Abstract
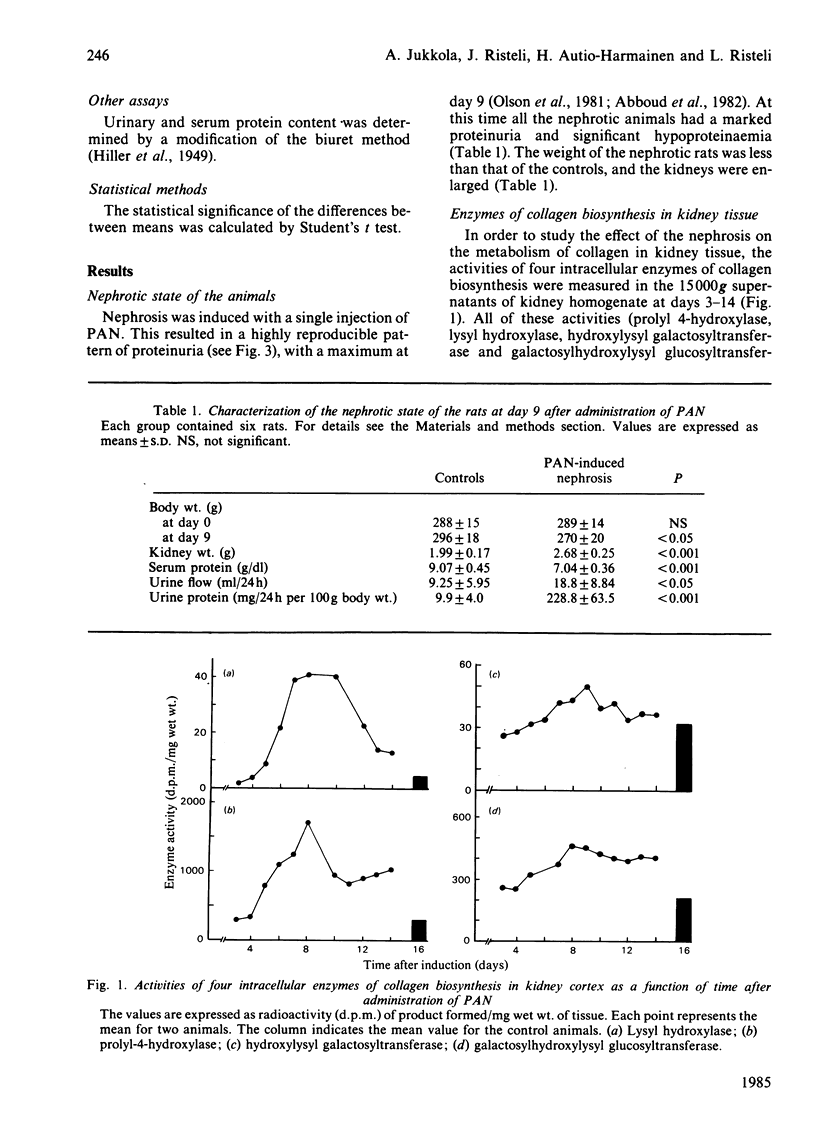
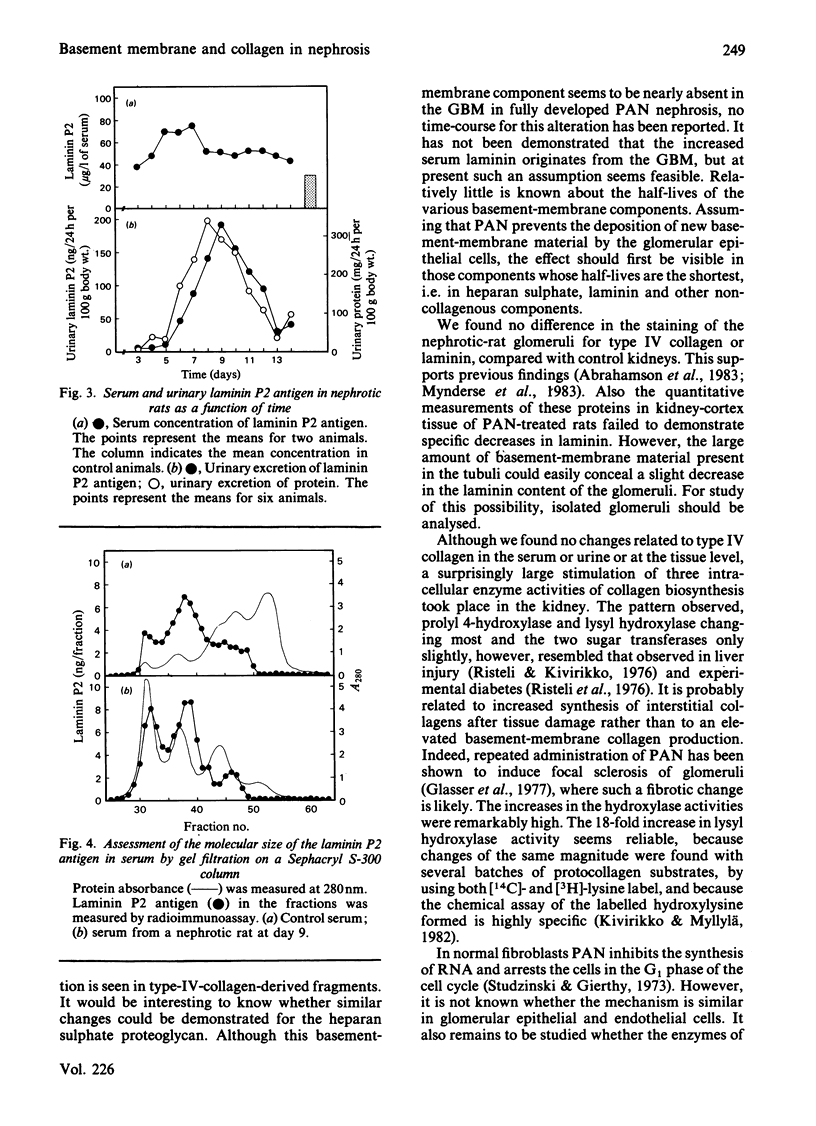
The aim of the present study was to find out whether the basement-membrane proteins laminin and type IV collagen are involved in the development of aminonucleoside-induced nephrosis. These proteins were measured by specific radioimmunoassays in serum, urine and kidney-cortex samples, and they were localized in the glomeruli by indirect immunofluorescence. Nephrosis was induced in rats with a single intraperitoneal injection of puromycin aminonucleoside. Serum laminin concentrations, detected by a radioimmunoassay for the P2 domain of the protein, increased to reach a maximum at days 5-7, and they remained elevated until at least day 14. The increase preceded the development of proteinuria, suggesting a role for laminin in glomerular function. Concomitant with proteinuria, increasing amounts of laminin antigenicity were also found in the urine. The size of the laminin antigen in serum was estimated by gel filtration, and the serum forms were found to contain both the P1 and the P2 regions of the intact laminin molecule. On the other hand, there were no changes in the serum or urinary concentrations of type-IV-collagen-derived antigens, as detected by a radioimmunoassay for the 7S collagen domain of this protein. The total content of laminin in kidney cortex, measured after digestion of the tissue with trypsin and collagenase, was, at day 9, still comparable with normal values, and the distribution of both basement-membrane proteins in the glomeruli, studied by indirect immunofluorescence, was similar to that in the controls. The tissue damage induced by aminonucleoside, however, seems to stimulate collagen biosynthesis, as the activities of prolyl 4-hydroxylase, lysyl hydroxylase and galactosylhydroxylysyl glucosyltransferase in kidney tissue increased significantly, with maxima at days 8-10.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abboud H. E., Ou S. L., Velosa J. A., Shah S. V., Dousa T. P. Dynamics of renal histamine in normal rat kidney and in nephrosis induced by aminonucleoside of puromycin. J Clin Invest. 1982 Feb;69(2):327–336. doi: 10.1172/JCI110456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson D. R., Hein A., Caulfield J. P. Laminin in glomerular basement membranes of aminonucleoside nephrotic rats. Increased proteinuria induced by antilaminin immunoglobulin G. Lab Invest. 1983 Jul;49(1):38–47. [PubMed] [Google Scholar]
- Bohrer M. P., Baylis C., Robertson C. R., Brenner B. M., Troy J. L., Willis W. T. Mechanisms of the puromycin-induced defects in the transglomerular passage of water and macromolecules. J Clin Invest. 1977 Jul;60(1):152–161. doi: 10.1172/JCI108751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Hostetter T. H., Humes H. D. Molecular basis of proteinuria of glomerular origin. N Engl J Med. 1978 Apr 13;298(15):826–833. doi: 10.1056/NEJM197804132981507. [DOI] [PubMed] [Google Scholar]
- Carlin B., Jaffe R., Bender B., Chung A. E. Entactin, a novel basal lamina-associated sulfated glycoprotein. J Biol Chem. 1981 May 25;256(10):5209–5214. [PubMed] [Google Scholar]
- Caulfield J. P., Farquhar M. G. Loss of anionic sites from the glomerular basement membrane in aminonucleoside nephrosis. Lab Invest. 1978 Nov;39(5):505–512. [PubMed] [Google Scholar]
- Caulfield J. P., Reid J. J., Farquhar M. G. Alterations of the glomerular epithelium in acute aminonucleoside nephrosis. Evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest. 1976 Jan;34(1):43–59. [PubMed] [Google Scholar]
- Glasser R. J., Velosa J. A., Michael A. F. Experimental model of focal sclerosis. I. Relationship to protein excretion in aminonucleoside nephrosis. Lab Invest. 1977 May;36(5):519–526. [PubMed] [Google Scholar]
- Hassell J. R., Robey P. G., Barrach H. J., Wilczek J., Rennard S. I., Martin G. R. Isolation of a heparan sulfate-containing proteoglycan from basement membrane. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4494–4498. doi: 10.1073/pnas.77.8.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Presence of heparan sulfate in the glomerular basement membrane. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1303–1307. doi: 10.1073/pnas.76.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Jakubowski M. L. Unaltered anionic sites of glomerular basement membrane in aminonucleoside nephrosis. Kidney Int. 1984 Apr;25(4):613–618. doi: 10.1038/ki.1984.65. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A., Forsell-Knott L. Structural changes in the protein and carbohydrate components of glomerular basement membrane in aminonucleoside nephrosis. Biochim Biophys Acta. 1970 Mar 17;203(1):62–66. doi: 10.1016/0005-2736(70)90035-0. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Posttranslational enzymes in the biosynthesis of collagen: intracellular enzymes. Methods Enzymol. 1982;82(Pt A):245–304. doi: 10.1016/0076-6879(82)82067-3. [DOI] [PubMed] [Google Scholar]
- Krakower C. A., Nicholes B. K., Greenspon S. A. Proteinuria and the fragility of normal and diseased glomerular basement membrane. Proc Soc Exp Biol Med. 1978 Dec;159(3):324–334. doi: 10.3181/00379727-159-40342. [DOI] [PubMed] [Google Scholar]
- Michael A. F., Blau E., Vernier R. L. Glomerular polyanion. Alteration in aminonucleoside nephrosis. Lab Invest. 1970 Dec;23(6):649–657. [PubMed] [Google Scholar]
- Mynderse L. A., Hassell J. R., Kleinman H. K., Martin G. R., Martinez-Hernandez A. Loss of heparan sulfate proteoglycan from glomerular basement membrane of nephrotic rats. Lab Invest. 1983 Mar;48(3):292–302. [PubMed] [Google Scholar]
- Olson J. L., Rennke H. G., Venkatachalam M. A. Alterations in the charge and size selectivity barrier of the glomerular filter in aminonucleoside nephrosis in rats. Lab Invest. 1981 Mar;44(3):271–279. [PubMed] [Google Scholar]
- Ott U., Odermatt E., Engel J., Furthmayr H., Timpl R. Protease resistance and conformation of laminin. Eur J Biochem. 1982 Mar;123(1):63–72. doi: 10.1111/j.1432-1033.1982.tb06499.x. [DOI] [PubMed] [Google Scholar]
- Risteli J., Bächinger H. P., Engel J., Furthmayr H., Timpl R. 7-S collagen: characterization of an unusual basement membrane structure. Eur J Biochem. 1980;108(1):239–250. doi: 10.1111/j.1432-1033.1980.tb04717.x. [DOI] [PubMed] [Google Scholar]
- Risteli J., Kivirikko K. I. Intracellular enzymes of collagen biosynthesis in rat liver as a function of age and in hepatic injury induced by dimethylnitrosamine. Changes in prolyl hydroxylase, lysyl hydroxylase, collagen galactosyltransferase and collagen glucosyltransferase activities. Biochem J. 1976 Aug 15;158(2):361–367. doi: 10.1042/bj1580361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risteli J., Koivisto V. A., Akerblom H. K., Kivirikko K. I. Intracellular enzymes of collagen biosynthesis in rat kidney in streptozotocin diabetes. Diabetes. 1976 Nov;25(11):1066–1070. doi: 10.2337/diab.25.11.1066. [DOI] [PubMed] [Google Scholar]
- Risteli J., Rohde H., Timpl R. Sensitive radioimmunoassays for 7 S collagen and laminin: application to serum and tissue studies of basement membranes. Anal Biochem. 1981 May 15;113(2):372–378. doi: 10.1016/0003-2697(81)90091-9. [DOI] [PubMed] [Google Scholar]
- Risteli L., Risteli J., Ihme A., Krieg T., Müller P. K. Preferential hydroxylation of type IV collagen by lysyl hydroxylase from Ehlers-Danlos syndrome type VI fibroblasts. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1778–1784. doi: 10.1016/0006-291x(80)91380-7. [DOI] [PubMed] [Google Scholar]
- Risteli L., Timpl R. Isolation and characterization of pepsin fragments of laminin from human placental and renal basement membranes. Biochem J. 1981 Mar 1;193(3):749–755. doi: 10.1042/bj1930749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde H., Bächinger H. P., Timpl R. Characterization of pepsin fragments of laminin in a tumor basement membrane. Evidence for the existence of related proteins. Hoppe Seylers Z Physiol Chem. 1980 Nov;361(11):1651–1660. doi: 10.1515/bchm2.1980.361.2.1651. [DOI] [PubMed] [Google Scholar]
- Stenman S., Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med. 1978 Apr 1;147(4):1054–1064. doi: 10.1084/jem.147.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studzinski G. P., Gierthy J. F. Selective inhibition of the cell cycle of cultured human diploid fibroblasts by aminonucleoside of puromycin. J Cell Physiol. 1973 Feb;81(1):71–83. doi: 10.1002/jcp.1040810109. [DOI] [PubMed] [Google Scholar]
- Timpl R., Dziadek M., Fujiwara S., Nowack H., Wick G. Nidogen: a new, self-aggregating basement membrane protein. Eur J Biochem. 1983 Dec 15;137(3):455–465. doi: 10.1111/j.1432-1033.1983.tb07849.x. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Timpl R., Wiedemann H., van Delden V., Furthmayr H., Kühn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem. 1981 Nov;120(2):203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- VERNIER R. L., PAPERMASTER B. W., GOOD R. A. Aminonucleoside nephrosis. I. Electron microscopic study of the renal lesion in rats. J Exp Med. 1959 Jan 1;109(1):115–126. doi: 10.1084/jem.109.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velosa J. A., Shah S. V., Ou S. L., Abboud H. E., Dousa T. P. Activities of lysosomal enzymes in isolated glomeruli. Alterations in experimental nephrosis. Lab Invest. 1981 Dec;45(6):522–526. [PubMed] [Google Scholar]