84.Dalkey N, Helmer O. An experimental application of the DELPHI method to the use of experts. Manag Sci. 1963;9(3):458–67. [Google Scholar]
- 85.Klepac Pogrmilovic B, O’Sullivan G, Milton K, Biddle SJH, Bauman A, Bellew W, et al. The development of the comprehensive analysis of policy on physical activity (CAPPA) framework. Int J Behav Nutr Phys Act. 2019;16(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gillis L, Tomkinson G, Olds T, Moreira C, Christie C, Nigg C, et al. Research priorities for child and adolescent physical activity and sedentary behaviours: an international perspective using a twin-panel Delphi procedure. Int J Behav Nutr Phys Act. 2013;10(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Healy S, Patterson F, Biddle S, Dumuid D, Glorieux I, Olds T, et al. It’s about time to exercise: development of the Exercise Participation Explained in Relation to Time (EXPERT) model. Br J Sports Med. 2024. 10.1136/bjsports-2024-108500. [DOI] [PubMed] [Google Scholar]
- 88.Brislane Á, Hayman MJ, Davenport MH. A Delphi study to identify research priorities regarding physical activity, sedentary behavior and sleep in pregnancy. Int J Environ Res Public Health. 2022;19(5):2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Williamson C, Kelly P, Tomasone JR, Bauman A, Mutrie N, Niven A, et al. A modified Delphi study to enhance and gain international consensus on the Physical Activity Messaging Framework (PAMF) and Checklist (PAMC). Int J Behav Nutr Phys Act. 2021;18(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hanson CL, Oliver EJ, Dodd-Reynolds CJ, Pearsons A, Kelly P. A modified Delphi study to gain consensus for a taxonomy to report and classify physical activity referral schemes (PARS). Int J Behav Nutr Phys Act. 2020;17(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Slade SC, Dionne CE, Underwood M, Buchbinder R, Beck B, Bennell K, et al. Consensus on Exercise Reporting Template (CERT): Modified Delphi Study. Phys Ther. 2016;96(10):1514–24. [DOI] [PubMed] [Google Scholar]
- 92.Brown DMY, Kwan MYW, King-Dowling S, Cairney J. Cross-sectional associations between wake-time movement compositions and mental health in preschool children with and without motor coordination problems. Front Pediatr. 2021;9: 752333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bourke M, Vanderloo LM, Irwin JD, Burke SM, Johnson AM, Driediger M, et al. Association between childcare movement behaviour compositions with health and development among preschoolers: Finding the optimal combinations of physical activities and sedentary time. J Sports Sci. 2022;40(18):2085–94. [DOI] [PubMed] [Google Scholar]
- 94.Verswijveren SJJM, Lamb KE, Martín-Fernández JA, Winkler E, Leech RM, Timperio A, et al. Using compositional data analysis to explore accumulation of sedentary behavior, physical activity and youth health. J Sport Health Sci. 2022;11(2):234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tremblay MS, Duncan MJ, Kuzik N, Silva DAS, Carson V. Towards precision 24-hour movement behavior recommendations—the next new paradigm? J Sport Health Sci. 2024;13:743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Asano Y, Nagata K, Shibuya K, Fujii Y, Kitano N, Okura T. Association of 24-h movement behaviors with phase angle in community-dwelling older adults: a compositional data analysis. Aging Clin Exp Res. 2023;35(7):1469–76. [DOI] [PubMed] [Google Scholar]
- 97.Bezerra TA, Clark CCT, Souza Filho AND, Fortes LDS, Mota JAPS, Duncan MJ, et al. 24-hour movement behaviour and executive function in preschoolers: a compositional and isotemporal reallocation analysis. Eur J Sport Sci. 2021;21:1064–72. [DOI] [PubMed] [Google Scholar]
- 98.Bianchim MS, McNarry MA, Holland A, Cox NS, Dreger J, Barker AR, et al. A compositional analysis of physical activity, sedentary time, and sleep and associated health outcomes in children and adults with Cystic Fibrosis. Int J Environ Res Public Health. 2022;19(9):5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Biddle G, Edwardson C, Henson J, Davies M, Khunti K, Rowlands A, et al. Associations of physical behaviours and behavioural reallocations with markers of metabolic health: a compositional data analysis. Int J Environ Res Public Health. 2018. 10.1002/central/CN-01702982/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blodgett JM, Mitchell JJ, Stamatakis E, Chastin S, Hamer M. Associations between the composition of daily time spent in physical activity, sedentary behaviour and sleep and risk of depression: compositional data analyses of the 1970 British cohort Study. J Affect Disord. 2023;320:616–20. [DOI] [PubMed] [Google Scholar]
- 101.Blodgett JM, Ahmadi MN, Atkin AJ, Chastin S, Chan HW, Suorsa K, et al. Device-measured physical activity and cardiometabolic health: the Prospective Physical Activity, Sitting, and Sleep (ProPASS) consortium. Eur Heart J. 2024;45(6):458–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cabanas-Sánchez V, Esteban-Cornejo I, García-Esquinas E, Ortolá R, Ara I, Rodríguez-Gómez I, et al. Cross-sectional and prospective associations of sleep, sedentary and active behaviors with mental health in older people: a compositional data analysis from the Seniors-ENRICA-2 study. Int J Behav Nutr Phys Act. 2021;18(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carson V, Tremblay MS, Chaput JP, Chastin SFM. Associations between sleep duration, sedentary time, physical activity, and health indicators among Canadian children and youth using compositional analyses. Appl Physiol Nutr Metab. 2016;41(6 Suppl 3):S294-302. [DOI] [PubMed] [Google Scholar]
- 104.Carson V, Tremblay MS, Chastin SFM. Cross-sectional associations between sleep duration, sedentary time, physical activity, and adiposity indicators among Canadian preschool-aged children using compositional analyses. BMC Public Health. 2017;17(5):848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chao L, Ma R, Jiang W. Movement behaviours and anxiety symptoms in Chinese college students: A compositional data analysis. Front Psychol. 2022;13: 952728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen H, Wang LJ, Xin F, Liang G, Zhou YL. Associations between 24-h movement behaviours and BMI in Chinese primary- and middle- school students. J Exerc Sci Fit. 2023;21(2):186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chong KH, Parrish AM, Cliff DP, Dumuid D, Okely AD. Cross-sectional and longitudinal associations between 24-hour movement behaviours, recreational screen use and psychosocial health outcomes in children: A compositional data analysis approach. Int J Environ Res Public Health. 2021;18(11):5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chong KH, Dumuid D, Cliff DP, Parrish AM, Okely AD. Changes in 24-hour domain-specific movement behaviors and their associations with children’s psychosocial health during the transition from primary to secondary school: a compositional data analysis. J Phys Act Health. 2022;19(5):358–66. [DOI] [PubMed] [Google Scholar]
- 109.Clarke AE, Janssen I. A compositional analysis of time spent in sleep, sedentary behaviour and physical activity with all-cause mortality risk. Int J Behav Nutr Phys Act. 2021;18(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Collings PJ, Backes A, Aguayo GA, Fagherazzi G, Malisoux L, the ORISCAV-LUX study group. Substituting device-measured sedentary time with alternative 24-hour movement behaviours: compositional associations with adiposity and cardiometabolic risk in the ORISCAV-LUX 2 study. Diabetol Metab Syndr. 2023;15(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Collings PJ, Backes A, Malisoux L. Arterial stiffness and the reallocation of time between device-measured 24-hour movement behaviours: a compositional data analysis. Atherosclerosis. 2023;379: 117185. [DOI] [PubMed] [Google Scholar]
- 112.Curtis RG, Dumuid D, Olds T, Plotnikoff R, Vandelanotte C, Ryan J, et al. The association between time-use behaviors and physical and mental well-being in adults: a compositional isotemporal substitution analysis. J Phys Act Health. 2020;17(2):197–203. [DOI] [PubMed] [Google Scholar]
- 113.Curtis RG, Dumuid D, McCabe H, Singh B, Ferguson T, Maher C. The association between 24-hour activity, sedentary and sleep compositions and mental health in Australian adults: a cross-sectional study. J Act Sedentary Sleep Behav. 2023;2(1):15. [Google Scholar]
- 114.de Faria FR, Barbosa D, Howe CA, Canabrava KLR, Sasaki JE, Dos Santos Amorim PR. Time-use movement behaviors are associated with scores of depression/anxiety among adolescents: a compositional data analysis. PLoS ONE. 2022;17(12): e0279401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Del Pozo CB, Alfonso-Rosa RM, McGregor D, Chastin SF, Palarea-Albaladejo J, Del Pozo CJ. Sedentary behaviour is associated with depression symptoms: compositional data analysis from a representative sample of 3233 US adults and older adults assessed with accelerometers. J Affect Disord. 2020;265:59–62. [DOI] [PubMed] [Google Scholar]
- 116.Domingues SF, Diniz da Silva C, Faria FR, de Sá Souza H, Dos Santos Amorim PR. Sleep, sedentary behavior, and physical activity in Brazilian adolescents: Achievement recommendations and BMI associations through compositional data analysis. PLoS ONE. 2022;17(4): e0266926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dumuid D. Adiposity and the isotemporal substitution of physical activity, sedentary time and sleep among school-aged children: a compositional data analysis approach. BMC Public Health. 2018;18:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dumuid D, Lewis LK, Olds TS, Maher C, Bondarenko C, Norton L. Relationships between older adults’ use of time and cardio-respiratory fitness, obesity and cardio-metabolic risk: a compositional isotemporal substitution analysis. Maturitas. 2018;110:104–10. [DOI] [PubMed] [Google Scholar]
- 119.Dumuid D, Maher C, Lewis LK, Stanford TE, Martín Fernández JA, Ratcliffe J, et al. Human development index, children’s health-related quality of life and movement behaviors: a compositional data analysis. Qual Life Res. 2018;27(6):1473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dumuid D, Wake M, Clifford S, Burgner D, Carlin JB, Mensah FK, et al. The association of the body composition of children with 24-hour activity composition. J Pediatr. 2019;208:43-49.e9. [DOI] [PubMed] [Google Scholar]
- 121.Dumuid D, Simm P, Wake M, Burgner D, Juonala M, Wu F, et al. The “Goldilocks Day” for children’s skeletal health: compositional data analysis of 24-hour activity behaviors. J Bone Miner Res. 2020;35(12):2393–403. [DOI] [PubMed] [Google Scholar]
- 122.Dumuid D, Wake M, Burgner D, Tremblay MS, Okely AD, Edwards B, et al. Balancing time use for children’s fitness and adiposity: evidence to inform 24-hour guidelines for sleep, sedentary time and physical activity. PLoS ONE. 2021;16(1): e0245501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dumuid D, Mellow ML, Olds T, Tregoweth E, Greaves D, Keage H, et al. Does APOE ɛ4 status change how 24-hour time-use composition is associated with cognitive function? An exploratory analysis among middle-to-older adults. J Alzheimers Dis JAD. 2022;88(3):1157–65. [DOI] [PubMed] [Google Scholar]
- 124.Fairclough SJ, Dumuid D, Taylor S, Curry W, McGrane B, Stratton G, et al. Fitness, fatness and the reallocation of time between children’s daily movement behaviours: an analysis of compositional data. Int J Behav Nutr Phys Act. 2017;14(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fairclough SJ, Tyler R, Dainty JR, Dumuid D, Richardson C, Shepstone L, et al. Cross-sectional associations between 24-hour activity behaviours and mental health indicators in children and adolescents: a compositional data analysis. J Sports Sci. 2021;39:1602–14. [DOI] [PubMed] [Google Scholar]
- 126.Farrahi V, Kangas M, Walmsley R, Niemelä M, Kiviniemi A, Puukka K, et al. Compositional associations of sleep and activities within the 24-h cycle with cardiometabolic health markers in adults. Med Sci Sports Exerc. 2021;53(2):324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Feter N, de Paula D, Dos Reis RCP, Alvim Matos SM, Barreto SM, Duncan BB, et al. Association between 24-hour movement behavior and cognitive function in Brazilian middle-aged and older adults: findings from the ELSA-Brasil. Innov Aging. 2023;7(3):igad030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Franssen WMA, Jermei J, Savelberg HHCM, Eijnde BO. The potential harms of sedentary behaviour on cardiometabolic health are mitigated in highly active adults: a compositional data analysis. J Act Sedentary Sleep Behav. 2023;2(1):6. [Google Scholar]
- 129.Gupta N, Dumuid D, Korshøj M, Jørgensen MB, Søgaard K, Holtermann A. Is daily composition of movement behaviors related to blood pressure in working adults? Med Sci Sports Exerc. 2018;50(10):2150–5. [DOI] [PubMed] [Google Scholar]
- 130.Gupta N, Korshøj M, Dumuid D, Coenen P, Allesøe K, Holtermann A. Daily domain-specific time-use composition of physical behaviors and blood pressure. Int J Behav Nutr Phys Act. 2019;16(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gupta N, Dencker-Larsen S, Lund Rasmussen C, McGregor D, Rasmussen CDN, Thorsen SV, et al. The physical activity paradox revisited: a prospective study on compositional accelerometer data and long-term sickness absence. Int J Behav Nutr Phys Act. 2020;17(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gupta N, Hallman DM, Dumuid D, Vij A, Rasmussen CL, Jørgensen MB, et al. Movement behavior profiles and obesity: a latent profile analysis of 24-h time-use composition among Danish workers. Int J Obes 2005. 2020;44(2):409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hofman A, Voortman T, Ikram MA, Luik AI. Substitutions of physical activity, sedentary behaviour and sleep: associations with mental health in middle-aged and elderly persons. J Epidemiol Community Health. 2022;76(2):175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kandola AA, del Pozo CB, Osborn DPJ, Stubbs B, Choi KW, Hayes JF. Impact of replacing sedentary behaviour with other movement behaviours on depression and anxiety symptoms: a prospective cohort study in the UK Biobank. BMC Med. 2021;19(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kastelic K, Šarabon N, Stanford T, Dumuid D, Pedišić Ž. Are reallocations of time between physical activity, sedentary behaviour and sleep associated with low back pain? A compositional data analysis. BMJ Open Sport Exerc Med. 2023;9(4): e001701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kitano N, Kai Y, Jindo T, Tsunoda K, Arao T. Compositional data analysis of 24-hour movement behaviors and mental health in workers. Prev Med Rep. 2020;20: 101213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kuzik N, Naylor PJ, Spence JC, Carson V. Movement behaviours and physical, cognitive, and social-emotional development in preschool-aged children: cross-sectional associations using compositional analyses. PLoS ONE. 2020;15(8): e0237945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Larisch LM, Kallings LV, Hagströmer M, Desai M, von Rosen P, Blom V. Associations between 24 h movement behavior and mental health in office workers. Int J Environ Res Public Health. 2020;17(17):6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lau PWC, Song H, Song D, Wang JJ, Zhen S, Shi L, et al. 24-Hour movement behaviors and executive functions in preschoolers: a compositional and isotemporal reallocation analysis. Child Dev. 2024;95(2):e110–21. [DOI] [PubMed] [Google Scholar]
- 140.Le F, Yap Y, Tung NYC, Bei B, Wiley JF. The associations between daily activities and affect: a compositional isotemporal substitution analysis. Int J Behav Med. 2022;29(4):456–68. [DOI] [PubMed] [Google Scholar]
- 141.Lee J, Walker ME, Matthews KA, Kuller LH, Ranjit N, Gabriel KP. Associations of physical activity and sleep with cardiometabolic risk in older women. Prev Med Rep. 2020;18: 101071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lemos L, Clark C, Brand C, Pessoa ML, Gaya A, Mota J, et al. 24-hour movement behaviors and fitness in preschoolers: a compositional and isotemporal reallocation analysis. Scand J Med Sci Sports. 2021;31(6):1371–9. [DOI] [PubMed] [Google Scholar]
- 143.Lin Z, Zhu S, Cheng J, Lin Q, Lawrence WR, Zhang W, et al. The mediating effect of engagement in physical activity over a 24-hour period on chronic disease and depression: using compositional mediation model. J Affect Disord. 2022;299:264–72. [DOI] [PubMed] [Google Scholar]
- 144.Lu Z, Qu X, Chang J, Xu M, Song G, Wang X, et al. Reallocation of time between preschoolers’ 24-h movement behaviours and executive functions: a compositional data analysis. J Sports Sci. 2023;41(12):1187–95. [DOI] [PubMed] [Google Scholar]
- 145.Lund Rasmussen C, Gába A, Stanford T, Dygrýn J, Dumuid D, Janda D, et al. The Goldilocks Day for healthy adiposity measures among children and adolescents. Front Public Health. 2023;11:1158634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Madden KM, Feldman B, Chase J. Sedentary time and metabolic risk in extremely active older adults. Diabetes Care. 2021;44(1):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Marshall ZA, Mackintosh KA, Gregory JW, McNarry MA. Using compositional analysis to explore the relationship between physical activity and cardiovascular health in children and adolescents with and without type 1 diabetes. Pediatr Diabetes. 2022;23(1):115–25. [DOI] [PubMed] [Google Scholar]
- 148.Marshall ZA, Mackintosh KA, McNarry MA. Investigating the influence of physical activity composition on arterial stiffness in youth. Eur J Sport Sci. 2023;23(4):617–24. [DOI] [PubMed] [Google Scholar]
- 149.Matricciani L, Dumuid D, Paquet C, Fraysse F, Wang Y, Baur LA, et al. Sleep and cardiometabolic health in children and adults: examining sleep as a component of the 24-h day. Sleep Med. 2021;78:63–74. [DOI] [PubMed] [Google Scholar]
- 150.McGee M, Unger S, Hamilton J, Birken CS, Pausova Z, Vanderloo LM, et al. Lean mass accretion in children born very low birth weight is significantly associated with estimated changes from sedentary time to light physical activity. Pediatr Obes. 2020;15(5): e12610. [DOI] [PubMed] [Google Scholar]
- 151.McGregor DE, Carson V, Palarea-Albaladejo J, Dall PM, Tremblay MS, Chastin SFM. Compositional analysis of the associations between 24-h movement behaviours and health indicators among adults and older adults from the Canadian Health Measure Survey. Int J Environ Res Public Health. 2018;15(8):1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McGregor DE, Palarea-Albaladejo J, Dall PM, Del Pozo CB, Chastin SFM. Compositional analysis of the association between mortality and 24-hour movement behaviour from NHANES. Eur J Prev Cardiol. 2021;28(7):791–8. [DOI] [PubMed] [Google Scholar]
- 153.Mellow ML, Dumuid D, Wade AT, Stanford T, Olds TS, Karayanidis F, et al. Twenty-four-hour time-use composition and cognitive function in older adults: cross-sectional findings of the ACTIVate study. Front Hum Neurosci. 2022;16:1051793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mellow ML, Dumuid D, Olds T, Stanford T, Dorrian J, Wade AT, et al. Cross-sectional associations between 24-hour time-use composition, grey matter volume and cognitive function in healthy older adults. Int J Behav Nutr Phys Act. 2024;21(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Migueles JH, Cadenas-Sanchez C, Esteban-Cornejo I, Torres-Lopez LV, Aadland E, Chastin SF, et al. Associations of objectively-assessed physical activity and sedentary time with hippocampal gray matter volume in children with overweight/obesity. J Clin Med. 2020;9(4):1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Migueles JH, Delisle Nyström C, Dumuid D, Leppänen MH, Henriksson P, Löf M. Longitudinal associations of movement behaviours with body composition and physical fitness from 4 to 9 years of age: structural equation and mediation analysis with compositional data. Int J Behav Nutr Phys Act. 2023;20(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mitchell JJ, Blodgett JM, Chastin SF, Jefferis BJ, Wannamethee SG, Hamer M. Exploring the associations of daily movement behaviours and mid-life cognition: a compositional analysis of the 1970 British Cohort Study. J Epidemiol Community Health. 2023;77(3):189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mota JG, Clark CCT, Bezerra TA, Lemos L, Reuter CP, Mota JAPS, et al. Twenty-four-hour movement behaviours and fundamental movement skills in preschool children: a compositional and isotemporal substitution analysis. J Sports Sci. 2020;38(18):2071–9. [DOI] [PubMed] [Google Scholar]
- 159.Murray RM, Doré I, Sabiston CM, Michael F, O’Loughlin JL. A time compositional analysis of the association between movement behaviors and indicators of mental health in young adults. Scand J Med Sci Sports. 2023;33(12):2598–607. [DOI] [PubMed] [Google Scholar]
- 160.Ng E, Wake M, Olds T, Lycett K, Edwards B, Le H, et al. Equivalence curves for healthy lifestyle choices. Pediatrics. 2021;147(4): e2020025395. [DOI] [PubMed] [Google Scholar]
- 161.Niemelä M, Kiviniemi A, Ikäheimo TM, Tulppo M, Korpelainen R, Jämsä T, et al. Compositional association of 24-h movement behavior with incident major adverse cardiac events and all-cause mortality. Scand J Med Sci Sports. 2023;33(5):641–50. [DOI] [PubMed] [Google Scholar]
- 162.Oviedo-Caro MÁ, Bueno-Antequera J, Munguía-Izquierdo D. Associations of 24-hours activity composition with adiposity and cardiorespiratory fitness: the PregnActive project. Scand J Med Sci Sports. 2020;30(2):295–302. [DOI] [PubMed] [Google Scholar]
- 163.Pina I, Mendham AE, Tomaz SA, Goedecke JH, Micklesfield LK, Brooks NE, et al. Intensity matters for musculoskeletal health: a cross-sectional study on movement behaviors of older adults from high-income Scottish and low-income South African communities. Int J Environ Res Public Health. 2021;18(8):4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Powell C, Browne LD, Carson BP, Dowd KP, Perry IJ, Kearney PM, et al. Use of compositional data analysis to show estimated changes in cardiometabolic health by reallocating time to light-intensity physical activity in older adults. Sports Med Auckl NZ. 2020;50(1):205–17. [DOI] [PubMed] [Google Scholar]
- 165.Rees-Punia E, Guinter MA, Gapstur SM, Wang Y, Patel AV. Composition of time in movement behaviors and weight change in Latinx, Black and white participants. PLoS ONE. 2021;16(1): e0244566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Runacres A, MacKintosh KA, Chastin S, McNarry MA. The associations of physical activity, sedentary time, and sleep with V˙O2max in trained and untrained children and adolescents: a novel five-part compositional analysis. PLoS ONE. 2023;18(3): e0275557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sandborg J, Migueles JH, Söderström E, Blomberg M, Henriksson P, Löf M. Physical activity, body composition, and cardiometabolic health during pregnancy: a compositional data approach. Med Sci Sports Exerc. 2022;54(12):2054–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Segura-Jiménez V, Pedišić Ž, Gába A, Dumuid D, Olds T, Štefelová N, et al. Longitudinal reallocations of time between 24-h movement behaviours and their associations with inflammation in children and adolescents: the UP&DOWN study. Int J Behav Nutr Phys Act. 2023;20(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Smith E, Fazeli F, Wilkinson K, Clark CCT. Physical behaviors and fundamental movement skills in British and Iranian children: An isotemporal substitution analysis. Scand J Med Sci Sports. 2021;31(2):398–404. [DOI] [PubMed] [Google Scholar]
- 170.Smith AE, Dumuid D, Goldsworthy MR, Graetz L, Hodyl N, Thornton NLR, et al. Daily activities are associated with non-invasive measures of neuroplasticity in older adults. Clin Neurophysiol. 2021;132(4):984–92. [DOI] [PubMed] [Google Scholar]
- 171.St Laurent CW, Rasmussen CL, Holmes JF, Cremone-Caira A, Kurdziel LBF, Desrochers PC, et al. Associations of activity, sedentary, and sleep behaviors with cognitive and social-emotional health in early childhood. J Act Sedentary Sleep Behav. 2023;2(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Su J, Wei E, Clark C, Liang K, Sun X. Physical exercise, sedentary behaviour, sleep and depression symptoms in Chinese young adults during the COVID-19 pandemic: a compositional isotemporal analysis. Int J Ment Health Promot. 2022;24(5):759–69. [Google Scholar]
- 173.Suorsa K, Gupta N, Leskinen T, Andersen LL, Pasanen J, Hettiarachchi P, et al. Modifications of 24-h movement behaviors to prevent obesity in retirement: a natural experiment using compositional data analysis. Int J Obes. 2023;47(10):922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Swindell N, Rees P, Fogelholm M, Drummen M, MacDonald I, Martinez JA, et al. Compositional analysis of the associations between 24-h movement behaviours and cardio-metabolic risk factors in overweight and obese adults with pre-diabetes from the PREVIEW study: cross-sectional baseline analysis. Int J Behav Nutr Phys Act. 2020;17(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Talarico R, Janssen I. Compositional associations of time spent in sleep, sedentary behavior and physical activity with obesity measures in children. Int J Obes 2005. 2018;42(8):1508–14. [DOI] [PubMed] [Google Scholar]
- 176.Tan SYX, Padmapriya N, Bernard JY, Toh JY, Wee HL, Tan KH, et al. Cross-sectional and prospective associations between children’s 24-h time use and their health-related quality of life: a compositional isotemporal substitution approach. Lancet Reg Health West Pac. 2023;41: 100918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Taylor R, Haszard J, Farmer V, Richards R, Te Morenga L, Meredith-Jones K, et al. Do differences in compositional time use explain ethnic variation in the prevalence of obesity in children? Analyses using 24-hour accelerometry. Int J Obes 2005. 2020;44(1):94–103. [DOI] [PubMed] [Google Scholar]
- 178.Taylor RW, Haszard JJ, Meredith-Jones KA, Azeem AA, Galland BC, Heath ALM, et al. Associations between activity, sedentary and sleep behaviours and psychosocial health in young children: a longitudinal compositional time-use study. J Act Sedentary Sleep Behav. 2023;2(1):3. [Google Scholar]
- 179.Tyler R, Atkin AJ, Dainty JR, Dumuid D, Fairclough SJ. Cross-sectional associations between 24-hour activity behaviours and motor competence in youth: a compositional data analysis. J Act Sedentary Sleep Behav. 2022;1(1):3. [Google Scholar]
- 180.Vanderlinden J, Biddle GJH, Boen F, van Uffelen JGZ. To be well or not to be well: compositional associations of physical activity, sedentary behaviour and sleep with mental well-being in Flemish adults aged 55+ years. J Act Sedentary Sleep Behav. 2023;2(1):9. [Google Scholar]
- 181.Verhoog S, Braun KVE, Bano A, van Rooij FJA, Franco OH, Koolhaas CM, et al. Associations of activity and sleep with quality of life: a compositional data analysis. Am J Prev Med. 2020;59(3):412–9. [DOI] [PubMed] [Google Scholar]
- 182.Walmsley R, Chan S, Smith-Byrne K, Ramakrishnan R, Woodward M, Rahimi K, et al. Reallocation of time between device-measured movement behaviours and risk of incident cardiovascular disease. Br J Sports Med. 2021;56(18):1008–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang S, Liang W, Song H, Su N, Zhou L, Duan Y, et al. Prospective association between 24-hour movement behaviors and mental health among overweight/obese college students: a compositional data analysis approach. Front Public Health. 2023;11:1203840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.